INTRODUCTION
Korea is a country with scarce agricultural resources. About two thirds of its land area is mountains and hills. The cultivated area only accounts for 22% of the total land area. It has one of the lowest per capita cultivated land areas in the world. The livestock industry accounts for almost 40% of total agricultural production in Korea [1]. With the development of the livestock industry, the forage industry has attracted increasing attention. The forage industry is the basis for the survival and development of the livestock industry. However, Korea’s current self-produced feed resources are relatively limited, and some feeds must still be imported from overseas. As the most basic production source of animal products, problems with the feed supply will affect the sustainable development of the whole livestock industry. To stabilize the livestock industry and agricultural production, the production of high-quality forage would reduce feed costs and have an import substitution effect.
Corn and sorghum-sudangrass hybrids are the two most common forage crops that are used mainly as summer-season forages in dairy and beef rations. They have low production costs, high yield, and a relatively high nutritional value. Proso millet (Panicum miliaceum L.) is a short growing, summer season crop (60 to 100 days) with unique agronomic properties such as high tolerance to heat and drought conditions, and is cultivated in abundance in Asian and African countries [2-4]. Proso millet crop has the potential to remain productive in areas with marginal lands and limited agricultural inputs, where cultivation of major crops such as corn is restricted [5-7]. Proso millet could be a viable alternative to main summer forages in areas where cultivation of corn or sorghum-sudangrass is restricted due to a longer growing season or poor agricultural conditions [8].
Ensiling has long been recognized as a simple and effective method of preserving moist forage, ensuring a continuous supply of forage to animals [9,10]. To our knowledge, few studies have investigated the fermentation dynamics of proso millet forage. The purpose of this research was to provide basic information about the ensiling feasibility of forage from proso millet in comparison to commonly cultivated summer crops (whole-plant corn and sorghum-sudangrass hybrid).
MATERIALS AND METHODS
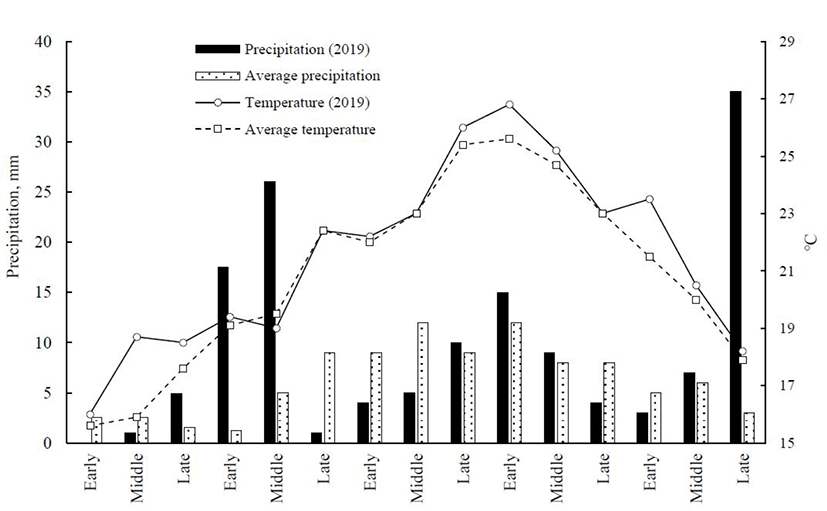
Establishment of experimental plots was made at the experimental site of Seoul National University, Pyeongchang Campus (located at 37° 32’ 40’’ N, 128° 26’ 33’’ E, average altitude is about 550 m above sea level) during the summer season of 2019. A detailed description of meteorological data including temperature and precipitation throughout the growing season (May to September, 2019) is illustrated in Fig. 1. During the growing season, temperature ranged from 15.9°C to 26.8°C (average = 21.5°C). Soil analysis on the 0-15-cm soil depth of the experimental site showed that it was slightly acidic (pH 6.55; soil:water suspension = 1:5), with 14.1% organic matter, 0.12% total nitrogen, and a cation exchange capacity of 16.5 cmol(+)/kg. Concentration of exchangeable cations including Ca, K, Mg, and Na averaged 1.75, 4.01, 0.92, and 0.10 mg/kg, respectively. For the three crops, nitrogen, phosphorus, and potassium fertilizers were applied at a rate of 200, 150, and 150 kg/ha, respectively. After preparation of seedbed, seeds were sown manually and grown on 3 replicate plots/each crop. Each plot was 3 m × 5 m in size. Proso millet (Panicum miliaceum L. var. Geumsilchal) was planted on June 8 at a seeding rate of 20 kg/ha, and harvested on September 5. Sorghum-sudangrass hybrid (Sorghum bicolor L. var. Turbo-gold) was sown at a seeding rate of 40 kg/ha on May 10 and harvested on September 10. Corn (Zea may L. var. Gwangpyeongok) was sown on May 10 at a plant-to-plant distance of 20 cm and