INTRODUCTION
Presently available allopathic contraceptives are costly and cause multiples of problems like hormonal imbalance and various other side effects [1]. An ideal alternative to allopathic contraceptives could be herbal extracts which could be applied topically when required and which would directly affect gamete function without causing any hormonal imbalance. Several medicinal plants have been investigated for various biochemical active components possessing contraceptive properties [2]. Owing to low side effects, they have gained considerable attention in recent years.
Plants with anti-fertility effects include Rubica cardiofolia, Artemisia vulgaris, Piper nigrum, Juniperus phoenica, Cuscuta reflexa, Tinospora cardiofolia, Acyranthes aspera etc. [2]. Ethanol extract of Tinospora cordifolia was found to be effective in producing reversible sterility, weight reduction of the reproductive organs like testis, epididymis, seminal vesicle and ventral prostate of treated male rats. The extract was found to cause significant reduction in the sperm density, motility and fertility in mice [3]. The induced infertility was completely reversed after withdrawal of the treatment. Alcoholic extract of Iranian neem (Azadirachta indica) seeds significantly reduced the motility and fertlity of mouse epididymal spermatozoa thus suggesting that the extract could be as a potential anti-fertility agent [4].
Artemisia is a diverse and large genus of plants, belonging to the family Asteraceae. Genus Artemisia comprises over 500 species of important medicinal plants, mainly found around Asia, Europe and North America. They are currently the subject of numerous phytochemical research and are found to possess varieties of essential oils [5]. A. vulgaris is one of the important perennial and aromatic medicinal herbs, 1–2 m tall, growing at the sides of paths and tracks and margins of cleared forests. It is known as mugwort, sailor’s tobacco, common wormwood, wild wormwood, felon herb, old Uncle Henry, St. John’s plant etc. and also known as ‘pati’ or ‘titepati (bitter-leaf plant)’ in Nepal. Leaves are 5–20 cm long, pinnate, sessile, alternate, simple and the margins are often rolled back. The plant possess a broad spectrum of therapeutic properties like antitumor antispasmodic, anti-steroidogenic, anti-fertility, antihypertensive, muscle relaxant, antiviral, antibacterial, antioxidant, cholinergic, diuretic etc. [6,7]. It is an important medicinal plant due to the presence of anti-malarial drug artemisinin [8], sesquiterpenes (derivative of artemisinin) and several essential oils [9]. Phytochemical screening of leaf extract of A. vulgaris, showed the presence of high amount of alkaloids, flavonoids, and terpenoids, steroids and tannin [10].
In addition to various therapeutic uses, A. vulgaris leaves extract shows anti-implantation, estrogenic activities in rats [11] and anti-fertility effect on Callosobruchus chinensis (weevil) through the effect on hatchability of pupae [12]. Alcoholic extract of A.vulgaris was found to induce an irregular estrous cycle and anti-implantation effect in female albino rats [13]. The herbal extract when fed to pregnant female mice as a dietary supplement in high dose did not cause gross malformations in pups, proving its non-toxic nature [13,14]. In the present work, we have shown that A. vulgaris extract causes high rate of precocious acrosome reaction in mouse spermatozoa, but lesser extent of membrane damage or viability loss. Intravaginal application of the extract reduces fertility significantly.
MATERIALS AND METHODS
All reagents and chemicals were purchased from Emplura-Sigma Aldrich, Bangalore, India unless otherwise mentioned.
Leaves were collected from plants before onset of flowering, growing wild in Kirtipur campus of Tribhuvan University, Kathmandu. They were washed by running tap water to remove the surface contamination. The leaves were dried in shade overnight followed by oven drying at 60°C. Dry leaves were grinded to fine powder using a mechanical blender (Phillips, India). 100 g of powder was mixed with 80% ethanol in 1 : 3 ratio in a dark bottle. The mixture was kept in shaker for three overnights. The extract was filtered through Whatman No. 1 filter paper (GE Healthcare, Mumbai, India). The filtered extract was then preserved in an airtight dark bottle in a refrigerator for further studies.
