Background
The Korean native pig (KNP) is generally believed to have come from northern China to the Korean peninsula approximately 2000 years ago [1]. KNP pigs have black coat color in general and were at the brink of extinction in the 1980s due to their smaller animal size and slower growth rate [2]. Since then, effort has been made to restore the breed by bringing together the remaining stocks from a few provinces in the Republic of Korea [2-4]. As a result, the restored KNP has been registered as a breed in 2006 [3]. Genetic relationships between KNP, Chinese Meishan [4] and Western [4,5] pig breeds have been determined using microsatellite markers. To find additional breed-specific markers that are distinct among pig breeds, several genes including O-linked N-acetylglucosamine transferase (OGT) were investigated. OGT is located on chromosome X and catalyzes the post-translational addition of a single O-linked-β-N-acetylglucosamine (O-GlcNAc) on the hydroxyl groups of Ser and/or Thr residues of target proteins [6]. GlcNAcylation regulates cellular signaling and transcription processes in response to nutrients and stress, and has extensive crosstalk with phosphorylation [6]. OGT is also involved in nutrient sensing [7]. OGT has been mapped within the quantitative trait locus (QTL) affecting backfat depth, boar plasma FSH, and testicular weight using a length polymorphism within the intron 20 of OGT between Chinese and the Western pig breeds [8]. This genomic region located on chromosome X was investigated using Meishan (MS) x White composite (WC) crossbred boars [9]. Further, boars with MS alleles at the region had smaller testicles and lower total daily sperm production than boars with WC alleles [10]. In MS x WC crossbred boars, the length polymorphism in OGT was applicable to genotyping and identifying the breed of origin [8]. Therefore, the role of OGT and differential contribution of the X chromosome from each breed in crossbred pigs is worthy of further investigation. The first objective of this study was to determine whether the length polymorphism in the intron 20 of OGT was present in KNP and this polymorphism could be used as a breed-specific marker among KNP, Chinese Meishan, and the Western pig breeds. In addition, KNP pigs have been used to produce specialty meat and they have been crossbred to Landrace or Yorkshire breeds for growth and meat quality trait studies in Korea [11-13]. Thus, the second objective of this study was to determine whether the length polymorphism can be used as a breed-specific marker in screening the breed of origin in crossbred pigs between KNP with known genotypes and the Western pig breeds.
Methods
The experimental protocol and standard operating procedures on experimental animals were reviewed and approved by the Institutional Animal Care and Use Committee of the National Institute of Animal Science, RDA (Suwon, Republic of Korea), in compliance with standard international regulations. Initially, 10 animals for each breed from the breeding stocks at the National Institute of Animal Science, Rural Development Administration, Republic of Korea were analyzed for the presence of the length polymorphism. The animals included 10 Duroc sows, 5 Landrace boars and 5 sows, 3 Yorkshire boars and 7 sows, 5 KNP boars and 5 sows, 1 Meishan boar, 8 sows and 1 unknown sex, and 10 Duroc x KNP crossbred sows. Subsequently, the number of pigs was increased to include 40 Duroc, 36 Landrace, 36 Yorkshire, 39 KNP, 10 Meishan, and 15 Duroc x KNP crossbred pigs (n = 176). Blood samples were collected and DNA was isolated.