an inter-row spacing of 75 cm. Whole-crop corn was harvested on September 10. Sorghum-sudangrass hybrid and proso millet were harvested when they reached soft-dough stage of the seedhead. Whole-crop corn was harvested at about the half milk-line stage, which is a reliable visionary criterion indicating the optimum time to harvest whole plant for silage making [11]. This was accomplished by splitting the corn ear in the center and visually inspecting the kernel milkline. Whole-crop corn was fractionated into cob (containing kernel and rachis) and stover component that was consisted of the remaining components of the plant after cob removal [12]. These fractions were separately weighed and approximately 1-kg representative subsamples were collected for dry matter (DM) determination. The proportion of these fractions in the whole plant was then calculated. Forage yield was determined by manually harvesting the forage material in the whole plot and calculating the fresh forage yield, which was then converted to units of fresh and DM/hectare.
At harvest, four whole-crop plants from center rows in each plot were randomly selected and chopped into approximately 2-3 cm long pieces using a chopper (Richi Machinery, Henan, China). The chopped crops were grouped into separate piles per each plot for silage experiment. The representative allotments were also collected for quality assessment of fresh biomass before ensiling. Ensiling was made by packing approximately 600 g chopped material into plastic film bags (28 cm × 36 cm). The bags were vacuum-sealed (Zhejiang Hongzhan Packing Machinery, Wenzhou, China) and stored in a dark and dry condition at room temperature (about 22°C). Bags were randomly opened on days 1, 2, 3, 5, 10, 15, 20, 30, and 45 of ensiling for quality assessment of silage fermentation. Silos were weighed at designated openings for DM loss determination [13]. Number of replicate silos for each crop at each opening was 3. Therefore, the design arrangement for the three forage types in the silage trial was as follows: 3 forage types × 9 silo openings × 3 replications, resulting in formation of a total of 81 silos. At each silo opening, the ensiled material inside each silo was emptied, mixed thoroughly and divided into 3 representative portions. The first portion was dried (65°C) to a constant weight and used for the chemical composition analysis. The second portion was stored in a freezer at −80°C (TSE400D, Thermo Fisher Scientific, Waltham, MA, USA) for quantification of organic acids and ammonia nitrogen (NH3-N). The third subsample was used for enumeration of microbial population in ensiled biomass.
A 10-g fresh silage sample was placed into a 250 mL conical flask and covered with 100 mL distilled water. The flasks were shaken for 1 h on a mechanical shaker (Green Sseriker, Vision Scientific, Daejeon, Korea) and stored in refrigerator for 24 h. The conical flasks were shaken by hand every 2 hours during refrigeration. The mixture was filtered through a filter paper (Whatman No. 6, Advantech, Zurich, Switzerland). Silage pH was determined in the filtrate with a pH meter (AB 150, Fisher Scientific International, Pittsburgh, PA, US). A 1.5 mL portion of the filtrate was used for analysis of the organic acid concentration using high performance liquid chromatography (HPLC, Agilent Technologies, Santa Clara, CA, US) equipped with a refractive index detector [8]. NH3-N was analyzed via the method described by Broderick and Kang [14]. The spread-plate method [15] was used to enumerate the population of microorganisms. In brief, a 10-g sample was diluted with 90 mL sterilized saline solution (8.50 g/L NaCl) and shaken for 1 h. Lactic acid bacteria (LAB), molds, and total microorganisms were enumerated on Rogosa, and Sharpe agar medium, potato dextrose agar, and plate count agar media, respectively. The limit of detection was 2 Log10 CFU/g fresh mass.