Male and female Swiss albino mice of 8–12 weeks were purchased from the Department of Plant Resources, Thapathali, Kathmandu. They were housed in polypropylene cages, in vivarium and maintained at a 12 h light–12 h dark cycle, 24°C–28°C temperature, supplied with water and solid pellet food (VRK Nutritional Solutions, Pune, India). All studies involving mice handling were done by strictly following the approved protocol issued by the Ethical Committee of the Central Department of Biotechnology/Tribhuvan University (protocol number 084/2019).
After one week of acclimatization, mice were used for experiments. Male mice were sacrificed by cervical dislocation and dissected with sterile dissection equipment. Testes with attached epididymis were harvested and epididymis were excised and immediately placed in 5 mL of Dulbecco’s Modified Eagle’s Media (DMEM) supplemented with 5 mg/mL bovine serum albumin (BSA), 20 mM hydroxyethyl piperazine ethane sulfonicacid (HEPES) buffer. Epididymis were minced and sperm samples were collected. For all experiments, the final sperm concentration was adjusted to 106 cells/mL. Growth media, supplements and buffers were purchased from HiMedia Laboratories, Mumbai, India.
Spermatozoa were incubated in 1 mL DMEM supplemented with 0.5 mg/mL BSA and 20 mM HEPES, taken in 1.5 mL eppendorf tubes. Required volumes of plant extract and medium were added to the tubes making 1 mL of mixture with extracts concentration equal to 0, 25, 100, 400, 500, 800, 1,000, and 2,000 µg/mL. Vehicle controls were prepared for 1,000 and 2,000 µg/mL-treatments by replacing extract with 80% ethanol in media. After equilibrating in a 37°C incubator, 100 µL aliquots of spermatozoa were added to each tube followed by incubation in the incubator for 2 h.
Coomassie brilliant blue (G-250) was purchased from Sigma-Aldrich (Baden-Württemberg, Germany) and fluorescein isothiocyanate (FITC) peanut agglutinin (FPNA) from Molecular Probes-Invitrogen (Middlesex County, MA, USA). After 2 h incubation, 100 µL of 40% formalin was added (final 4%) to each tube and left at room temperature for 15 min. Centrifugation was done at 500×g for 3 minute. The supernatant was aspirated until a loose pellet of 100 µL was left at the bottom of the tube. A thin layer of saliva was applied on coverslips and allowed to dry completely. 50 µL of sperm suspension was applied on each coverslip and left to air dry. Each coverslips was stained with Coomassie blue for 5 minutes and then washed properly [15]. Finally after complete drying the coverslip were attached on slides with dibutylphthalate polystyrene xylene (DPX) with cells side up. Spermatozoa with acrosome and without acrosome were counted under a bright field microscope under 100X objective.
For FPNA labeling, the tubes were centrifuged at 500×g for 3 minute. The supernatants were aspirated leaving behind 100 µL of loose pellets. Thin smear of saliva was applied on coverslips and dried. 50 µL of resuspended sperm suspension was added and allowed to settle for 3–5 minutes. Unattached spermatozoa were poured off from the edges and then the coverslips were immersed in 4% formalin for 30 minutes. After washing with phosphate buffered saline (PBS), the coverslips were treated with 1% Tween 20 in PBS for 30 minutes. Tween was poured off and 50 µL of 250-times diluted FPNA was added followed by incubation at 37°C for 1 hr (covered with aluminum foil to avoid light). After removing FPNA, 50 µL of ethidium bromide (EtBr, 100 µg/mL) was added and incubated for 10 minutes at room temperature in dark. EtBr was removed by washing with PBS and mounted with a drop of 10% glycerol (in PBS) on a clean slide and the edges sealed with DPX mountant.
For Trypan blue staining, the eppendorf tubes containing extract treated and control spermatozoa were centrifuged at 500×g for 3 minutes. Supernatant is aspirated and discarded leaving about 20–30 µL of sperm pellet at the bottom. The tubes were immediately kept in ice bucket. From each tube 5 µL of sperm suspension was applied on a clean slide and mixed with 5 µL of 0.4% Trypan-blue stain [16]. The mixture was covered with coverslip and observed under 100X objective of a microscope. The percentage of dead spermatozoa (stained blue) was calculated from total 200 counts.
