INTRODUCTION
Sugarcane bagasse is a by-product of the sugar industry after the juice was extracted, approximately 300 kg of sugarcane bagasse (SB) is produced from the raw sugarcane of one ton [1]. The SB has low protein and high fiber content consisting of less than 3% crude protein, 40% to 45% cellulose, 28% to 30% hemicellulose, and 19% to 21% lignin [2]. This biomass is potential for ruminant feeding.
Lactic acid bacteria (LAB) fermentation is a well-known technique used to preserve high moist forages under low pH and high lactic acid conditions from bioconversion of sugar [3,4]. Silage additives such as molasses and cellulase are normally added when low moist and sugar content feedstuffs are fermented with LAB inoculants. Molasses and cellulase greatly affect the growth of LAB by providing more soluble sugars as the substrate for lactic acid production [5]. Current studies have been reported on the effect of LAB and additives for improvement of silages [3,6,7]. Lactobacillus casei TH14 (L. casei TH14) belongs to facultative heterofermentative groups and a local strain isolated from sweet corn stover silage [8], and it has been used for improvement of tropical silage. Khota et al. [5] reported that ensiled sorghum with Lactobacillus plantarum Chikuso 1, L. casei TH 14, and Acremonium cellulase resulting in an increase of LAB count and lactic acid content and a decrease of pH and ammonia nitrogen (NH3-N). Few studies on the application of L. casei TH14 in combination with additives to improve the nutritive value of high indigestible fiber feedstuffs have been reported. Cherthong et al. [9] revealed that the nutritive value of rice straw was improved with L. casei TH14 as inoculant and additives fermentation. However, the study on the effect of L. casei TH14, molasses, and cellulase on fermentation characteristics and degradation of nutrients especially the fiber fractions of SB silage has not yet been explored.
Therefore, the study aimed to study the effect of L. casei TH14, cellulase, molasses, and their combination of fiber reduction, fermentation products, and microorganism count of SB silage.
MATERIALS AND METHODS
Sugarcane bagasse was kindly supplied by Khon Kaen Sugar Power Plant, one of the operation plants of Khon Kaen Sugar Industry Public Company Limited (KSL), located in Khon Kaen province, which is one of the major provinces in the Northeastern Thailand and accomodate the four major cities in Isan, Thailand.
Commercial L. casei TH14 as a silage starter (composed of 80% trehalose, 15% lactose, and 5% LAB; Bio Ag Khon Kaen, Khon Kaen, Thailand), cellulase enzyme (acid; powder, 5 × 105 U/g activity, CAS number: 9004-34-6, Sinobios Imp. & Exp., Thanghai, China), and molasses were purchased from a local supplier in Khon Kaen province, Thailand, were used.
The treatments were arranged according to a factorial arrangement (2 × 2 × 2) + 1, in a complete randomized design (CRD). The first factor consisted of two levels of L. casei TH14 (TH14, 0 and 0.05 g/kg fresh matter [FM]); the second factor consisted of two levels of cellulase enzyme (C, 0 and 104 U/kg FM); and the third factor consisted of two levels of molasses (M, 0 and 5 g/ 100 mL distilled water). A treatment (+1) referred to the use of rice straw without any treatments, which was used for comparison purposes with untreated SB with the addition of distilled water at a respective ratio of 1.5 liters to 1 kg of FM SB. A total nine treatments were performed: 1) untreated rice straw (RS, +1), 2) untreated SB, 3) treated molasses (M), 4) treated cellulase (C), 5) treated TH14 (TH14), 6) treated molasses and cellulase (M:C), 7) treated TH14 and molasses (TH14:M), 8) treated TH14 and cellulase (TH14:C), and 9) treated TH14, cellulase, and molasses (TH14:C:M). A total of 3 kg of SB was prepared for all treatments with triplications and was divided into three vacuum bags (7.5 × 11 inch, Zhongshan, China) containing 300 g of treated SB and sealed using a vacuum sealer machine (Brother, Zhejiang Brother Packing Machinery, Zhejiang, China). The sealed bags were kept at room temperature, and every seven days, three bags from each treatment were opened to assess for the change of pH and dry matter. The remaining bags were opened on day 30 of fermentation for further analysis, including chemical and biological properties.