DM concentration in ensiled material was determined in triplicate at 65°C in a forced drying oven for 72 h. The dried samples were ground to pass through a 1 mm screen (Thomas Scientific, Swedesboro, NJ, USA) for nutrient composition analysis. Total nitrogen was quantified via the Dumas method [16], and crude protein (CP) was calculated as nitrogen × 6.25. Acid detergent fiber (ADF) and neutral detergent fiber (NDF) were measured following the method of Van Soest et al. [17]. Water-soluble carbohydrate (WSC) was analyzed via a modification of the anthrone method proposed by Yemm and Willis [18].
In vitro DM digestibility (IVDMD) was performed in triplicate using an Ankom DaisyII incubator (ANKOM Technologies, Fairport, NY, USA) [19], as described by Goering and Van Soest [20]. Ground samples (0.5-0.6 g) were weighed into F57 filter bags and sealed using a heat sealer. Samples were evenly distributed on both sides of the digestion jars. Then, 1,330 mL buffer solution A and 266 mL buffer solution B were added to each jar. Two ruminally cannulated Holstein steers were selected and their rumen fluid was collected before the morning feed and passed through four layers of cheesecloth. Then, 400 mL rumen fluid was added to the buffer solution and samples. The digestion jar was purged with CO2 gas for 30 s and then closed with a lid. The jars were incubated at 39°C for 48 h. Undigested NDF residues in original bags were extracted using an ANKOM2000 fiber analyzer.
Field experiment was arranged in a completely randomized block design with three replications. Data were subjected to analysis of variance (ANOVA) using the general linear model (GLM) in SPSS (IBM SPSS Statistics, Version 24.0, Armonk, NY, USA). Individual plot was regarded as the experimental unit in the model for analysis of data from the field experiment (Table 1). Individual silo served as the experimental unit in the model for analysis of data from silage experiment. Prior to statistical analysis, microbial data (Table 4) were logarithmically transformed. Mean treatment differences were obtained by Duncan’s multiple range tests, with a statistical significance level of 5%.
TDN, total digestible nutrients. For proso millet and sorghum-sudangrass hybrid, TDN was calculated according to the following equation: [889 - (0.79 × ADF, g/kg DM)]. For corn plant, TDN was claculated using the following equation: [878.4 - (0.70 × ADF, g/kg DM)] [46]; RFV, relative feed value calculated according to the following equation: [(dry matter intake × digestible dry matter)/1.29], where dry matter intake = 120/(NDF%) and digestible dry matter = 88.9 - (0.779 × ADF%) [47].
RESULTS AND DISCUSSION
Yield and forage quality of experimental forage crops are presented in Table 1. Forage DM concentration was greatest in proso millet (303 g/kg), intermediate in corn (277 g/kg), and lowest in sorghum-sudangrass hybrid (193 g/kg). Whole-plant corn had the highest relative feed value (RFV) of 117, which was 20 and 40 units higher on average than proso millet and sorghum-sudangrass hybrid, respectively. A forage crop with an RFV between 103 and 124 is considered a high-quality forage [21], indicating the superiority of corn over sorghum-sudangrass hybrid and proso millet forage. Similar to our observations, Jahansouz et al. [22] also reported a similar trend in fresh forage yield. Concentration of total digestible nutrients was highest in corn (667 g/kg DM), intermediate with proso millet (631 g/kg DM), and lowest with sorghum-sudangrass hybrid (541 g/kg DM). In general, the forage nutritive value of proso millet is comparable to the value reported by Kim et al. [23] harvesting “Geumsilchal” variety in reclaimed lands located in Sihwa (Korea).