Hypo-osmotic swelling tests [17] were performed for investigating membrane damage of spermatozoa. After incubating with different concentrations of plant extract and control medium for 2 h at 37°C, centrifugation was performed for 3 minutes at 700×g. Pellets of 100 µL sample were collected. Sperm suspension in pellet and hypo-osmotic solution were mixed in 1 : 10 ratio, followed by 15 min incubation at 37°C. The tubes were centrifuged at 500×g for 4 minutes, supernatant discarded leaving around 100 µL of loose sperm pellet at the bottom. 1, 2 drops of sperm sample was placed on slide, covered by a coverslip. The edges were sealed by DPX and then tail swellings of spermatozoa were observed under microscope [18].
After one week acclimatization in vivarium, estrus cycle was checked each day in each female mouse. A direct effect of A. vulgaris extract on fertilization was studied by injecting extract into the vagina of estrus females and allowing them to mate with fertile males. Female mice of 6–8 weeks old and male of 8–10 weeks old were used in these experiments.
Experimentations were performed on five sets of mice. For each set of experiment, five mice were used for extract treatment and five as blank control. On the day in which they were found to be in estrus cycle, 10 µL of 1,000 µg/mL of Artemisia extract was injected through vagina while for blank control no treatment was done. Immediately, extract injected female mice were caged with male mice in 2 : 1 ratio. Extract injection was performed for three consecutive days in the evening and returned back to the males. In the morning after three days mating, the female mice were checked for the evidence of copulation. Mice with closed copulatory plug and waxy, whitish substances around vaginal area were considered mated. Microscopic examination was performed with the vaginal swab of mated mice for confirming the presence of spermatozoa in their vagina. The female mice were separated and that day was considered day one of pregnancy. Mice were routinely fed throughout the gestation period (21 days) and after delivery, number of pups delivered by control mice and extract injected mice were compared [19].
Each experiment was done in triplicate repeats. Microsoft Excel program (MS Office 2013) was used for statistical processing of data, for calculating mean, standard deviations, standard errors, and drawing graphs. Data are expressed as Mean ± SD. For statistical analysis of the data, two-tailed t test was employed wherever required. p < 0.05 was considered significant and was calculated from MEDCALC® website [20].
RESULTS
The nuclei of sperm heads appeared red due to EtBr staining. Spermatozoa labeled with FPNA displayed green, crescent shaped acrosome on the dorsal side of the sperm heads (Fig. 1A). Acrosome reacted spermatozoa lacked such green labeling (Fig. 1B). Acrosomes of mouse spermatozoa were also studied by staining with Coomassie blue. Spermatozoa with intact acrosome displayed crescent-shaped staining on the dorsal surface of head (Fig. 1C, arrow) whereas acrosome reacted spermatozoa lacked such crescent staining (Fig. 1C, arrowhead).
