INTRODUCTION
Foot-and-mouth disease (FMD) is a highly contagious disease, associated with high morbidity in cloven-hoofed animals, including cattle, goats, sheep, and pigs. FMD causes substantial economic and production losses worldwide, and it has been listed first in the class A diseases of the animal health code by the World Organisation for Animal Health [1–3]. The etiological agent, FMD virus (FMDV), is a small, non-enveloped, single stranded, positive-sense RNA virus belonging to the genus Aphthovirus of the family Picornaviridae. The virus is grouped into seven serotypes (O, A, C, SAT1, SAT2, SAT3, and Asia1), and its high mutation rate could lead to extensive variation within serotypes [3,4]. There is no significant difference in the clinical signs of infection by these serotypes, but there is almost no cross-immunity [5]. Among the seven serotypes, outbreaks of serotype A and O FMDV are frequently reported in various Asian countries, including Vietnam, Korea, and China [6].
The clinical signs commonly observed in adult FMDV-infected pigs are vesicular epithelial lesions, high morbidity (up to 100%), and negligible mortality rate (approximately 1%) [1,7,8]. However, the mortality rate has been reported to be approximately 40% in piglets during an FMDV epidemic in Taiwan and China [9]. Viral myocarditis, which results in heart failure, is known to be the major cause of death in acute FMDV infection in piglets [9,10]. A previous case study reported that two FMDV-exposed piglets (weighing approximately 20 kg) died 3 and 5 days after exposure, respectively [10]. Considering these facts, we hypothesized that immunological response at the initial phase of FMDV infection differs between piglets and adult pigs.
The host immune response against viral infection has been demonstrated to be modulated by complex and divergent pathway [11]. Therefore, understanding the immunological interactions between the host and the pathogen is essential to provide a novel approach for the potential control of FMDV infection. During the early phase of viral infection, the innate immune response is initiated, mediated largely by white blood cells such as natural killer (NK) cells, dendritic cells (DCs), and macrophages [12,13]. Peripheral blood mononuclear cells (PBMCs) are known to play a significant role in the immune system, as they contain a mixed population of T-cells, B-cells, NK cells, DCs, and macrophages. Therefore, PBMCs are key cells that induce an innate and adaptive immune response through the expression of various cytokines [12].
The objective of this study was to investigate the effect of age on the immunological response against FMDV in porcine PBMCs. Furthermore, we compared the time-series of quantities of FMDV RNAs in PBMCs from pigs of different age groups. To the best of our knowledge, this is the first study to report the age-dependent differences in the expression of cytokines in FMDV-inoculated porcine PBMCs. This study could provide a better understanding of the mechanisms underlying the host immune responses to evaluate the age-dependent immune responses in FMDV-infected pigs.
MATERIALS AND METHODS
The protocols used for the experimental procedures were reviewed and approved by the Institute Animal Care and Use Committee of National Institute of Animal Science (approval number: NIAS 2020-465).
Three adult Vietnamese pot-bellied pigs (age 35 weeks and average weight 75 kg) and three piglets (age 8 weeks and average weight 7 kg), not vaccinated against FMDV, were obtained from the same herd from a commercial pig farm. All pigs were confirmed serologically negative for the following five pathogens: FMDV, porcine circovirus 2, porcine reproductive and respiratory syndrome virus, classical swine fever virus, and African swine fever virus. Animals were maintained with a commercial diet twice daily, and water was provided ad libitum. Blood samples (10 mL) were collected aseptically from pigs into a K2 ethylene-diamine-tetraacetic acid (EDTA) vacuum tube by puncturing the vena cava, and immediately transported to the biosafety laboratory of the National Institute of Veterinary Research, Vietnam.
The collected blood sample was diluted 1:1 with phosphate-buffered saline (Sigma-Aldrich, St. Louis, MO, USA), and then used to obtain PBMCs by density-gradient centrifugation with Ficoll-Paque™ Plus (1.077 g/mL, Cytiva Life Sciences, Uppsala, Sweden). Five milliliters of diluted blood was added to 4 mL of Ficoll-Paque™ Plus (Cytiva Life Sciences) and centrifuged at 1,200×g for 20 min at 20°C. After centrifugation, the PBMC layer found at the interface between the plasma and Ficoll-Paque™ Plus (Cytiva Life Sciences) was collected. Cells were stained with trypan blue and counted using a plastic disposable C-Chip Neubauer Improved hemocytometer (DHC-No. 1, InCyto, Cheonan, Korea). The PBMCs were resuspended in RPMI 1640 medium (R7388, Sigma-Aldrich) containing 10% fetal bovine serum (FBS) and 100 U/mL penicillin and 100 μg/mL streptomycin mixture (Sigma-Aldrich), and seeded in 24-well plates at a density of 2.0 × 106 cells/mL.
