Background
Diarrhea is one of the major causes of mortality in neonatal and young piglets. It attributes to the approximately half (49%) of the pig death [1]. One of the most detrimental diseases resulting in diarrhea and death in swine is coliba-cillosis that is caused by pathogenic Escherichia coli (E. coli) infection [2]. The main symptoms of coliba-cillosis include vomiting, severe diarrhea, and dehydration leading to high mortality [2]. Though it occurs in pigs regardless of age, young piglets under 1 week of age are most vulnerable [3]. E. coli infection is transmitted mostly by the oral route through feed or water contaminated with pathogenic E. coli. In the case of newborn piglets, the infection is commonly transmitted through the feces or the nipple [3].
As the preventive and therapeutic measures against colibacillosis, antibiotics, such as antibiotic growth promoters (AGPs), have long been used. Therefore, the stable supply of effective antibiotics for pigs has been thought to be essential for the enhanced health and well-being of the animals [4]. As such, approximately 1,000 tons of macrolide and tetracycline antibiotics alone were added to pig feed each year in the U.S [5]. Despite the beneficial effects of antibiotics in pig production, there is a concern that the use of antibiotics in food animals may lead to the selection for antibiotic-resistant bacteria and the spread of increased antibiotic resistance gene pool in commensal bacteria in humans as well as animals [4].
In addition, the long-term use of antibiotics in agriculture makes it difficult to cure animal diseases caused by bacteria that are highly fertile and resistant [6]. Because the risks of antibiotics usage outweigh cost savings in livestock production [7], many countries have limited the use of antibiotics, and banned AGPs in food animal production. Consequently, probiotics, prebiotics or synbiotics have been presented as the most desirable alternatives to antibiotics due to their beneficial effects [8].
Probiotics are feed additives that can be used to replace antibiotics in animal feed [9]. They refer to a group of beneficial bacteria that contribute to the health and well-being of the host when administered in sufficient amounts [3]. As an antibiotic substitute, probiotics enhance the productivity of livestock by improving resistance to pathogens, helping the growth of beneficial microbiota in the gut and boosting immune activity. Prebiotics, such as lactulose, oligosaccharides, and lactitol are non-digestible feed supplements with constructive effects on the host as they assist the growth and activity of beneficial microbiota in the gut [9]. Prebiotics can selectively stimulate the growth of beneficial microbiota in the gut, therefore improve the immunity of livestock and durably maintain intestinal microbiota. Synbiotics, a mixture of probiotics and prebiotics, were developed to further enhance the effects of probiotics. Synbiotics, are ingested as feed supplements to provide health benefits through synergistic effects [10]. One mechanism of actions of synbiotics is to enhance the intestinal attachment of probiotics resulting in improved livestock productivity and feed efficiency [11,12]. As demand for synbiotics continuously grows because of their beneficial effects on livestock health, there are immense needs to develop synbiotics. Therefore, in this study, we produced the stable quality feed additives, synbiotics, and evaluated synbiotics as gut health improvement agents in the mouse model challenged with Shiga toxin-producing E. coli (STEC) isolated from piglets.
Materials and Methods
A total of 40 3-week old BALB/c mice were purchased from RaonBio Inc. (Gyeonggi-do, Korea), and randomly assigned into four groups (n = 10): control group without any treatment, treatment group 1 treated with synbiotics based on Pediococcus acidilactici GB-U15, treatment group 2 treated with synbiotics based on Lactobacillus plantarum GB-U17, and treatment group 3 treated with synbiotics based on Lactobacillus plantarum GB 1-3. Each synbiotics treated groups were daily administrated with 5.0 × 106 CFU/mL of one synbiotics for the first week, and every 3 days during the second week. Mice were challenged with 8.0 × 108 CFU/mL of STEC 5 days after animals began to receive synbiotics. Mice were reared in confinement with controlled light, temperature (25°C ± 2). All the mice in this experiment were fed with the same feed ad libitum. The general health status of mice was evaluated by recording fecal index, daily weight gain, mortality and clinical symptoms. Fecal index criterions that indicate stool hardness or softness are as follows. 1: Hard, dry pellets in a small, hard mass, 2: Hard, formed stool that remains firm and soft, 3: Soft, formed and moist stool that retains its shape, 4: Soft, unformed stool that assumes the shape of the container, 5: Watery, liquid stool that can be poured.