Forage yield was significantly different by forage types, with proso millet producing the least DM. The forage DM yield was greater in the order of sorghum-sudangrass hybrid (23.5 t/ha), corn (18.7 t/ha), and proso millet (7.68 t/ha). Forage yield of proso millet (fresh or DM basis) agrees with the values reported by Shin et al. [24]. Calamai et al. [4] also reported that total dry biomass in proso millet averaged 6.43 t/ha. Data of NDF and CP concentration of these forage crops is previously reported [8]. NDF was highest in sorghum-sudangrass hybrid, intermediate in proso millet, and lowest in corn. No difference existed in CP concentration among crops, averaging 58 g/kg DM.
Changes in DM loss and chemical composition of the three forage crops during ensiling are reported in Table 2. As ensiling progressed, DM loss occurred in all crops, with proso millet losing the most DM than corn or sorghum-sudangrass hybrid, most likely because a higher number of epiphytic molds existed on proso millet biomass. Loss of DM was faster in proso millet during the first day of fermentation, which may be justified by the significantly greater population of total microorganisms in fresh mass of proso millet than in corn or sorghum-sudangrass hybrid (Table 4). Microbial degradation of nutrients into carbon dioxide and water could possibly explain loss of DM with ensiling [25,26]. CP concentration displayed a downward trend during the ensiling process, which is suggestive of protein degradation with ensiling. A downward trend was also observed in NDF concentration of all forage crops with ensiling. From day 0 to 45, NDF concentration of proso millet decreased from 607 to 591 g/kg DM, which is less than the corresponding values in corn and sorghum-sudangrass hybrid. Chen et al. [26] suggested that hemicellulose degradation during the ensiling process is mainly responsible for NDF reduction with ensiling. This loss could be due to a combination of enzymatic and acid hydrolysis of the more digestible cell-wall fractions during the fermentation [10,27]. After 45 days of ensiling, ADF concentration of proso millet silage declined by about 20 g/kg DM. Similar decreases also occurred for corn and sorghum-sudangrass hybrid.
Values with different lowercase letters within each row show significant difference among ensiling days with the same forage type.
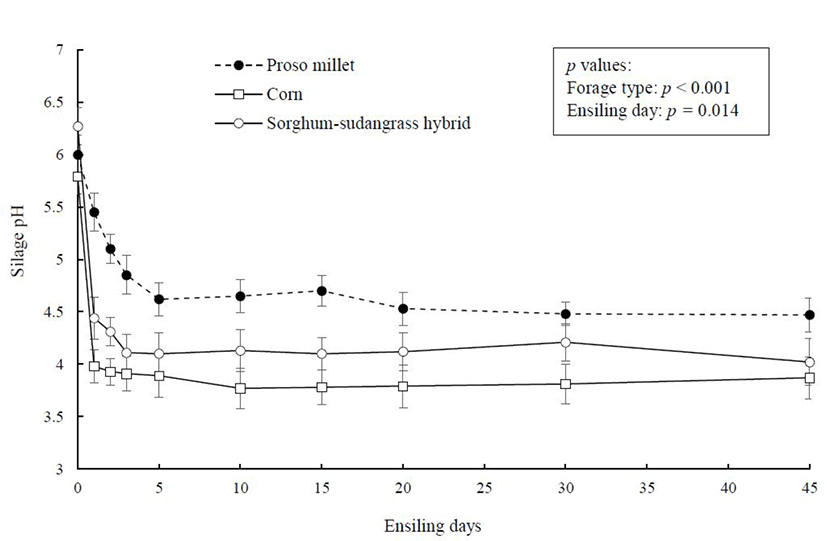
Changes in silage pH as a function of fermentation time are illustrated in Fig. 2. The day-0 pH of corn crop (5.80) was generally lower than proso millet or sorghum-sudangrass hybrid (mean 6.05), which is in agreement with the mean values (5.50 to 6.0) reported for the different forages after chopping [28,29]. Silage pH of corn and sorghum-sudangrass hybrid fell rapidly to below 5 within 24 hrs of ensiling, but it took 3 days for proso millet pH to decline below this value. During the late phase of ensiling, silage pH remained stable and was significantly lower in corn than in proso millet or sorghum-sudangrass hybrid (p < 0.05), possibly due to the higher population of LAB in corn silage biomass (Table 4). During the 45-day ensiling period, silage pH of corn, proso millet, and sorghum-sudangrass hybrid decreased by 1.94, 1.65, and 2.04 units, respectively. Buffering capacity, WSC concentration, and moisture level have been identified as critical parameters influencing the ensilability of forages if epiphytic LAB exist in sufficient numbers [30]. Buffering capacity was lowest in corn (24.2 mEq/kg DM), intermediate in proso millet (32 mEq/kg DM), and highest in sorghum-sudangrass hybrid (55.5 mEq/kg DM) [8]. Forages with higher buffering capacity require more acids for pH reduction. This supports the faster pH reduction in corn plant at the initial phase of ensiling than proso millet or sorghum-sudangrass hybrid.