The chemical composition (dry matter, DM; ash; organic matter, OM; crude protein, CP; ether extract, EE; neutral detergent fiber, NDF; acid detergent fiber, ADF; and lignin) were assessed. Raw and fermented at day 30, the SB was assessed under an oven at 60°C, and the particle size was reduced through a 1 mm mesh screen. The full procedure for assessing DM (viz. 934.01), OM (viz. 942.05), CP (viz. 976.05), EE (viz. 920.39), and ash was mentioned in the AOAC [10] method. A fiber analyzer (ANKOM 200, ANKOM Technology, New York, NY, USA) was used for NDF and ADF analysis according to the detailed procedure of Van Soest [11], and acid detergent lignin (ADL) analysis was done based on the method of Faichney and White [12]. Also, a bomb calorimeter (AC 500, LECO, St. Joseph, MI, USA) was conducted for the gross energy (GE) analysis of raw and fermented SB at day 30.
The products at day 30 of fermentation, including pH, lactic acid (LA), acetic acid (AA), propionic acid (PA), butyric acid (BA), NH3-N, and ethanol, were assessed. The pH was performed using a pH meter (HI83141, 0608064N S/N, HANA instruments, Romania), following the procedure mentioned by Chen et al. [3]. High-pressure liquid chromatography (HPLC, Water, and Novapak model 600E, water model I484 UV detector) was performed for organic acid (LA, AA, PA, and BA) analysis, with the detailed procedure for LA analysis being described by Pruksatrakul et al. [13]. The AA, PA, and BA analysis followed the procedure of Samuel et al. [14], and the NH3-N analysis method was described by Fawcett and Scott [15] using a spectrophotometer (UV/VIS Spectrometer, PG Instruments, London, UK). Gas chromatography (Model HP6890, Hewlett Packard; Headspace, Model HP 7694E, Hewlett Packard; Flame Ionization Detector [FID], Capillary Column HP-1 [Methyl Siloxane], 30 m length, 320 nm diameter) was assessed for ethanol concentration following the procedure of Luangkriangkrai [16].
The procedure of Kozaki et al. [17] was used for the biological property assessment. The microorganism assessment was focused on LAB, yeast, mold, aerobic bacteria, and the coliform count. A series of dilutions (10−1, 10−2, 10−3, 10−4, and 10−5) were formed for the dilutant of 10 g of raw and fermented SB in 90 mL of sterilized distilled water. The colony counts were enumerated at a selective dilution of 10−1, 10−3, and 10−5 and expressed as a log colony form unit per g of FM (Log CFU/g FM). The detailed steps are described in the work of Khota et al. [5].
The chemical and biological data were subjected to a 2 × 2 × 2 (+1) factorial in a CRD and analyzed (Window version 6.2.9200, SAS Institute, Cary, NC, USA) with the following model:
Where Yijkl = observation values of treatment combination at ijk, replication l when l = 1, …, r, μ = overall mean; αi = effect of main effect A at i when i = 1, …, a; βj = effect of main effect B at j when j = 1, …,b; γk = effect of main effect C at k when k = 1, …, c; αβij = interaction of A and B at ij; αγik = interaction of A and C at ik; βγjk = interaction of B and C at jk; αβγijk = overall interaction of A, B, and C at ijk; and εijkl = error term. The means difference was compared using Duncan’s new multiple range tests [18] at p < 0.05, which is considered to be a statistical difference. Also, orthogonal contrasts were performed to study the difference of each factor.
RESULTS
Table 1 shows the nutritive values of raw and fermented SB. The raw SB was composed of 785.3 g/kg of DM, 72.2 g/kg of ash, 927.8 g/kg of OM, 26.7 g/kg of CP, 3.1 g/kg of EE, 744.7 g/kg of NDF, 689.6 g/kg of ADF, and 142.9 g/kg of ADL, respectively. The GE was 6.93 kcal/g of FM (described in Table 1). The DM (306.4–317.7 g/kg of DM), NDF (773.0–721.6 g/kg of DM), and GE (7.20–7.31kcal/g of FM) after 30 days of fermentation were significantly different among treatments, whereas OM (935.97–933.59 g/kg of DM), ash (64.03–66.41 g/kg of DM), ADF (601.15–641.62 g/kg of DM), and ADL (110.28–138.53 g/kg of DM) were not significant (p > 0.005) among treatments. The SB and RS treatments differed significantly in terms of OM, ash, ADF, and ADL values.
