INTRODUCTION
Rinse & Chill® technology (RCT) was developed and patented by MPSC (Hudson, Wisconsin, United States). Immediately upon exsanguination, a sanitized catheter is inserted into the carotid artery (or heart) of an animal (Fig. 1), and a chilled isotonic solution is then infused through the cardiovascular system. The vasculature is rinsed at a rate up to 10% of the carcass weight and as a result improves residual blood removal from the carcass [1–4]. The RCT process lasts approximately 3 to 4 minutes on each beef carcass, and approximately 15 seconds on a lamb carcass. The catheter is then removed, and the suspended carcass continues along the chain as normal.
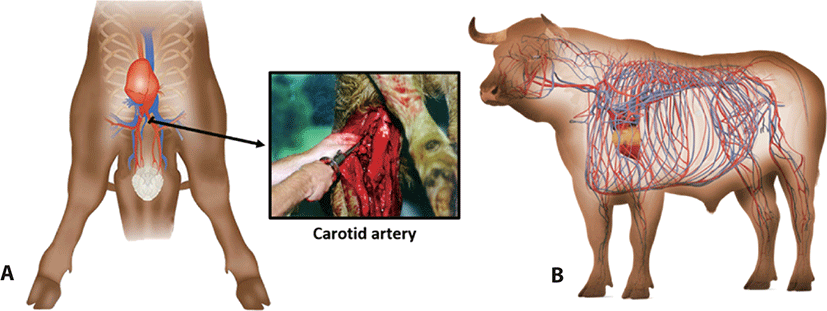
On the kill floor, an automated process control system weighs each carcass and calculates the amount of rinse required. Appropriate Hazard Analysis Critical Control Point (HACCP) and sanitary procedures are followed for equipment and operators for each catheter insertion and removal. Once the equipment is installed, there is a full-time, trained and certified RCT technician in every customer facility. They monitor the process and conduct regular tests to confirm that RCT achieves the highest levels of safety, wholesomeness, and performance. In detail, an RCT system is designed with multiple catheters to meet existing plant production speeds so that multiple rinses can occur simultaneously as carcasses move along the production chain. According to MPSC’s technical engineer, the RCT process has been able to accommodate production speeds up to 200 carcasses per hour in beef and up to 780 lamb carcasses per hour. Typically, beef plants using RCT process 200 to 1,200 carcasses per 8 hours shift, and lamb plants process 2,000–6,000 carcasses.
The RCT solution is prepared daily, filtered, and sanitized by an in-line ultraviolet (UV) light system prior to infusion. First, the RCT ingredients are inspected for any contamination, and the incoming water (the carrying agent) is filtered to remove any inadvertent foreign contamination. Throughout these procedures, the chemical, physical, and microbial hazard risks are mitigated, and the risks are further mitigated via regular Cleaning-in-place (CIP) and following Sanitation Standard Operation Procedures (SSOP). Although unlikely, if there was an apparatus failure, a real-time programmable logic controller, which monitors and controls the entire RCT process, initiates protocols via instrument feedback, followed by either shut down, or the fault alert on the RCT process to protect the safety of the product and overall RCT system and its sanitary integrity. Therefore, through the combination of all of the processes (SSOP, filtering, UV application, and system controls) the risk of a contaminated RCT solution being infused is greatly mitigated. To further acknowledge the safety of the process, the RCT solution in itself has significant antimicrobial activity.
The novel postmortem (PM) process referred to as RCT has matured enough that the benefits of vascular rinsing have become a technical and financial reality for an increasing number of beef and lamb processors. Thus, the objective of this review is to provide an overview of the effects of carcass vascular rinsing and chilling on various physical, chemical, biological and sensory traits compared to meat from non-rinsed carcasses.