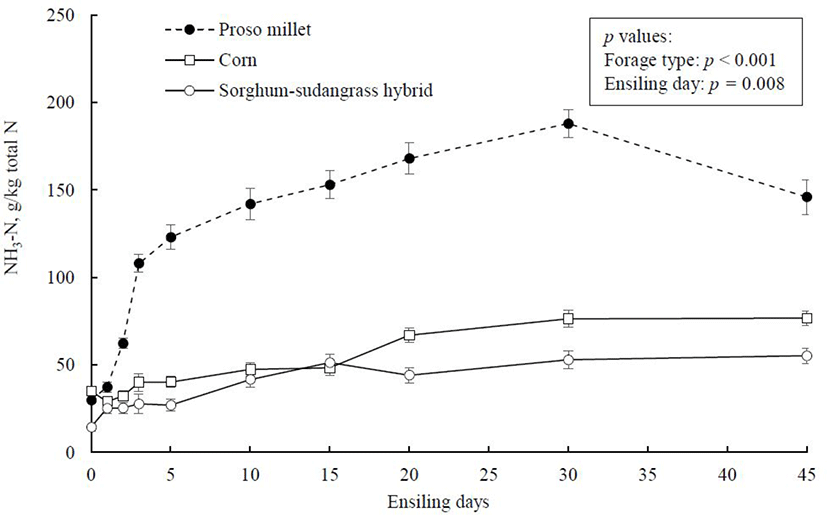
Time-course of silage ammonia-nitrogen development, expressed as a proportion of total N is illustrated in Fig. 3. Initial NH3-N (g/kg total N) level before ensiling was highest in corn (35), intermediate in prose millet (30), and lowest in sorghum-sudangrass hybrid (14.4). Ammonia-N concentration increased in three forage crops as ensiling progressed, with proso millet exhibiting the highest rise. This indicates that protein fractions in proso millet were degraded to a greater extent during ensiling, perhaps because of accelerated rate of proteolysis and deamination [31]. The NH3-N concentration of less than 70 g/kg total N indicates successful silage fermentation, whereas amounts greater than 100 g/kg total N have been linked to poor silage fermentation [32]. This criterion indicates more degradation of protein in proso millet than corn and sorghum-sudangrass hybrid. The rapid acidification of silage mass is known to inhibit growth and activity of undesirable microorganisms as well as proteolytic activity [10,33]. The higher NH3-N concentration in proso millet silage could be attributed to its higher pH during ensiling, which was likely insufficient to effectively suppress enzymes and microorganisms involved in protein degradation during fermentation.
Concentration of WSC in silage mass over the course of the 45-d fermentation is presented in Fig. 4. Initial WSC concentration (before ensiling) was higher in proso millet than in corn or sorghum-sudangrass hybrid (170 vs. mean 141 g/kg DM). An initial WSC concentration between 60 and 80 g/kg DM has been suggested as an adequate amount to promote an efficient silage fermentation [34]. This indicates that the forage crops evaluated in this study contained sufficient WSC to promote a good-quality silage fermentation. The exhaustion of WSC was faster in corn plant as ensiling progressed, reaching a minimum of 6.70 g/kg DM after 3 days of ensiling, after which WSC concentration decreased slightly until day 45 of ensiling (5.20 g/kg DM). Proso millet experienced a comparatively slower rate of decline in WSC during ensiling, decreasing to 18.2 g/kg DM on day 15 of ensiling and reaching a mean value of 5.9 g/kg DM after 45 days of ensiling.