The serotype O FMDV, isolated in 2003 from pigs in Gia Lai Province, Vietnam, was provided by the National Institute of Veterinary Research, Vietnam. Virus titration in BHK-21 cells was used to determine the median tissue culture infectious dose (TCID50) per milliliter of FMDV according to a previous study [14]. PBMCs, except the mock infection control group, were infected with 2.0 × 102 TCID50 of FMDV in RPMI 1640 and incubated for various time periods (0, 1, 3, 6, 12, 24, and 48 h) in a humidified incubator with 5% CO2 at 37.0°C.
The total RNA was isolated from the infected and non-infected PBMCs using the RNeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. RNA concentration was determined using a Nanodrop I spectrophotometer (Thermo Fisher Scientific, Hanover Park, IL, USA). RNA from each group was reverse transcribed into cDNA in a 20-µL reaction mixture using the RT2 First Strand Kit (Qiagen) containing random primers following the instructions provided in the user’s manual.
FMDV RNA in PBMCs were detected using the ABI 7500 real-time polymerase chain reaction (PCR) System (Applied Biosystems, Foster City, CA, USA) with a commercial FMDV diagnostic kit (VDx FMDV qRT-PCR Kit, Median Diagnostics, Chuncheon, Korea). RNA (5 µL) was added into a tube containing 10 µL of 2× master mix and 5 µL of 4× oligo mix, and the reaction conditions were as follows: step 1, 50°C for 30 min; step 2, 95°C for 15 min; step 3, 40 cycles at 95°C for 10 s and 60°C for 30 s. The probe was labeled with a 5′-reporter dye (fluorescein amidites).
The relative expression of genes in PBMCs was quantified and compared at each time point (0, 1, 3, 6, 12, 24, and 48 h) after FMDV infection. The amplification reactions were carried out in a customized RT2 Profiler PCR Array (Qiagen) manufactured with primer sets for the specified genes pre-dispensed into a 96-well PCR plate. After mixing the cDNA with RT2 SYBR Green ROX qPCR Master Mix (Qiagen), 25 μL of the mixture containing cDNA was dispensed into each well of PCR arrays. Real-time PCR was performed on an ABI 7500 Real-Time PCR System (Applied Biosystems) under the following conditions: 10 min at 95°C, 40 cycles at 95°C for 15 s and at 60°C for 1 min. Dissociation curve analyses were performed immediately after each PCR run using the instrument’s default setting. The gene list for the custom RT2 Profiler PCR Array in this study is presented in Table 1. Expression fold change was determined using the 2−∆∆Ct method with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as the endogenous reference gene to normalize the level of target gene expression [15].
All experiments had three replicates. The results are expressed as mean ± SEM. Statistical analyses were performed using SPSS version 26.0 (IBM, Armonk, NY, USA). Data were analyzed using an unpaired Student’s t-test to compare the mean values between two experimental groups at the same time point. Results with p < 0.05 were considered statistically significant.
RESULTS
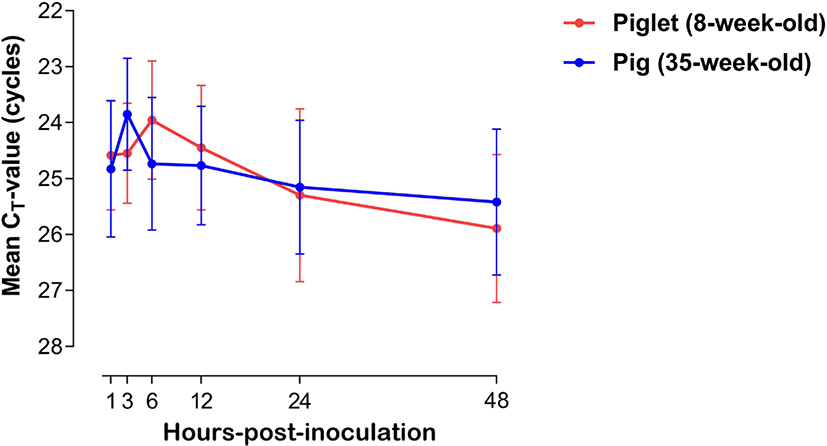
The expression of viral mRNA in PBMCs of piglets (8-week-old, n = 3) and pigs (35-week-old, n = 3) was analyzed after FMDV inoculation. The dynamics of cycle threshold-value (cycle threshold [CT]-value, mean ± SEM) of PBMCs of the piglets and pigs are shown in Fig. 1. The FMDV mRNA level in PBMCs from piglets (8-week-old) increased from 1 (average CT-value = 24.58 ± 0.97) to 6 hpi (23.95 ± 1.05), and then the concentration substantially decreased until 48 hpi (25.89 ± 1.32). In PBMCs from pigs (35-week-old), the viral mRNA level substantially increased from 1 (average CT-value = 24.58 ± 1.21) to 3 hpi (23.84 ± 0.99), and then steadily decreased until 48 hpi (25.41 ± 1.30).