HOW RINSE & CHILL® TECHNOLOGY WORKS: MAJOR ADVANCEMENTS
RCT is a thoroughly tested, safe, effective and a proven process where the US experience includes two decades of pilot programs, academic research and several years of commercial trials on a variety of animal types (beef, bison, pork, lamb, and goat). Much of the published scientific research on RCT has been generated by researchers at Michigan State University, the University of Minnesota, Kansas State University, South Dakota State University, and the University of Wisconsin-Madison. In addition, Dr. Claus at the UW-Madison has collaborated with Dr. David Hopkins and his group [1] at the Centre for Red Meat and Sheep Development and Dr. Robyn Warner at the University of Melbourne to add additional information on the applicability of RCT on Australian lamb. Table 1 presents a summary from previous studies which have investigated the effects of carcass vascular rinsing and chilling on PM metabolic changes and meat quality (tenderness, sensory traits, and color) during the PM period.
| Animal | Muscle/cuts | Effects | Reference |
|---|---|---|---|
| Dairy cows | SS, LL, ST |
• 37% reduction in toughness • Improve protein extractability □ Non-rinsed: 42.0% □ Rinsed: 43.5% |
Farouk et al. [11] |
| Lamb | LL, IS |
• Lower carcasses temperature in first 3 h PM □ Non-rinsed: 39.7°C (0 h) to 23.1°C (3 h) □ Rinsed: 36.6°C (0 h) to 21.7°C (3 h) • Glycolysis complete: non-rinsed (12–24 h), rinsed (6 h) |
Farouk & Price [9] |
| Steer | LL, ST, QF |
• 4% higher dressing percentage • Rapid pH decline rate before 24 h PM |
Dikeman et al. [20] |
| Steer | LL, OSM, ISM, PM |
• Rapid pH decline rate before 4 h PM • Lighter cherry-red initial color scores in LL and OSM steaks |
Hunt et al. [25] |
| Lamb | LL |
• 50% reduction in toughness • CIE L* and CIE b* colored muscle |
Fowler et al. [1] |
| Lamb | LL, SM |
• Reduce cold shortening up to 5% in rinsed carcasses with electrical stimulation applied before the rinse • Lower pH values during the first 5 h PM |
Mickelson et al. [26] |
| Lean dairy cows | LL, SM |
• 2.7% higher dressing percentage • No differences in moisture or fat content between the non-rinsed and rinsed ground beef • Higher CIE a*, higher deoxymyoglobin (DMb), and lower metmyoglobin (MMb) in the rinsed sample on day 7 display □ Non-rinsed: CIE a*,13.1; DMb 1.12; MMb,1.11 □ Rinsed: CIE a*, 15.8; DMb, 1.29; MMb, 0.94 • Carcass aerobic plate counts: 57% less with RCT |
Moreira et al. [19] |
| Cows | LL | • 20% reduction in toughness | Hite et al. [12] |
| Bison | LL, TB |
• 24% reduction in toughness • More red (CIE a*) and greater DMb on day 1 and 4 than the non-rinsed vacuum packaged ground bison |
Mickelson & Claus [4] |
| Market hogs | LL, TB |
• Lower pH values during the first 4 h PM • Redder, lighter, greater DMb, and less MMb • Not affect the moisture content when assessed on moisture on a fat-free basis (MFF basis), water holding capacity (WHC), purge, and cook loss |
Kethavath et al. [3] |
| Lean dairy cows (LE), Light dairy cows (LI) |
• Lower pH values during the first 24 h PM • More red (CIE a*) □ Non-rinsed: LE 16.87; LI 15.86 □ Rinsed: LE 13.62; LI 14.07 • Longer sarcomere length than non-rinsed □ Non-rinsed: LE 1.42 µm; LI 1.40 µm □ Rinsed: LE, 1.80 µm; LI, 1.80 µm • 58% (LE) and 56% (LI) reduction in toughness, respectively • Lower lipid oxidation (thiobarbituric acid reactive substances) □ Non-rinsed: 1.23 mg MDA/kg meat □ Rinsed: 0.83 mg MDA/kg meat |
Kethavath et al. [2] |
Although exsanguination removes approximately 50% of total blood volume from the carcass which is equal to 3.0%–3.5% of the animals’ live body weight [5,6], the blood that remains in the carcass is the ideal medium for bacteria to grow in and spread. Since the residual blood enables pathogenic bacteria such as Escherichia coli and Salmonella to survive and grow by providing essential nutrients (e.g., nitrogenous compounds, moisture, minerals, and vitamins), effective blood removal helps inhibit the growth of these organisms on the carcass. Therefore, it is important to remove as much blood from the carcass as possible during slaughter.