Prebiotics, lactulose, was formulated with each 5.0 × 106 CFU/mL of Pediococcus acidilactici GB-U15, Lactobacillus plantarum GB-U17, and Lactobacillus plantarum GB 1-3, to produce 3 types of syn-biotics. Probiotics were randomly selected lactic acid producing strains through evaluation of antimicrobial activity in vitro, and selected strains had excellent inhibitory effects against STEC, which is one of the major health problems in young piglets resulting in poor health and death. Three synbiotics containing lactulose and one of each Pediococcus aci-dilactici GB-U15, Lactobacillus plantarum GB-U17, and Lactobacillus plantarum U1-3 were manufactured by the Genebiotech Co., Ltd. (Seoul, Korea). Each synbiotics contains at least 5.0 × 106 CFU/mL of one probiotics selected.
STEC JOL576 was incubated in Luria-Bertani (LB) Broth (LPS SOLUTION, Daejeon, Korea) for 48 hours at 37°C. All the mice (n = 5 per group) were challenged with 150 uL of bacterial culture media containing 8.0 × 108 CFU/mL of STEC by oral gavage.
Two mice in each group were sacrificed by cervical dislocation one week after STEC oral challenge, and all other individual mice were autopsied at the end of the experiment, two weeks after STEC oral challenge. For histological examination, ileum and colon samples were fixed in 10% neutral buffered formalin, and stained with hematoxylin and eosin (HE). The staining was carried out by the Abion Inc. (Seoul, Korea).
Results
Mice treated with synbiotics based on Pediococcus acidilactici GB-U15 and Lactobacillus plantarum GB-U17 significantly improved daily weight gain compared to mice in other groups. In particular, mice treated with synbiotics based on GB-U15 showed the best growth performance (Table 1).
| DPI1) | Control2) | Group 13) | Group 24) | Group 35) | p-value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Average | Standard error | Average | Standard error | Average | Standard error | Average | Standard error | ||
| 0 | 100a | 0.00 | 100a | 0.00 | 100a | 0.00 | 100a | 0.00 | |
| 1 | 100.75b | 0.42 | 102.62ab | 0.68 | 103.45a | 0.50 | 101.50b | 0.18 | 0.004 |
| 2 | 101.55b | 0.42 | 105.07a | 1.14 | 106.85a | 1.01 | 105.23a | 0.43 | 0.0016 |
| 3 | 91.32b | 0.41 | 94.03ab | 0.72 | 94.42a | 1.29 | 92.72ab | 0.15 | 0.0402 |
| 4 | 90.50a | 0.70 | 91.93a | 0.84 | 91.85a | 1.52 | 90.15a | 0.78 | 0.4999 |
| 5 | 96.88a | 1.00 | 98.53a | 0.91 | 98.27a | 1.53 | 94.90a | 0.57 | 0.0924 |