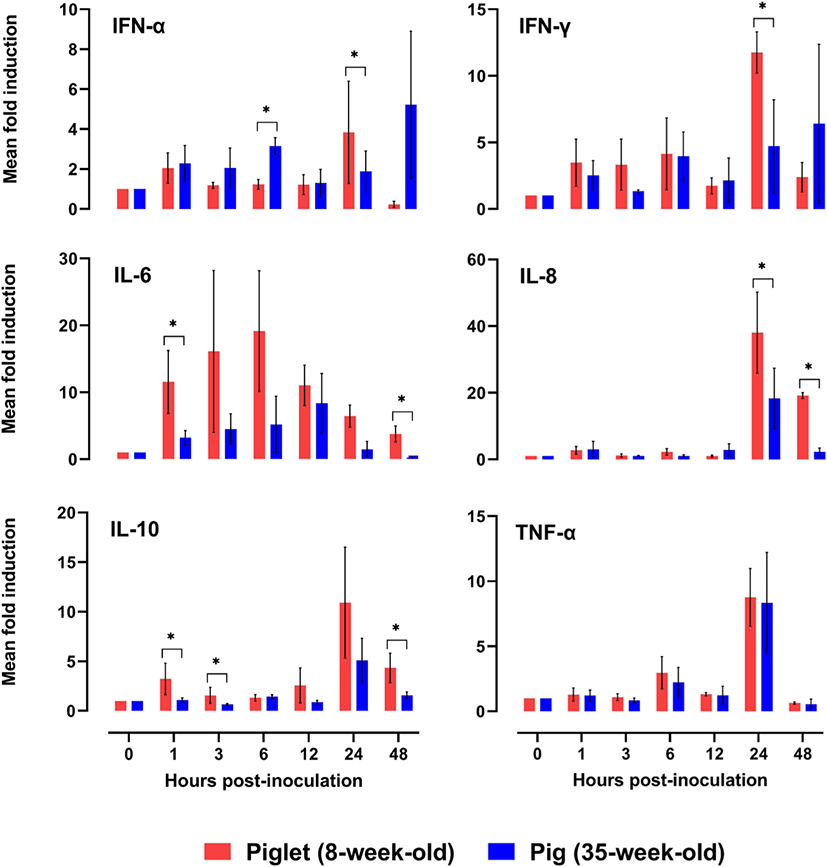
The dynamics of mRNA transcription of the six tested cytokines (interferon [IFN]-α, IFN-γ, interleukin [IL]-6, IL-8, IL-10, and tumor necrosis factor [TNF]-α) in porcine PBMCs in response to FMDV infection were analyzed at various time points to determine any difference between the experimental age groups (Fig. 2). The level of each cytokine was assessed at 0, 1, 3, 6, 12, 24, and 48 h using an SYBR Green-based real-time PCR assay, and all of them were induced by FMDV inoculation within 1 h in PBMCs from both groups. All tested cytokines (except TNF-α) derived from PBMCs showed age-dependent differences in the pattern of induction. The mean fold induction of IFN-α expression in the PBMCs from piglets was rapidly increased from 12 (1.22 ± 0.50) to 24 hpi (3.84 ± 2.56) and declined at 48 hpi (0.23 ± 0.16 fold). Regarding IFN-γ expression in piglets, the mean fold induction was substantially increased at 24 h (11.76 ± 1.54). In PBMCs from pigs, IFN-α mRNA expression varied throughout the experiment period, and rapidly increased at 48 hpi (5.22 ± 3.69 fold). IFN-γ expression in PBMCs from pigs showed an increasing trend until 48 h after FMDV infection. The fold induction values in IFN-α showed significant differences between the age groups at 6 and 24 hpi, and those in IFN-γ were significantly different at 24 hpi. IL-6 expression in PBMCs of both age groups increased progressively, with the maximum fold induction at 6 hpi in piglets (19.15 ± 9.03) and 12 hpi in pigs (8.37 ± 4.44 fold), and then steadily declined until 48 hpi (piglets [3.77 ± 1.17] and pigs [0.23 ± 0.05 fold]). At 1 and 48 hpi, the mean fold induction of IL-6 mRNA expression in the two age groups showed a significant difference. The IL-8 and IL-10 gene expression levels in PBMCs of both age groups peaked at 24 hpi (IL-8 in piglets [38.05 ± 12.16] and pigs [3.04 ± 1.05], IL-10 in piglets [18.29 ± 9.08] and pigs [5.10 ± 2.23 fold]) and decreased at 48 hpi (IL-8 in piglets [19.16 ± 0.87] and pigs [2.31 ± 1.11], IL-10 in piglets [4.34 ± 1.48] and pigs [1.59 ± 0.40 fold]). The fold induction values of IL-8 showed significant differences between the groups at 24 and 48 hpi, and those of IL-10 were significantly different at 1, 3, and 48 hpi. The fold induction of TNF-α gene expression showed similar trends in both groups, showing a substantial increase from 12 to 24 hpi and a decline at 48 hpi.