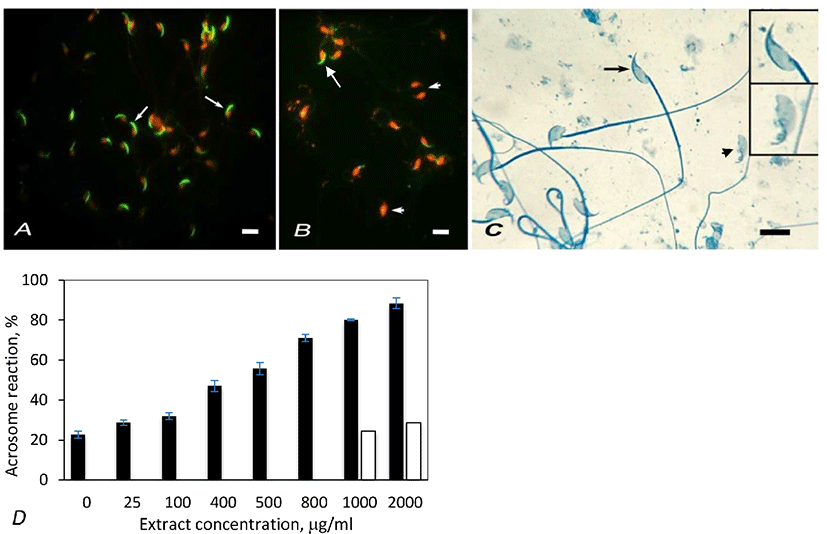
Artemisia leaf extract induced acrosome reaction in spermatozoa. The percentage of acrosome reacted spermatozoa was estimated by counting 200 spermatozoa in the control and treatment samples, labeled with FPNA. In control samples, the percentage of spontaneously acrosome reacted spermatozoa was found to be 22.64% (Fig. 1D). The proportion of acrosome reacted spermatozoa linearly increased with increasing extract concentration from 25 µg/mL to 1,000 µg/mL. The percentage of acrosome reacted spermatozoa further increased at 2,000 µg/mL extract concentration, but the value was not appreciably higher than that of 1,000 µg/mL. Hence the optimum concentration of the extract that induces acrosome reaction in mouse spermatozoa was considered to be 1,000 µg/mL. In vehicle control experiements that were done by adding ethanol equivalent to 1,000 µg/mL and 2,000 µg/mL of extract concentrations, the acrosome reaction rates were not significantly different than that of untreated samples (Fig. 1D). Hence a possibility that ethanol (vehicle) contained in the extracts might cause appreciable of acrosome reaction is ruled out. The effect of extract on acrosome reaction in mouse spermatozoa was also studied by staining spermatozoa with Coomassie blue. This study also showed similar result as that with FPNA staining (Table 1).
Trypan blue staining is a simple noninvasive rapid technique to determine sperm viability. When stained with Trypan blue, viable spermatozoa with intact cell membrane remain colorless, excluding the stain. Spermatozoa that have damaged membrane (dead) show dark blue staining in the heads due to dye penetration into them (Fig. 2A) [16]. Hence, the percentage of unstained spermatozoa denotes viability.
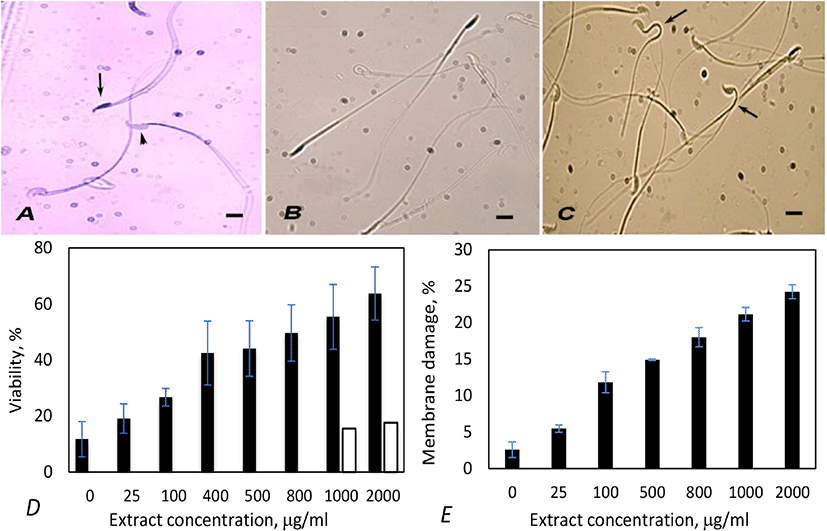
Mouse spermatozoa treated with hypoosmotic buffer differentiated into two populations - one population with apparently normal looking with straight structure (Fig. 2B) and the other with variously bent neck or tails (Fig. 2C). The latter population comprised live spermatozoa with intact plasma membrane and the former one were dead spermatozoa with damaged plasma membrane.