Using a 454 kg cattle as an example, there is a total of 32 kg of blood in the animal, which is 7% of live weight. According to MPSC research, the estimated blood yield in a non-rinsed animal is equivalent to 18 kg fresh blood. Thus, 14 kg would be left in the animal without rinsing. MPSC also confirmed that rinsing an animal through RCT results in 5.6 kg additional blood removed in comparison to the non-rinsed animal, while 8 kg of blood remains. The average blood yields are; non-rinsed = 56% (18 kg); RCT-processed = 75% (24 kg). This means that RCT effectively removes about 40% more residual blood from the carcass. Erazo-Castrejón et al. [7] observed the same result that RCT removed 40% more blood from the pork muscle when compared to the conventionally chilled carcasses. It should be noted that blood removed by vascular rinsing becomes more diluted as a result of the rinse solution, so utilization of the blood is impacted.
Carcasses should be chilled to an internal temperature of 7°C in the deep round before cutting or dispatch. Although no time is specified in the regulations, with current technology these temperatures can be achieved in 16–24 hours in small carcasses and in less than 48 hours in large carcasses (center of the hind leg). Effective chilling is important to slow microbial proliferation. Extensive research trials have shown that in a conventional chilling system, beef sides, pig carcasses, and lamb carcasses require at least 24, 16, and 10 hours, respectively to cool to 7°C [8].
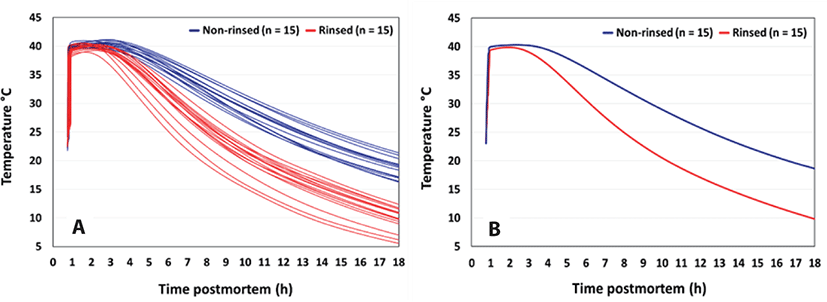
RCT facilitates lowering internal temperatures rapidly as much as 5°C since the vascular system serves as an effective conduit to allow the chilled rinse solution to reach all areas throughout the musculature of the carcass. Early work demonstrated that vascular infusion reduced the time required to achieve deep leg temperatures of 20°C from 2.6 to 1.3 hours, which is a significant time reduction [8]. Additionally, the infusion of the RCT solution lowered the temperature by ~2°C compared to non-rinsed carcasses during the early PM period (3 hours) but also increased the rate of pH decline [9]. Work done by MPSC at a client plant in 2019 demonstrated that RCT processed beef carcasses chilled significantly faster in the deep round muscle. RCT beef carcasses were 8.8°C colder at 18 hours of chill than non-rinsed carcasses (Fig. 2).