Means with different superscript lowercase letter within column showed statistically (p <0.05) different of the treatments.
DM, dry matter; OM, organic matter; CP, crude protein; EE, ether extract; NDF, neutral detergent fiber; ADF, acid detergent fiber; ADL, acid detergent lignin; GE, gross energy; RSB, raw sugarcane bagasse; RS, untreated rice straw; SB, untreated sugarcane bagasse; M, fermented with molasses; C, fermented with cellulase; TH14, fermented with L. casei TH14; M:C, fermented with molasses and cellulase; TH14:M, fermented with L. casei TH14 and molasses; TH14:C, fermented with L. casei TH14 and cellulase enzyme; TH14:M:C, fermented with L. casei TH14; molasses and cellulase.
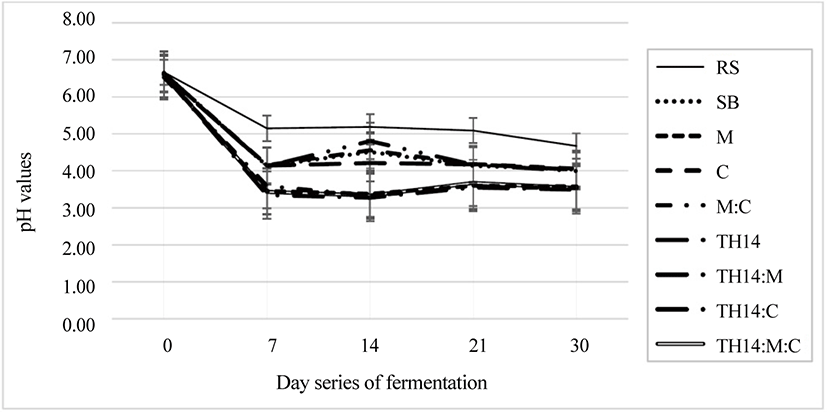
Fig. 1 shows the pH change of un-inoculated RS, un-inoculated SB, and inoculated SB during fermentation on days 0, 7, 14, 21, and 30. Table 2 shows the products of fermented SB after 30 days, including pH (< 5), LA (14.3–78.7 g/kg of DM), AA (10.80–24.14 g/kg of DM), PA (not detected), BA (not detected), NH3-N (3.7–2.3 g/kg of DM), and ethanol (12.7–10.3 g/kg of DM). The pH, LA, AA, NH3-N, and ethanol values were significantly (p < 0.05) different among treatments, whereas the BA and PA values were not detected in the current work. The pH, LA, and AA values were significantly different between SB and RS treatments.
Means with a different superscript lowercase letter within column showed statistically (p <0.05) different from the treatments.
LA, lactic acid; AA, acetic acid; PA, propionic acid; BA, butyric acid; NH3-N, ammonia nitrogen; DM, dry matter; RS, untreated rice straw; ND, not detected; SB, untreated sugarcane bagasse; M, fermented with molasses; C, fermented with cellulase; TH14, fermented with L. casei TH14; M:C, sugarcane bagase fermented with molasses and cellulase; TH14:M, fermented with L. casei TH14 and molasses; TH14:C, fermented with L. casei TH14 and cellulase enzyme; TH14:M:C, fermented with L. casei TH14; molasses, and cellulase.
The microorganism count in raw SB was 4.93 Log CFU/g of LAB, 5.20 Log CFU/g of yeast, 2.69 Log CFU/g of molds, 5.69 Log CFU/g of aerobic bacteria, and 3.87 Log CFU/g of FM of coliform (described in Table 3). After 30 days of fermentation, the microorganism count was 3.47–7.91 Log CFU/g FM of LAB, 4.63–3.96 Log CFU/g of FM of yeast, and 6.19–4.45 Log CFU/g of FM of aerobic bacteria. The LAB, yeast, and aerobic bacteria count differed significantly among treatments. The mold and coliform counts were not detected in the current work. The LAB, yeast, and aerobic bacteria counts were different between the RS and SB treatments (Table 3).