Incubation with extract caused loss of viability of spermatozoa. The extent of effect was proportional to the concentration of extract (Fig. 2D). Optimum effect was observed at 1,000 µg/mL concentration, as in acrosome reaction. Spermatozoa with Coomassie positive nuclei (dead spermatozoa) observed at this concentration was 55.4%. Treatment with 2,000 µg/mL of extract caused higher viability loss (63.7%), but the difference in comparision to 1,000 µg/mL was not remarkable in spite of two-folds increase in concentration. Vehicle control experiments were done by incubating spermatozoa with two concentrations of ethanol, equivalent to 1,000- and 2,000 µg/mL extract treatments. Ethanol caused slightly higher viability loss (15.5; 17.62%) than blank controls (11.71%; Fig. 2D). These observations prove that the viability loss was caused by Artemisia extract, but not due to ethanol.
Treatment with Artemisia leaf extract also caused membrane damage in spermatozoa in a dose dependent manner, but the effect was less conspicuous than viability loss or acrosome damage. At the optimum concentration of extract (1,000 µg/mL), membrane damage was only 21.17% (Fig. 2E).
To study a direct effect on fertility, the extract was injected into the vagina of estrus female mice and conjugated with males. In the beginning, extract treated and blank control female mice were conjugated for one night and separated. But such mice often failed to be impregnated due to unknown reason. Therefore, the estrus females were intravaginally injected with the extract for three consecutive evenings and kept with males for three nights. By this method the control and treated mice were successfully impregnated and gave birth to healthy pups. The extract caused low fertility in mice. The litter size of the treated mice was nearly half in comparison to the blank control (5.2 verses 9.4, Table 2). The pups developed into healthy and fertile adults some of which were used for breeding or for spermatozoa study. Hence the reduced fertility of Artemisia leaf extract treated mice is not due to ethanol or other stress incurred during the experimental procedure.
| Set No. | Number of litters born | |
|---|---|---|
| Control | Treatment | |
| 1 | 9 | 0 |
| 2 | 10 | 8 |
| 3 | 9 | 7 |
| 4 | 10 | 5 |
| 5 | 9 | 5 |
| Mean± SE | 9.4±0.21* | 5±2.22* |
DISCUSSION
In the present work, potential contraceptive effect of Artemisia extract was evaluated by analyzing acrosome reaction, viability loss, membrane damage in mouse spermatozoa and by evaluating fertility loss by intravaginal application. Acrosome plays very important role in fertilization by binding and proteolytically digesting zona pellucida thus helping spermatozoa to enter into oocytes [21]. During normal fertilization process, acrosome intact or recently acrosome reacted spermatozoa bind zona surface through specific ligand-receptor interaction followed by vesiculation and release of acrosomal content. Spermatozoa that have lost acrosome long before encountering zona pellucida cannot perform these two functions hence fail to fertilize. Moreover spermatozoa die shortly after acrosomal exocytosis [22]. The present work has shown that Artemisia leaf extract causes precocious acrosome exocytosis in mouse spermatozoa. The rate of acrosome reaction increased linearly with increase in concentration of the extract, the optimum effect being observed at the concentration 1,000 µg/mL. The optimum acrosome reaction observed was 80.4% which is similar to the optimum effect of Calcium ionophore in mouse spermatozoa and ram spermatozoa [23,24]. Calcium ionophore causes massive influx of Ca2+ into cells and kills them if the treatment is prolonged. Spermatozoa are quickly immobilized by calcium ionophore and hence cannot fertilize oocyte. However they regain hypermobility and resume fertilizing competence if ionophore is promptly removed [25].
FPNA stains the acrosome matrix by binding to glycoconjugates in the acrosome and produces bright green fluorescence under fluorescence microscope. Hence FPNA labeling is a standard method to study presence or absence of acrosome in spermatozoa [26]. Spermatozoa with disrupted acrosome do not label with FPNA (Fig. 1B). FPNA labeling can be used to assess the acrosomal status and the zona-pellucida induced acrosome reaction in stallion [27]. Spermatozoa sample were stained with FPNA, and counterstained with the DNA dye ethidium homodimer. Acrosome-intact stallion spermatozoa were found to display intensively green fluorescence over the acrosomal cap, whereas reacting spermatozoa showed a patchy disrupted image of fluorescence.