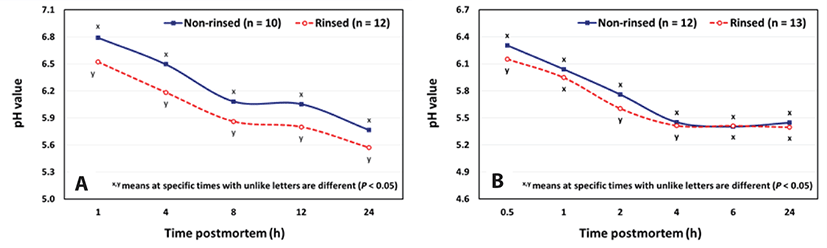
The pre-rigor infusion of the carcass manipulates the rate of glycolysis and thus the pH decline. In a lamb study by Farouk and Price [9], they reported glycolysis was completed in 6 hours compared to 12–24 hours for the non-rinsed carcass. More recent studies [2,3] showed carcasses infused with the MPSC solution exhibited more rapid pH declines during 24 hours PM on cull dairy cows and before 4 hours PM on market hogs (Figs. 3A and 3B). The faster pH decline could be explained by the ingredients in the isotonic solution and their effect of glycolytic enzymes. The phosphates likely serve to facilitate stimulating anaerobic metabolism and the saccharides (dextrose, maltose) serve as a source of glucose which under anaerobic conditions lead to the formation of lactic acid. Thus, these compounds likely regulate critical steps in the glycolytic pathway to control the rate and absolute decline in pH [9].
There is an interesting contrast associated with early PM carcass temperature and pH. Known for decades is that a rapid drop in pH while the carcass temperature is still warm can lead to undesirable effects on color (pigment denaturation) and reduction in water holding capacity. These quality problems are more associated with pork but have also been reported in beef [11]. On the other hand, if the carcass is chilled too quickly (< 15°C) while the pH is high (when the muscle still has available energy) cold-induced toughening can occur. Interestingly, although RCT infuses a cold solution, it appears the more rapid pH decline is capable of preventing cold-induced shortening [2]. The process likely induces using up a sufficient amount of energy before calcium is released that would trigger excessive sarcomere shortening. In addition, despite a more rapid pH decline, use of the chilled RCT solution and its effect on efficiently removing heat out of the carcass helps protect the meat pigments from being denaturated as mentioned previously RCT improves the red color stability.
Shear force trials conducted by Michigan State University [12] found RCT-processed carcasses were significantly more tender. While prime cattle showed improvement, cows showed much greater improvement in tenderness. The trials also showed the improvement in tenderness was realized sooner after processing. The RCT-processed carcasses were significantly more tender than the non-rinsed group at 14 days aging. At 28 days both groups had improved but the non-rinsed group was still less tender than the RCT-processed group at 14 days. Published studies on the carcass vascular rinsing have postulated that mechanisms involved in tenderization were likely associated with: (1) disruption of the muscle structure as a result of the internal fluid pressure when the carcass was vascularly rinsed with the RCT solution; (2) enhanced proteolytic activity by substrates in the solution; and (3) solubilization of actomyosin by the phosphates in the solution. This improvement in meat tenderness was without any negative effect on water holding capacity and protein extractability of the meat. Several recent studies have also reported improvement in tenderness as a result of RCT processing. Based on a reduction in mechanical shear, tenderness was improved by 50% in lamb chops [1], 20% in cow striploin steaks [13], 24% in bison steaks [4], 56% in steaks from light dairy cows [2], and 58% in steaks from lean dairy cows [2].
The technology positively affects meat color and stability. Numerous studies (Table 1 and Fig. 4) report increased red color in ground meat and intact steaks and increase levels of oxymyoglobin. In general, RCT results in lighter (higher CIE L*) and redder (higher CIE a* and deoxymyoglobin [DMb], lower metmyoglobin [MMb]) of the meat color in the triceps brachii (shoulder), longissimus lumborum (loin), and semimembranosus (ham) from a variety of animal species [1–4]. The lighter colored meat likely results from the additional blood removal from the carcass. The increased CIE b* might correspond to the higher lightness, resulting in the greater light scattering [9]. Although overall red color enhanced, in Table 1 cited lighter cherry-red initial color scores in steer [14] steaks (longissimus thoracis et lumborum, LL; outside semimembranosus, OSM) and lamb [1] and more yellow which it is unknown how consumers might perceive that change. Recent results from cull dairy cow studies [2] suggest RCT produces meat that has greater oxygen consumption ability which would be particularly beneficial in meat to be vacuum packaged. The reason for this is that eliminating oxygen in anaerobic package meat promotes DMb. Moreover, if a minute amount of oxygen is present that promotes MMb formation.