Primers were designed to amplify across the intron 20 based on the porcine OGT cDNA (GenBank accession no. DQ400859) and the gene sequence in the Genome Browser for pig (http://genome.ucsc.edu/). The forward (2968F) and reverse (3083R) primers correspond to bases 2968–2987 and 3083–3059 of the porcine OGT cDNA (GenBank accession no. DQ400859), respectively, and the expected size of the PCR amplicon across intron 20 was 631 bp. PCR reactions were carried out in a 20-μl volume containing 30 ng of genomic DNA, 2 mM MgCl2, 10 pmol of each primer (OGT-2968F: 5′-GCACACCACAGGGATGGATG-3′ and OGT- 3083R: 5′- GCTCAAGACAACCTAAACAAGTAAG-3′), 200 μM dNTP, and 2 U Top-Taq™ DNA polymerase (Qiagen, Germany). Amplification was performed under the following PCR conditions: 10 min at 95°C; 35 cycles of 30 sec at 95°C, annealing for 30 sec at 60°C, and 1 min at 72°C; and a final extension of 5 min at 72°C. Length polymorphism among the pig breeds was determined after gel electrophoresis. Genotypes of the OGT gene among the Duroc, Landrace, Yorkshire, KNP, Meishan, and Duroc x KNP pigs (n = 176) were determined. A number of pigs were sequenced to confirm the OGT genotypes of Duroc, KNP, and Meishan pigs.
Results and discussion
Length polymorphism in the intron 20 of OGT among the pig breeds was determined by PCR and gel electrophoresis (Figure 1A). Both strands of the genomic DNA corresponding to the intron 20 of OGT were amplified from KNP, Duroc, and Meishan boars, sequenced, and then submitted to the GenBank (accession no. JQ045376-7 and JQ579450). Amplification of a fragment containing the intron 20 of OGT by PCR revealed variations between KNP, Chinese Meishan and the Western pig breeds, including Duroc, Landrace, and Yorkshire. Fragments containing the intron 20 of OGT appeared to be the same among Duroc, Landrace, and Yorkshire pigs and were longer than those from either Meishan or KNP pigs (Figure 1A). In the initial study, using 10 purebred pigs from each breed, amplicons from all 10 pigs from each breed were the same size, except KNP pigs (data not shown). Amplicons from 8 out of 10 KNP pigs were the same, but those from the remaining two were different. The size of the fragment containing the intron 20 of OGT from Duroc boars was 631 bp (GenBank accession no. JQ045377), whereas those of Meishan (GenBank accession no. JQ579450) and 8 out of 10 KNP pigs were 350 bp (GenBank accession no. JQ045376). Sequencing indicated that the sizes of OGT intron 20 of the Duroc boars were 514 bp, while those from Meishan or KNP boars were 233 bp, resulting in a difference of 281 bp. The size and sequence of the intron 20 OGT from Duroc boars were identical to the one in the Genome Browser for pig (http://genome.ucsc.edu/). The 281-bp difference in the intron between Duroc and Meishan or KNP was due to an inserted 276-bp element near the beginning of the intron and the 5-bp ACTTG insertion in the middle of the intron (Figure 1B). These results are in agreement with a previous study on Meishan and White composite pigs. In that study, using 83 boars of 16 litters from inter se matings of 1/2 Meishan (MS) × 1/2 White composite (WC) parents, the breeds of origin of X chromosome QTL were confirmed based on this OGT polymorphism in the boars. The boars had either MS allele with AA genotype or WC allele with BB genotype (Kim JG, Nonneman D, Rohrer GA, unpublished observations).
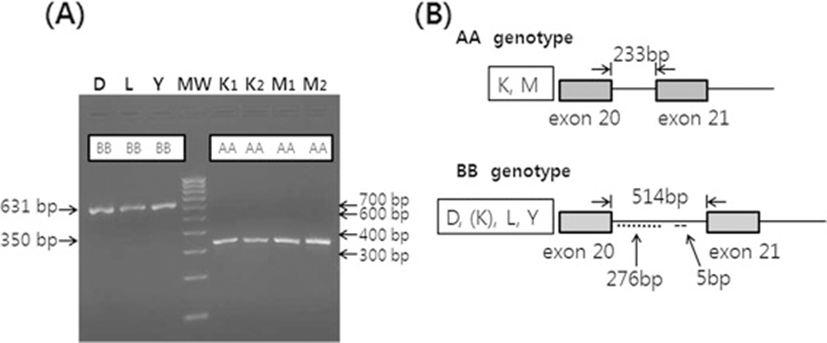
When the entire intron 20 sequence of OGT from Duroc (GenBank accession no. JQ045376) was compared using the algorithm “Basic Local Alignment Search Tool (BLAST)”, it was revealed that several homologous regions to this sequence existed in the human chromosome X and in pig chromosomes. However, when the inserted 276-bp element of the intron 20 was compared using the BLAST, homologous regions existed only in several pig chromosomes, but not in any other species. The sizes of the OGT intron 20 from human, cattle, and mice were 240, 243, and 262 bp (http://genome.ucsc.edu/), respectively, and thus, they are similar to the 233-bp intron from either Meishan or KNP boars. Sequence homologies of the OGT intron 20 of Meishan and KNP boars, which do not contain the inserted 276-bp element and the 5-bp ACTTG of the OGT intron 20, with those of the human, cattle and mice were 76.3%, 69.5%, and 62.0%, respectively. This suggests that the 276-bp element and the 5-bp ACTTG of the OGT intron 20 in Duroc and other Western pig breeds may have been inserted differentially during the domestication process in different regions.