| 6 | 100.15b | 0.17 | 103.05a | 0.77 | 102.83a | 0.72 | 99.75b | 0.28 | 0.0014 |
| 7 | 102.98b | 0.25 | 105.70a | 0.72 | 105.65a | 0.83 | 102.28b | 0.39 | 0.0022 |
| 8 | 103.18b | 0.63 | 107.93a | 1.13 | 107.53a | 1.41 | 102.70b | 0.50 | 0.0036 |
| 9 | 105.40a | 0.68 | 109.83a | 1.77 | 109.80a | 1.56 | 106.50a | 1.74 | 0.1303 |
| 10 | 108.15ab | 0.62 | 112.10a | 1.62 | 111.10ab | 1.45 | 106.68b | 0.50 | 0.0207 |
| 11 | 107.25ab | 0.66 | 122.53a | 1.51 | 111.00ab | 1.89 | 106.43b | 0.58 | 0.0159 |
| 12 | 109.18b | 0.55 | 114.85a | 1.81 | 114.60ab | 1.62 | 109.48ab | 0.78 | 0.0114 |
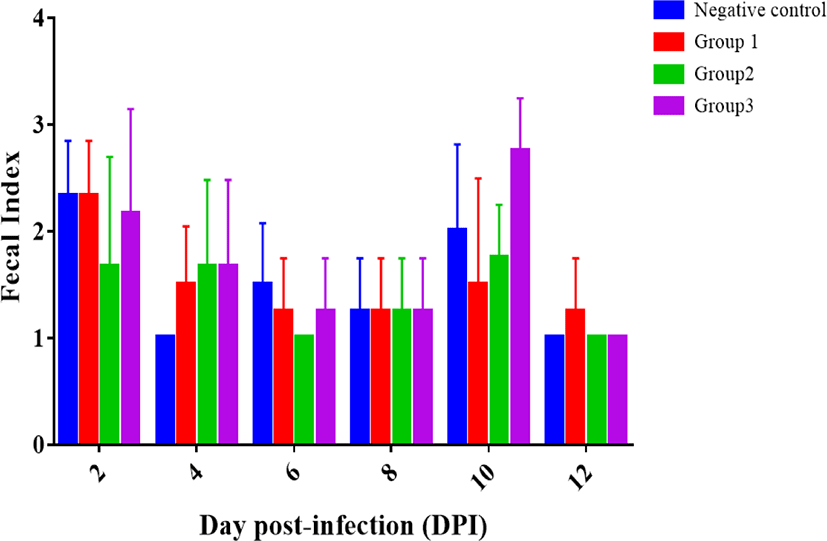
While mice treated with GB-U15 showed better fecal index, no significant differences were observed among groups (Fig. 1). Feces from the mice challenged with STEC were softer and more moisture compared to those of normal mice. It took a shorter time for the mice treated with synbiotics (group 1, 2, and 3) after ETEC challenge to return to the fecal index 1 than the mice without the synbiotics treatment.
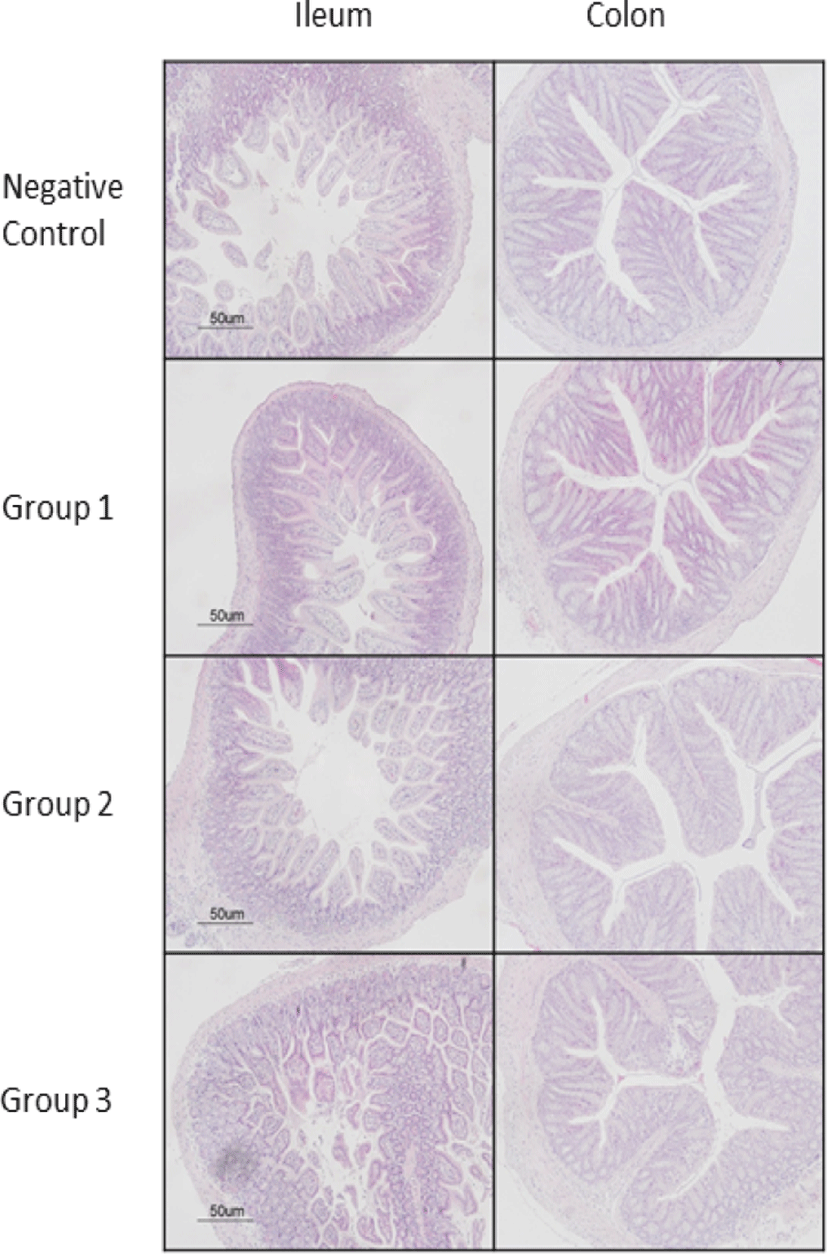
In all groups, the gross lesion and histopathological evaluations showed that the mice treated with synbiotics (group 1, 2, and 3) moderately improved recovery from STEC infection compared to those without the synbiotics treatment. The mice treated with GB-U 15 showed generally improved recovery compared to the other groups treated with GB-U17 and GB 1-3. Also, gross lesion and histopathological evaluations showed that mice treated with GB-U15 moderately improved recovery from STEC infection compared to other groups of mice. However, there were no significant differences were observed (Fig. 2).
Discussion
While the nutritional content of the animal feed is adjusted to optimize the impact on animal health and growth, feed additives including probiotics, prebiotics, and synbiotics are used to enhance livestock productivity [7,13]. When the host experiences high stress usually with a slower growth rate or weakened immune systems, probiotics are used to restore or strengthen natural gut microbial balance [14]. By improving intestinal microbial balance, probiotics including Lactobacilli and Bacilli heighten livestock productivity [8,15,16]. When prebiotics is used as feed supplements in livestock production, they assist the growth and activity of beneficial microbiota in the gut resulting in boosting the host's immune system [2]. A mixture form of probiotics and prebiotics, synbiotics, are