As mentioned before, RCT reduces the amount of hemoglobin and non-heme iron that can act as pro-oxidants and have a negative impact on flavor [7]. Previous works reported steaks (semitendinosus) from the non-rinsed cattle had higher cardboard flavor than those from the RCT-processed cattle. Also, cooked ground beef from the RCT-processed cattle had higher beef flavor identity and lower soapy/chemical flavor (Table 2) than those of the non-rinsed cattle [15].
Bacteria have the ability to double in their numbers every 20 minutes [16]. Flagellated bacteria can adhere readily to the carcass surface which will result in the difficulty of eliminating them later on. RCT helps prevent bacteria from attaching to the carcass surface by accelerating pH and temperature decline more rapidly while rinsing out additional blood from the vasculature. RCT-processed carcasses and by-products that are microbiologically cleaner and freer of blood have been observed. In addition, according to in vitro studies conducted by the University of Minnesota [17], certain pathogenic bacteria did not survive in the absence of metal cations found in the blood. This is especially true for coliform bacteria and Escherichia coli. They also reported the RCT solution has antibacterial properties against Escherichia coli, Salmonella typhimurium, and Pseudomonas fragi. The antimicrobial properties of the MPSC solution, when tested by itself, are quite effective in a short amount of time, killing all organisms present within 4–6 hours at low inoculation levels (Figs. 5A and 5B). At a high inoculation level, the antimicrobial properties are still present and significantly reduced the number of pathogens. Recently, the University of UW-Madison determined the ability of carcass vascularly rinsing to reduce Salmonella prevalence in various lymph nodes from intradermally infected goats [18]. The Salmonella infectivity model was successful by providing sufficient counts in the lymph nodes to assess the rinse solution effects. RCT was able to demonstrate a 1.3 log reduction in Salmonella in the medial iliac lymph node (RCT, 1.56 log CFU/g vs non-rised, 2.94 log CFU/g).
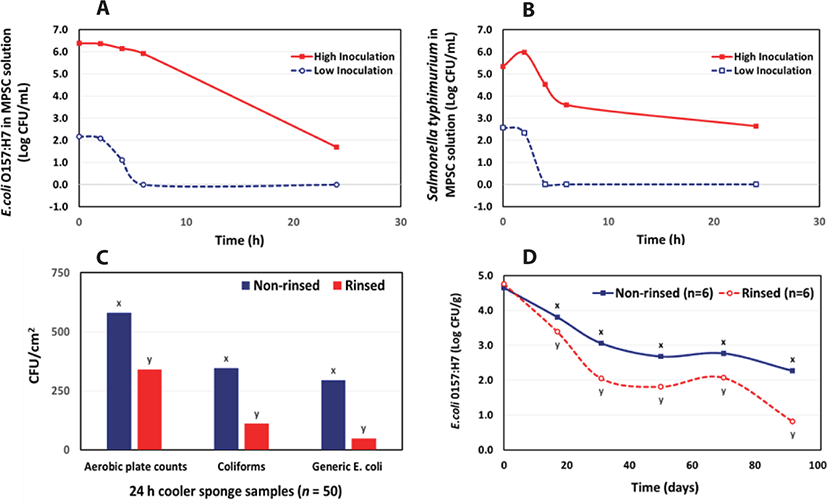
RCT effectively lowered aerobic plate counts, coliform bacteria, and Escherichia coli on beef carcasses after 24 hours in the cooler by 41%, 67%, and 83%, respectively (Fig. 5C) [17]. Additional research indicated that RCT also provided a continuous intervention in the reduction of Escherichia coli O157:H7 in vacuum packaged ground beef and the shelf-life of these products were considerably extended (Fig. 5D) [19]. In a recent study by Moreira et al. [20], they also confirmed a reduction (57%) in carcass aerobic plate counts.