The microorganism count presented in raw sugarcane bagasse was 4.93 LAB, 5.20 yeast, 2.69 molds, 5.69 aerobic bacteria, and 3.87 Log CFU/g FM.
Means with different superscript lowercase letters within column showed statistically (p <0.05) different from the effect of the treatment compared to RS.
CFU, colony form unit; FM, fresh matter; LAB, lactic acid bacteria; RS, untreated rice straw; ND, not detected; SB, untreated sugarcane bagasse; M, fermented with molasses; C, fermented with cellulase; M:C, fermented with molasses and cellulase; TH14, fermented with L. casei TH14; TH14:M, fermented with L. casei TH14 and molasses; TH14:C, fermented with L. casei TH14 and cellulase; TH14:M:C, fermented with L. casei TH14, molasses, and cellulase.
DISCUSSION
The DM contents were in the range of approximately 302 to 317 g/kg DM, which was the proper range for fermentation [19], for long-term storage and for inhibiting unfriendly microorganisms [20]. DM was increased by 4% compared to untreated SB when fermented with TH14, cellulase, and molasses. This increase was due to the action of cellulase enzyme in breaking down the cell wall and releasing more soluble carbohydrates and molasses as an additional nutrient supply. This also was available for LAB fermentation resulting in pH reduction and DM loss prevention [21,22]. This finding was similar to the report of Chen et al. [3], who reported that fermented sugarcane tops with LAB, molasses, and cellulase that resulted significantly in increasing the DM content when fermented with LAB and molasses, but this content decreased with cellulase treatment. Also, Li et al. [7] reported that the DM increased in most varieties of rice straw when fermented with LAB, except for the Zhenxian96 breed. However, SB fermented with cellulase as an additive had a significantly (p < 0.05) lower DM compared with other treatments. The reason for this significant change could be the action of cellulase as the main factor in breaking down the cell wall components, failing to form a maximum LAB count, and the growth of yeast and aerobic bacteria. Other findings were similarly reported [3,23–25], with other researchers discovering a reduction in the DM of fermented materials when treated with an enzyme. The reasons could be due to the application of an enzyme positively responded to the ensiled SB due to its low water-soluble carbohydrate content, and it subsequently improved fermentation using a released substrate [26]. The CP and EE contents of fermented SB failed to differ (p > 0.05) when compared with untreated SB and RS, which was similarly reported by Kim et al. [27], who found that various strains of Lactobacillus plantarum did not affect the CP content of rice straw silage.
A variety of enzymes were purposely added to ensiled fibrous materials to decrease the indigestible components and enhance the nutritive value. This mainly released more soluble carbohydrates and later enabled LAB to produce lactic acid. The NDF, ADF, and ADL values in fermented SB were lower than in the SB treatment (Table 1). The NDF was decreased by 2% when compared to untreated SB when fermented with a combination of TH14, cellulase, and molasses. Enzymes were often added with a variety of bacterial inoculations. Thus, the responses to fermented materials were to either enzymes or bacterial inoculations [21]. Some works have interpreted that this combination positively and synergistically improved fermented material quality, which resulted in more soluble carbohydrates, and further improves feed efficiency [28]. Notably, the response of enzyme utilization in anaerobic fermentation was positive and suitable for fibrous materials with less water-soluble carbohydrates (WSC). Gado et al. [25] found that fiber fraction and DM biological degradation were enhanced with enzyme cocktails during anaerobic fermentation.
The GE was significantly (p < 0.05) increased by the interaction of TH14, cellulase, and molasses. The combination of additives contributed to a higher GE value when compared with added additive alone and untreated treatments. This increase could be described by the contribution of molasses providing highly available soluble carbohydrates as an energy source, and the action of cellulase on the outer components released more usable carbohydrates. Moreover, Derwhust et al. [29] stated that organic acid output in the fermented materials contributed mainly to the increase of GE, approximately 10%–14%, in which BA represented 24.93 MJ/kg of DM, and LA represented 15.16 MJ/kg of DM in terms of the GE value. The recent finding was in agreement with McDonald et al. [30], who reported that fermented materials had a higher GE value compared with fresh materials.