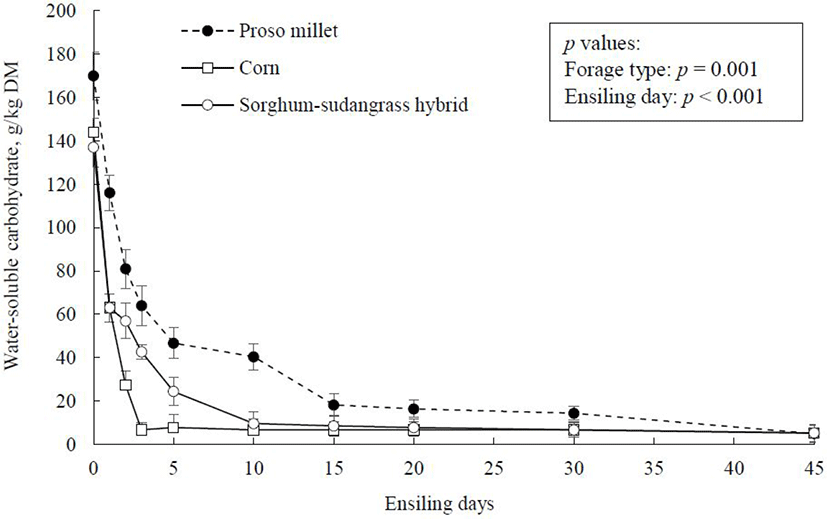
During the ensiling fermentation, LAB consume WSC as a readily available source of energy and primarily convert it to lactic acid, which is associated with silage mass acidification and inhibition of the activities of undesirable microorganisms [26]. Variations in WSC consumption rates amongst forage crops during the early phase of ensiling might be ascribed to differences in microbial activity and plant enzymes in the crops prior to ensiling. In general, WSC supplies the energy required to drive silage fermentation [35]. A sufficient quantity of WSC has been identified as an important factor in fast acidification during the initial phase of ensiling, which is associated with DM loss reduction and improvement of silage quality [10]. In our experiment, the faster reduction of WSC in corn compared to proso millet forage represented a faster decline in silage pH, which was associated with less DM loss and NH3-N production during ensiling.
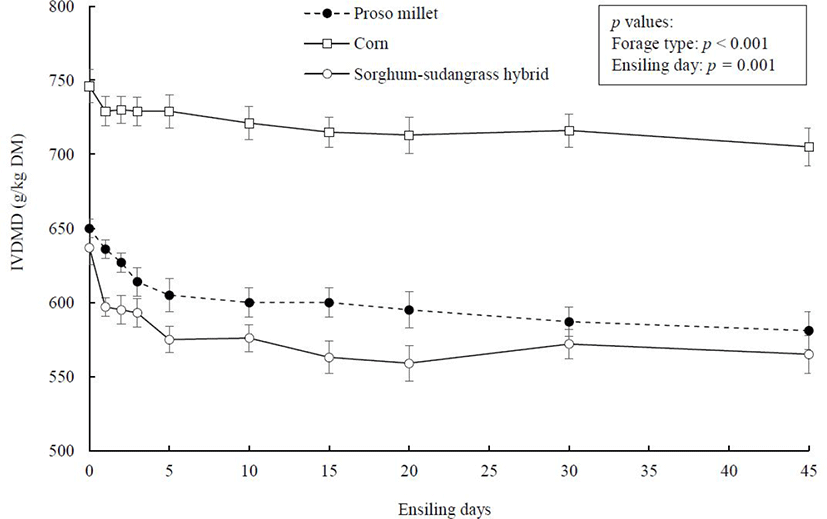
The IVDMD of the experimental forage crops as a function of ensiling duration are illustrated in Fig. 5. Before ensiling, IVDMD of proso millet and sorghum-sudangrass hybrid was not different, averaging 643 g/kg DM, which was approximately 16% less than corn (746 g/kg DM). All crops experienced a decline in IVDMD with ensiling. Previous studies have identified that ADF and NDF concentrations correlate negatively with IVDMD [36]. This supports findings of the current study because corn had less NDF and ADF fractions than proso millet or sorghum-sudangrass hybrid, resulting in the higher digestibility of corn than the other two crops.