Vascular infusion has improved dressing percentages to approximately 2%–4% as compared to non-rinsed carcasses [20–22]. The greater dressing percentage is associated with the hide being pulled off cleaner and the absence of excessively bloody areas in the neck region that normally required extensive trimming. With RCT, there is less incidental subcutaneous fat that gets pulled off during hide removal. Processors and boning room operators report beef carcasses from RCT are easier to debone, increasing yield by as much as 2% and improve worker safety and ergonomics. Interestingly, meat separates and peels off the bone cleanly.
VALIDATION TESTING OF POTENTIAL IN MEAT ASSOCIATED WITH THE RINSE SOLUTION
The RCT solution consists of approximately 98.5% water and a blend of dextrose, maltose, and sodium phosphates. The saccharides simply provide a source of glucose which is a normal substrate in the muscle used for metabolism. Similarly, various forms of phosphate are found in the muscle to facilitate metabolism. All of the ingredients in the RCT solution are approved by the U.S. Food & Drug Administration (FDA) and are internationally Generally Recognized as Safe (GRAS)-listed, common food-grade ingredients. They are classified as substrates and are completely metabolized, leaving no detectable residues in meat.
The University of Minnesota determined whether or not there are differences between non-rinsed cattle carcasses and the RCT-processed carcasses in terms of dextrose and phosphorus. The study was conducted on muscle tissue collected from 216 cattle: 108 controls and 108 rinsed cattle. At 24 hours PM, longissimus muscles were collected at commercial packing plants. Dextrose was analyzed with high performance liquid chromatography (HPLC). Phosphorus was determined using inductively coupled plasma (ICP); results were expressed as both phosphorus and phosphate.
The results demonstrated there was no measurable amount of dextrose in any of the samples from the non-rinsed carcasses or the RCT-processed carcasses. No differences between the non-rinsed and the rinsed carcasses were seen as residual dextrose levels were below the detection limit of the analytical procedure. In addition, measurable quantities of dextrose were not even found in the sample extracts that were concentrated five-fold, while dextrose content was detected in the positive controls. The inability to recover and detect any of the small amount of dextrose added by the RCT procedure is not surprising as glucose will rapidly be metabolized to lactic acid, CO2, and H2O in the early PM period. Mean values for phosphorus were as follows: non-rinsed = 2,113 μg/g; RCT-processed = 2,079 μg/g. The results of phosphorus expressed as phosphate (PO4) were; non-rinsed = 6,466 μg/g; RCT-processed = 6,362 μg/g. Although not statistically significant, the RCT-processed samples tended to contain less phosphorus and phosphate than the non-rinsed samples (possibly due to blood removal). In a very comprehensive study by Mateescu et al. [22], they reported standard deviations of 249 μg/g (Iowa steer beef, n = 309) and 278 μg/g (Iowa cow beef, n = 231).
A recent independent study was conducted by the UW-Madison to determine the effects of vascular rinsing and chilling temperatures on the quality attributes of meat from cull dairy cows [21]. Carcasses from lean grade, cull dairy cows were conventionally chilled (non-rinsed; n = 12) or RCT processed (n = 28). Immediately after exsanguination at a commercial plant, carcasses were vascularly rinsed with the chilled solution (RCT3, rinse solution, 3°C; n = 13; RCT14, rinse solution, 14°C; n = 15). Longissimus muscles were excised for residue testing. Total phosphorus and sodium were analyzed using ICP optical emission spectrometry (ICP-OES, AOAC 982.14 Modified). Glucose content was conducted using a glucose assay kit (GAHK20, Sigma Chemical, St. Louis, MO, USA) with the hexokinase method [24].