Anaerobic fermentation or silage, a method of forage crops, byproducts, and grass preservation, was practiced as well [31]. The process happened without the presence of oxygen and is characterized by an increasing LAB number and decreasing pH [32]. A rapidly decreasing pH could preserve ensiled materials longer. Also, the loss of nutrients was not significant [33]. The pH and NH3-N were significantly dropped to 3.5 and 2.3 g/kg DM. Moreover, LA and AA were increased by 64.93% and 53.31% compared to untreated SB, which found in the treatment combination of TH14, cellulase, and molasses, respectively. After 30 days under anaerobic fermentation, the acidity of fermented SB became more acid (4.03–3.5) compared with the raw SB. The pH after fermentation dropped significantly under the interaction of the combination of the three additives. This implied that the combination had a positive synergistic effect on the acidity of the fermented materials. The dropping of the pH was due to the increase of LA and AA values. These values were considerably high in treatments fermented with TH14 inoculation, with the highest LA (78.70 g/kg of DM) and AA (24.14 g/kg of DM) values being found in the treatments fermented with TH14, C, and M. This increase was mainly dominated by the increase in the LAB count, ranging from 3.47–7.91 Log CFU/g of FM. Molasses and cellulase addition supplied more direct and indirect substrates for promoting the anaerobic fermentation resulting in a high LA concentration and LAB count [34,35]. Adding these additives promoted better qualities when compared with no additives and indicated a successful fermentation process for SB. This addition was the most suitable for SB fermentation to succeed due to its low WSC value; in spite of this, it would not be necessary for materials with high WSC values [35]. The AA concentration was increased significantly in response to additives compared with the SB treatment, and the highest value of 24.14 g/kg of DM was found in the treatment of TH14, cellulase, and molasses. This increase could be due to the slow sealing process since it was naturally produced in silage. The slow sealing process could be an advantage of enterobacteria and heterolactic-bacteria growth resulting in the accumulation of the AA concentration [36]. Another reason could be the high ambient temperature during storage increasing the AA concentration [37] with the presence of Lactobacillus buchneri growth [38]. The AA directly affected the yeast and mold growth on an opening day at the end of fermentation [21]. The NH3-N (3.7–2.3 g/kg DM) and ethanol (12.7–10.3 g/kg DM) concentrations were significantly reduced when compared with the SB treatment, while the lowest values of NH3-N and ethanol were found in the treatment of TH14, cellulase, and molasses. This decrease could be due to the increase in the LAB count, LA concentration, and AA concentration, as well as the decline in the pH, which depressed the action of Clostridia and other microorganisms, including yeast and mold. The presence of Clostridia affected the protein content in the fermented material by degrading to NH3-N [39]. The PA and BA concentrations were not detected; even though, mold and coliform were not found in this work.
Regarding evaluating the quality of anaerobic fermentation, pH is always viewed as the important consideration factor [40]. Naturally, epiphytic LAB is present in almost all living plants and contributed mainly to the fermentation process, where the fermented materials were considered to be well preserved at a count number of 5 Log CFU/g of FM [41,42]. The LAB count was significantly increased by 28% as compared to untreated SB, and the non-important bacteria in the fermentation process was inhibited. An increase of LAB count could be explained by the physiological growth of LAB and the adequate supply of molasses as a substrate. Homofermentative LAB isolated in the tropical area normally could grow well under pH less than 4, and during the first stage of fermentation, while LAB could grow fast with an adequate amount of WSC [8,43]. L. casei TH14 was one of homo-fermentative LAB which may grow well under pH 4 when compared to other epiphytic LAB and produced high lactic acid concentration with lowering the pH value.
CONCLUSION
The current study could be concluded that additives could enhance the biochemical qualities of SB when compared to untreated SB and RS. Fermenting with L. casei TH14, cellulase and molasses in combination resulted in the promotion of high quality of SB with DM loss prevention, fiber component reduction, pH reduction, and enhanced LA production and LAB count. Further studies both in vitro and in vivo are recommended to elucidate the effect of fermented SB on nutrients digestibility and rumen fermentation.