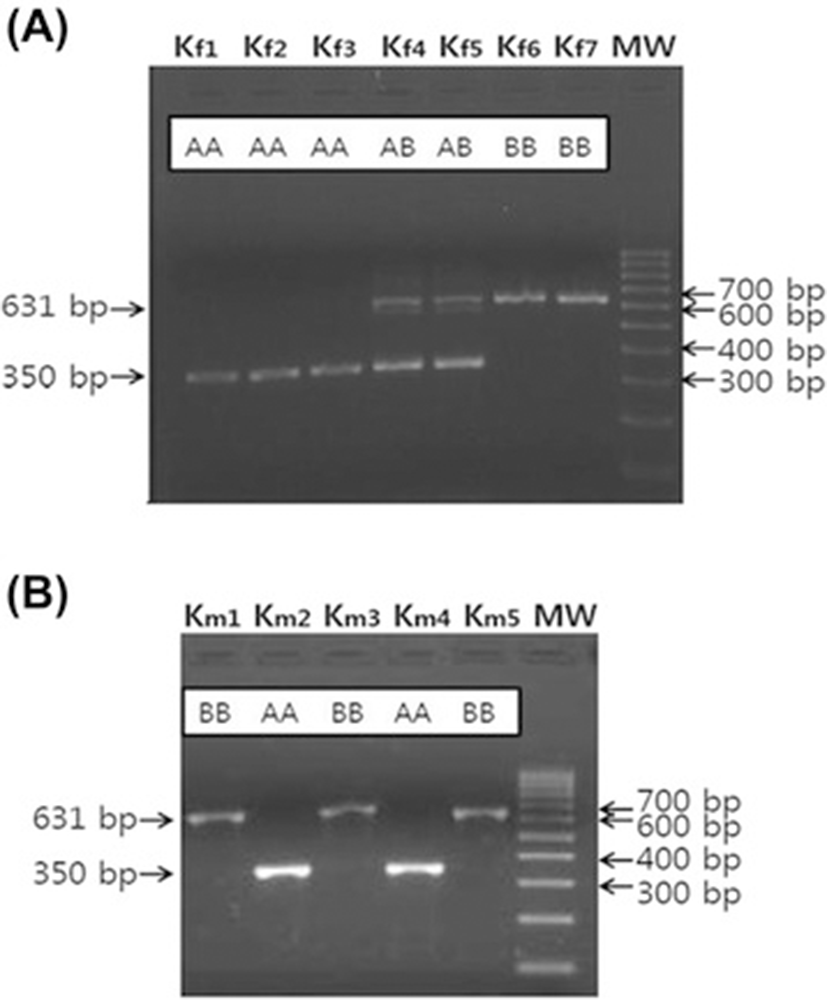
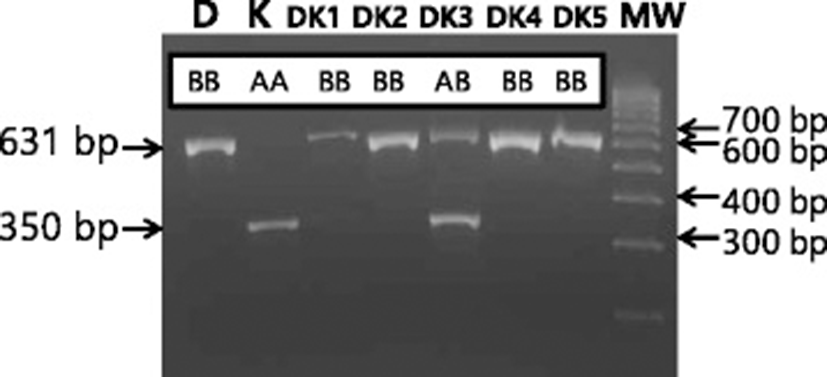
There was a variation in the intron 20 OGT among the 10 KNP pigs in the initial study. Unexpectedly, 8 out of the 10 KNP pigs had a short intron, which is the same as the Meishan pigs, whereas 2 KNP pigs had a long intron, which is the same as the Western pigs (data not shown). When additional pigs were analyzed, KNP pigs had short and long intron 20 of OGT, and others were heterozygotes, and thus the genotypes were designated as AA, BB, and AB, respectively (Figure 2A, B). In contrast to the KNP pigs, the genotypes of OGT in Meishan pigs and the Western breeds were fixed as AA and BB, respectively (Table 1). When genotypes of 39 KNP pigs were analyzed, there were 16 AA (41.0%), 13 AB (33.3%), and 10 BB (25.6%). When we compared the genotypes of OGT between KNP pigs born in 2009 and in 2010 or 2011, the frequency of allele A decreased slightly in 2010 or 2011 in comparison to 2009. The genotypes of 18 pigs born in 2009 were 8 AA (44.4%), 6 AB (33.3%), and 4 BB (22.2%). However, the genotypes of 21 pigs born in 2010 or 2011 were 8 AA (38.1%), 7 AB (33.3%), and 6 BB (28.6%). The results suggest that the locus is not fixed near OGT in KNP pigs. The observed frequency reduction of genotype AA among KNP pigs born in 2011 or 2012 could be due to the sampling bias of including more sows born from parents of AB or BB genotypes (Table 1). However, we cannot rule out that the reduction is due