Formation of lactic acid and acetic acid as a function of ensiling duration is illustrated in Table 3. Butyric acid was undetectable during the 45-day ensiling period, which indicates a well-fermented silage and a lack of clostridial activity during ensiling process [10,26,29]. High silage pH, typically greater than 4.5, low DM concentration, and high buffering capacity have been identified as probable factors which contribute to clostridia growth and proliferation during ensiling [32,37]. This suggests that among the forage types evaluated in this experiment, prose millet had a greater susceptibility to clostridial activity and, thus butyric acid production. However, such an effect was not observed in this experiment and the absence of butyric acid detection during silage fermentation of proso millet indicates its low susceptibility to putrefaction by clostridial fermentation.
Lactic acid formation increased as silage fermentation progressed, and the magnitude of this increase was generally greater in sorghum-sudangrass hybrid, intermediate in corn, and lowest in proso millet. During the 45-day ensiling period, lactic acid concentration displayed an upward trend and reached a maximum on day 45, with values of 42.5 g/kg DM for proso millet, 67.7 g/kg DM for corn, and 127 g/kg DM for sorghum-sudangrass hybrid. Lactic acid is typically found in concentrations ranging from 20 to 40 g/kg DM in commonly used silages [29], which indicates that all forages in the present experiment underwent an adequate lactic acid fermentation. Similar to lactic acid production, acetic acid also increased with ensiling, the rate of its production was generally larger in the earlier phase of silage fermentation. During ensiling process, acetic acid was usually lower in proso millet than in corn and sorghum-sudangrass hybrid. Acetic acid concentration in sorghum-sudangrass hybrid reached a maximum concentration of 100 g/kg DM on day 45 of silage fermentation. The higher lactic acid and acetic acid production in sorghum-sudangrass hybrid during silage fermentation could be explained by its higher moisture concentration than the other two crops, which accelerates microbial activity and acid production during the ensiling process. This explanation is supported by the findings of a previous study identifying that a lower moisture level limits silage fermentation [38]. Although no consistent trend was seen in lactic acid: acetic acid ratio, there was a general downward trend for each crop, which is likely indicative of a shift from homo- to hetero-fermentative pattern. This observation is consistent with results reported by Shao et al. [39,40]. The higher ratio of lactic acid: acetic acid in corn is most likely suggestive of the dominance of homofermentative LAB during the ensiling process.
Changes in microbial population as a function of ensiling duration are shown in Table 4. The pre-ensiling population of LAB, mold, and total microorganisms is presented in our companion paper [8]. Briefly, the highest LAB count was detected in corn (6.15 Log10 CFU/g), followed by proso millet (5.91 Log10 CFU/g), and sorghum-sudangrass hybrid (5.88 Log10 CFU/g). An LAB count of 5.0 Log10 CFU/g biomass has been suggested as a minimum number to enable the dominance of the epiphytic LAB during ensiling [41,42]. This suggests that the forage crops had sufficient epiphytic LAB population to initiate an efficient silage fermentation. Number of mold was highest on proso millet biomass (4.53 Log10 CFU/g fresh mass), which was 0.23 and 1.23 Log10 CFU/g fresh mass greater than corn and sorghum-sudangrass hybrid, respectively. Forage species, maturity stage, weather, and field wilting have all been identified as factors causing differences in the population of epiphytic microorganisms in forage crops [43]. During the 45-day of fermentation, LAB count was generally lower in proso millet than corn or sorghum-sudangrass hybrid. Number of LAB increased during the early ensiling period and peaked on day 10 of ensiling. Low pH and the exhaustion of fermentable substrates have been identified as the primary factors contributing to the decline of LAB population as ensiling proceeds [44].