DISCUSSION
The severity of clinical signs of FMDV infection is inversely related to the age of pig [9,16]. Piglets aged up to 14 weeks, and particularly those aged less than 8-weeks may die without developing any clinical signs of FMD, due to heart failure, characterized by acute or hyper-acute myocarditis [17]. A comparison of cytokine mRNA expression in 8- and 35-week-old pigs could explain the age-related difference in the severity of FMDV infection. In this study, we investigated the changes in viral load in porcine PBMCs after FMDV infection and analyzed the dynamics of expression of relevant cytokines, such as IFN-α, IFN-γ, IL-6, IL-8, IL-10, and TNF-α, in PBMCs from piglets (8-week-old) and pigs (35-week-old) to understand the age-related difference in immune response against FMDV infection in the initial stages.
In general, in vitro analysis of cytokine expression in PBMCs against viral infection is a widely used method to study immune responses in host animals [18]. Although PBMCs are not regarded as a main target of FMDV, they are reportedly suitable for characterizing host immune responses against FMDV infection [9,18]. In this study, the time-series amounts of RNAs from FMDV-infected porcine PBMCs were compared between piglets and pigs. No significant changes were observed between them, and FMDV RNAs could be detected in the PBMCs of both age groups within an hour after FMDV inoculation. Through these results, we could confirm that PBMCs were successfully infected with FMDV, and the infected cells would appropriately respond to viral infection via secreting various cytokines. Peak detection of FMDV antigens was comparatively delayed in the PBMCs of piglets (maximum at 6 hpi) compare with those of the pigs (maximum at 3 hpi). The mean CT-value in porcine PBMCs inoculated with FMDV steadily decreased in both age groups after their respective peak time points. These findings suggest that there was an age-related difference in the intracellular infection period of the virus or virus suppression by the immune response. However, a general conclusion could not be drawn regarding the age-dependent FMDV susceptibility in pigs, as these results were obtained only from in vitro experiments. Therefore, further in vivo studies are needed to clarify the pattern of viral replication in FMDV-infected pigs of different age groups.
Innate immunity is the first host defense system against infectious diseases [19]. PBMCs are the gatekeepers against virus spread resulting from systemic viral infections and include many subpopulations that might cooperate in the immune activation processes [20]. In case of FMDV, the virus has been demonstrated to modulate the host immune response by using several mechanisms, implying that it is important to understand the host-pathogen interactions [11]. Cytokine response should be analyzed to understand the host immunological mechanisms against viral infections [21,22].
Type-I interferons are known to be the first line of host cell defense against viral infections, which inhibit viral replication in susceptible cells [23–25]. IFN-α is known to be a key factor for rapid resistance to FMDV infection [26,27]. Early IFN-α production induces virus-susceptible cells refractory to infection, thereby preventing viral replication and spread within the host, and resulting in a rapid reduction of viremia [27]. In this study, IFN-α mRNA expression in PBMCs was more pronounced in the 35-week-old pigs than in the 8-week-old piglets until 12 h after FMDV inoculation. Delayed IFN-α response in PBMCs from piglets caused adverse effects on the initial immune response against FMDV infection. IFN-γ is another important cytokine that regulates the immune response against FMDV infection and inhibits FMDV replication in vitro [28]. The dynamics of IFN-γ mRNA expression in the two age groups showed similar patterns with those of IFN-α. This result was compatible with the fact that IFN-α activates NK cells, which kill the FMDV-infected cells and secrete IFN-γ [29]. IFN-γ and TNF-α are the major pro-inflammatory cytokines that activate T-cells, the main effector cells for viral clearance, via the Th1 pathway [30]. Therefore, TNF-α is known to be an essential mediator of inflammation and is known to facilitate the transition from innate to adaptive immunity [19]. The current results showed that there was no significant difference in TNF-α mRNA expression between the two age groups, and it was the most pronounced in both groups at 24 hpi.