The crude extract of A. vulgaris at very low concentration, induced high rate of acrosome reaction. The putative substance(s) that induced acrosome reaction might be present in very low proportion in the crude extract. At present, it is not possible to speculate about its chemical nature or molecular pathway of the acrosome reaction. Future investigation should address whether the extract induced acrosome reaction is Ca2+ dependent, whether it affected Ca2+ uptake during capacitation of spermatozoa, whether addition of anion transport inhibitor during extract treatment obstructs Ca2+ uptake [28], or whether it overrides the cAMP/PKA pathway in inducing acrosome reaction [25].
Whereas Artemisia extract induced acrosome reaction at high rate (80.12% by 1,000 µg/mL conc) but caused lesser viability loss (55.39%) and even lower rate of membrane damage (21.17%). These observations signify that some spermatozoa whose acrosomes have been damaged by the extract are viable and possess intact cell membrane. Quite possibly extract induced acrosome reaction is similar to calcium ionophore induced acrosome reaction in which majority of spermatozoa retain viability [25, 28] that can fertilize oocytes when ionophore is removed [25].
In a study carried out by Alvarez et al. [29], ethanol was found to cause loss of acrosome as well as the the equatorial membrane that are needed for gamete fusion. So it was imperative that the effect of ethanol which was used as the sovent (vehicle) of Artemisia extract, be evaluated. Experiments were carried out by replacing extract by 80% ethanol. Acrosome reacted spermatozoa was found to be 21.68% and 24.1% when treated with amount of ethanol equivalent to the extract concentration of 1,000 µg/mL and 2,000 µg/mL (vehicle controls) respectively which were slightly higher than the effect of blank control (18.15%). These observations prove that low concentrations of ethanol used in the present experiment had negligible effect on acrosome reaction.
The extract may exert effect on spermatozoa by generating reactive oxygen species (ROS). As spermatozoan plasma membrane is rich in polyunsaturated fatty acids which can easily undergo lipid peroxidation in the presence of ROS leading to change in fluidity and then degeneration [30]. Therefore, the effect of extract on sperm membrane integrity or viability was analyzed by hypo-osmotic swelling test. Membrane damage increased from 5.47% in control to 21.17% on treatment with extract concentration of 1,000 µg/mL. These data signify that extract damaged the sperm membrane to some extent but the effect was remarkably less than acrosome damage (Compare Fig. 2E with Fig. 1D) or viability loss (compare with Fig. 2D).
Intravaginal application of Artemisia extract significantly reduced the litter size. Since the extract would directly interact with spermatozoa deposited in vagina after copulation, reduction in litter size would imply that the extract exerts a direct contraceptive effect by interfering in fertilization or implantation. But the treatment resulted only 49% reduction in litter size. This much effect does not qualify the crude ethanol extract of Artemisia to be used as used as a contraceptive. Nevertheless, there is a scope for further evaluation by treating females with higher dose of the extract since presently injected extract amount (10 µL at 1,000 µg/mL) is totally harmless to mice and mice could possibly tolerate higher dose. Furthermore the active contraceptive principle of extract should be purified and screened. To this end, in vitro fertilization techniques would provide more resolution. This technique would allow studying the effect extract on various aspects of fertilization such as sperm-zona binding, zona penetration, sperm-oocyte fusion and early cleavages.
The results provided strong evidence that the extract could be an efficient contraceptive if applied intravaginally. But we have performed tests only on five sets of mice. For irrefutable conclusion, more experiments are needed to be performed with more sets of mice. Moreover several toxicological studies must be performed before it could be tested for human use or veterinary use. In a similar study, hexane fraction of the two plants Achyranthes aspera and Stephania hernandifolia in combination was found to be an effective spermicidal and when applied as a vaginal contraceptive in rats resulted 100% infertility [31].