to the heterogametic state of boars or the result of genetic drift in males. After two generations of crossbreeding between Duroc with KNP pigs, all five Duroc x KNP (DK) crossbred boars and eight of 10 DK crossbred sows had BB genotypes, which is the same as Duroc boars, while the remaining two DK crossbred sows had AB genotypes (Table 1) (Figure 3).
| AA | AB | BB | Total | |
|---|---|---|---|---|
| Duroc | 0 | 0 | 40 | 40 |
| Landrace | 0 | 0 | 36 | 36 |
| Yorkshire | 0 | 0 | 36 | 36 |
| KNP | 16 | 13 | 10 | 39 |
| (KNP boar)a | (7) | (0) | (3) | (10) |
| (KNP sow)b | (9) | (13) | (7) | (29) |
| Meishan | 10 | 0 | 0 | 10 |
| Duroc x KNP | 0 | 2 | 13 | 15 |
| Total | 28 | 13 | 135 | 176 |
a,bBoars and sows of KNP pigs were calculated within ( ).
Conclusions
In conclusion, the length polymorphism in the intron 20 of OGT may be used as an additional marker for determining the breed of origin among Chinese Meishan and the Western pig breeds including Duroc, Landrace, and Yorkshire. The polymorphism identified in this study suggests that the locus near OGT is not fixed in KNP pigs, and this marker may supplement the restoration effort of KNP as an additional mean to verify the origin of the breed near this locus. It would be also relevant in determining the breed of origin in crossbred pigs between KNP with known genotypes and Duroc or other Western breeds with BB genotypes, and thus confirming the contribution of the X chromosome from each breed.
