IL-6 is a pleotropic cytokine that is produced in response to tissue damage and infections [31]. The biological consequences of IL-6 production have been associated with both pro- and anti-inflammatory effects [32], highlighting the pivotal role of IL-6 in the activation and regulation of immune response [33]. The upregulation of IL-6 was observed in the PBMCs of piglets at all tested time points, and the corresponding mean fold induction was significantly higher in piglets than in the pigs, at 1 hpi. Increased levels of IL-6 may exacerbate the immunopathology by cytokine secretion and cellular recruitment. In fact, this condition of increased inflammation may be an advantage for some viruses by providing them with new cellular targets for subsequent viral infections [33]. To date there is no scientific evidence to support the causal relationship between IL-6 levels and pathogenicity of FMDV. However, a recent study suggested that infection by Picornaviridae members induces IL-6 expression in various cardiac-resident cells, which might contribute to damage to cardiomyocytes via acute inflammation [9]. This implies that IL-6, which reacted more strongly in piglets than in pigs, may be a clue to explain the age-related difference in pathogenicity against FMDV. IL-8 is a chemotactic factor for all migratory immune cells. The level of IL-6 along with IL-8 increases in pigs vaccinated against FMDV [34,35]. Here, the IL-8 mRNA expression level showed no substantial variation until 12 hpi; thereafter, it rapidly increased at 24 and 48 hpi in FMDV-inoculated PBMCs from piglets and pigs, respectively. The mRNA expression level of IL-8 was considerably higher in PBMCs from the piglets than those from the pigs, after 24 hpi. IL-10 has important anti-inflammatory functions to prevent unnecessary tissue damage caused by the immune system [36]. In addition, the diminished T cell response during FMDV infection is known to be related to the elevated level of IL-10 produced by DCs [11]. Given that IL-10 mRNA expression was observed to be significantly higher in piglet PBMCs than in pig PBMCs in the early stages of viral infection (1 and 3 hpi) in this study, piglets may have a weaker T-cell-mediated cellular response than pigs.
Here, we investigated age-dependent cytokine expression patterns against FMDV infection in porcine PBMCs and elucidated the immune responses to FMDV infection in pigs. These findings imply that the immune response against FMDV infection is age dependent. However, the study had two major limitations. First, FMDV-inoculated pigs are known to show peak viremia at 24–48 hpi, with full clearance by 96 hpi [27]. However, the present study was only performed until 48 hpi, which may not fully reflect the immune response against FMDV infection. Therefore, further studies are needed to analyze the dynamics of cytokine mRNA expression until at least 96 hpi. Second, the number of animals, from which PBMCs were obtained, was relatively small to establish a definitive conclusion. In addition, as this study involved an in vitro experiment targeting PBMCs from pigs, it may not accurately reflect the overall age-dependent immune responses of pigs infected with FMDV. Future in vivo studies with a large number of animals are essential to understand the host defense mechanisms against FMDV infection in pigs, and further transcription analyses could also provide valuable information for improving our awareness of host cell-FMDV interactions.
In conclusion, FMDV mRNA expression in PBMCs was not consistent between 8- and 35-week-old pigs, and this may be associated with the differences in the expression patterns of cytokine mRNAs in porcine PBMCs between the two age groups. The expression dynamics of immune-regulating cytokine genes, except that of TNF-α, in the FMDV-inoculated porcine PBMCs were different in pigs of the two age groups. Only IFN-α showed a rapid initial response in adult pigs, and all other cytokine mRNAs showed higher expression in the PBMCs of piglets than in those of adult pigs. In addition, IL-6 mRNA expression was more pronounced in piglets than in pigs in the very early stage of FMDV infection, which may be related to the occurrence of acute myocarditis in young pigs. The results suggest that there is a difference in the cytokine-mediated immune response against FMDV infection between piglets and pigs, which may explain the age-related differences observed in pigs with respect to the pathogenesis of FMDV. Further in vivo studies may help validate this conclusion.