commonly used to further enhance the effects of probiotics to provide livestock with health benefits through synergistic effects. Synbiotics selectively stimulate the passage of probiotic bacteria through the gut and help growth and/or colonization of the beneficial bacteria in the intestines [17]. As AGPs are limited or banned in livestock production worldwide, alternatives including probiotics, prebiotics and synbiotics are becoming more on demand in livestock farming because of their beneficial effects on animal health and well-being [7,18,19].
Disparate studies have shown the beneficial effects of probiotics on pig growth performance and health [20,21]. The mixture of fructooligosaccharides and L. paracasei has been shown to stimulate the growth of beneficial bacteria in the gut, to reduce the number of harmful bacteria including E. coli, and to improve the morphology of intestinal villi [22–24]. Other studies show that synbiotics are more effective when fed to suckling piglets [8,24]. While mortality decreased, the gut microbial diversity was increased [22]. It has been proved to be useful in maintaining host intestinal microflora and improving animal health [8]. As such, our results showed that mice treated with synbiotics based on Pediococcus acidilactici GB-U15 and Lactobacillus plantarum GB-U17 with lactulose significantly improved daily weight gain compared to mice in other groups. In particular, mice treated with synbiotics based on GB-U15 showed the best growth performance. While mice treated with GB-U15 showed better fecal index, no significant differences were observed among groups. Gross lesion and histopathological evaluations showed that mice treated with GB-U15 moderately improved recovery from STEC infection. Similar to the results from various studies, our results suggest that the synbiotics formulated with lactulose and Pediococcus acidilactici GB-U15 has potential benefits to prevent and improve colibacillosis in weaned piglets.
However, the exact effects of synbiotics formulated with lactulose and Pediococcus acidilactici GB-U15 are still needed to be elucidated as the effects of probiotics and prebiotics on livestock are often incongruent among different studies [25]. Understanding of the precise mechanisms for proving the beneficial effects of synbiotics in animal health and well-being will also lead us to understand the unknown interactions between feed additives and intestinal flora [7,16,20].