| Microbial count1) | Forage type | Ensiling days | SEM | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 5 | 10 | 15 | 20 | 30 | 45 | |||
| Lactic acid bacteria | Proso millet | 6.48bB | 6.88aA | 6.94aB | 6.93aB | 6.96aB | 6.48bB | 5.78cC | 5.78cB | 5.34dB | 0.11 |
| Corn | 6.84fA | 6.85fA | 7.23cdA | 7.30cA | 7.77aA | 7.61abA | 7.04eA | 6.60gA | 6.08hA | 0.08 | |
| Sorghum-sudangrass hybrid | 5.08dC | 5.68cB | 6.60bc | 6.53a-hBC | 6.95aB | 6.60bB | 6.95aB | 6.62bA | 5.89cA | 0.12 | |
| Molds | Proso millet | 3.49dA | 4.21cA | 4.30baA | 4.20cA | 5.00abA | 5.38aA | 4.34bcA | 4.04cB | 4.80bA | 0.30 |
| Corn | 3.18cB | 3.00cC | 4.00bB | 3.00cC | 4.05bB | 4.00a-hBC | 3.85bB | 4.67aA | 4.18abB | 0.41 | |
| Sorghum-sudangrass hybrid | 3.48cdA | 3.30dB | 3.85bB | 3.70bcB | 3.29dC | 5.11aB | 3.60bcC | 3.31dC | 3.00eC | 0.13 | |
| Total microorganisms | Proso millet | 7.43bA | 7.51bA | 7.44bA | 7.79aB | 7.86aB | 7.26bcA | 7.04cB | 6.30dC | 6.60eB | 0.10 |
| Corn | 7.05cB | 7.29cA | 7.18cB | 8.10bA | 8.85aA | 7.11cAB | 7.85bA | 7.04cB | 7.12cA | 0.19 | |
| Sorghum-sudangrass hybrid | 6.57cC | 6.88bB | 6.48cC | 7.01bc | 7.40aC | 6.95bB | 7.04bB | 7.32aA | 6.51cB | 0.08 | |
Microbial count was expressed as the logarithmic number of colony-forming units per gram fresh mass.
Mold was always present in each crop during fermentation, with a lower number existing on corn than proso millet or sorghum-sudangrass hybrid. The lower mold population in corn biomass is likely related to the rapid acidification (lower pH) of corn silage, inhibiting the growth of undesirable microorganisms [26,42]. Another factor inhibiting mold growth during ensiling is a high acetic acid concentration [45]. Less formation of acetic acid and lactic acid (higher pH) during ensiling fermentation of proso millet could possibly explain the higher mold number in proso millet biomass during ensiling. The count of total microorganisms was generally higher in corn than in sorghum-sudangrass hybrid or proso millet. Total microorganisms reached the maximum number on day 10 of ensiling, and then followed a downward trend, which could be explained by pH reduction at this time point, limiting the growth of microorganisms.
CONCLUSION
Silage fermentation of proso millet forage resulted in a significant increase in NH3-N generation and a larger loss of DM when compared to corn or sorghum-sudangrass hybrid, perhaps because of its higher buffering capacity and silage pH. However, butyrate was undetectable during its ensiling fermentation. Further research is needed to optimize the fermentation quality of proso millet forage, possibly by using the appropriate silage additives to minimize ammonia-nitrogen formation during fermentation, as well as to promote greater lactate production, which is associated with a further decline in silage pH and mold growth inhibition, and thus with a reduction in DM loss. Despite the lower productivity (less forage production per unit of cultivated land) than corn and sorghum-sudangrass hybrid, nutrient value of proso millet was comparable to sorghum-sudangrass hybrid. Proso millet could be harvested in a shorter period of time, making it a potential summer crop in situations where cultivation of other major summer crops is limited.