No differences in residuals (phosphorus, sodium, and glucose; p > 0.05) were found between the non-rinsed controls and either of the rinse temperature beef samples (Fig. 6). As documented in the early residue validation work in which meat from RCT carcasses that were vascularly rinsed with the cold solution (3°C), the concentration of residual phosphorus was not different (p > 0.05) than the non-rinsed samples (Fig. 6A). In addition, the beef from carcasses rinsed at the higher rinse solution temperature (14°C) was also not different than the non-rinsed control. In a very comprehensive study by Mateescu et al. [23], they analyzed the phosphorus content (wet basis) naturally found in the loin from conventionally chilled beef carcasses, wherein the phosphorus contents were 1,850 μg/g in bull, 1,945 μg/g in cow, and 2,056 μg/g in steer, respectively. Furthermore, the concentration of residual sodium was not different (p > 0.05) than the non-rinsed control samples and were very similar to those reported by Mateescu et al. [23] in meat from the Angus (bull 439 μg/g, cow 530 μg/g, steer 517 μg/g). The residual glucose content in the loin from the cull dairy cows was 4.91 µmol/g in the non-rinsed control, 4.56 µmol/g for RCT3, and 4.58 µmol/g for RCT14. The residual glucose content of the samples from the two RCT carcass treatments was not different (p > 0.05, Fig. 6C) than the non-rinsed samples. The level of residual glucose determined in these samples was similar to that reported by Antonelo et al. [24] in the loin muscle which contained 4.11 μg/g at day 7 PM (n = 15, conventionally chilled carcasses).
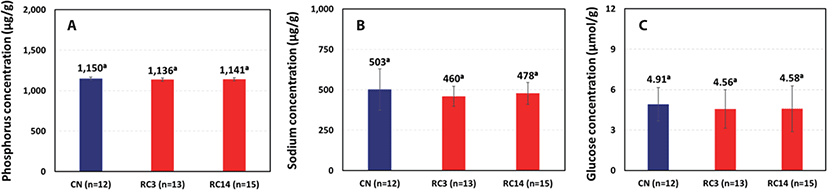
At the time of vascular rinsing and early PM, pre-rigor muscle is physiologically active and therefore capable of metabolizing endogenous as well as added sources of glucose (maltose and dextrose). In addition, endogenous phosphate is also involved in normal muscle metabolism. As such, the minor amount of these substances used to rinse out the blood from the vasculature, even if none of them drained from the carcass, would be readily metabolized by the muscle. Based on the inherent amount in sodium naturally found in beef and the rinse solution is allowed to drain, the diminutive contribution associated with the phosphates does not result in a difference in the sodium content of the beef. Therefore, the results based on the early validation testing combined with this validation work confirmed that after PM storage, no differences in glucose and phosphorus residuals between the non-rinsed beef and the RCT beef exist.
RETAINED MOISTURE DECLARATION (LABELLING) FOR MEAT PRODUCTS
Many advanced nations require moisture declaration labeling for raw meat products, however, retained water below 0.5% does not need to be declared in the United States. Data from the University of Minnesota showed the average moisture fat free (MFF) percent of non-rinsed samples was 72.30% whereas the percent in RCT-processed carcasses was 72.64%. The difference of +0.34% was not scientifically significant. It is expected that some muscles will naturally vary in MFF. In the United States, the longissimus muscle is extensively used as a representative muscle to predict the overall eating quality of the meat from the carcass. As such RCT utilizes this muscle in order to monitor compliance with MFF compliance. Since there is no significant moisture gain, RCT is compliant with retained moisture regulations in the United States. Recent works have also reported RCT processing in market hogs [3] did not affect MFF in pork longissimus muscle and in the ground pork shoulder muscles compared to the non-rinsed carcasses. Based on rigorous continuous in-plant sampling of beef carcasses, meat from RCT does not require any labeling claim associated with moisture. RCT meets government requirements in countries including the United States, Canada, Japan, New Zealand, and Australia, which have approved the installation and commercialization of this technology, as well as the import of RCT-processed meat. RCT results in some variety meats (e.g., liver, heart, kidneys) that retain the rinse solution, and such that special labeling declarations are required in some countries.
CONCLUSION
After decades of extensive research and technological advances, the RCT is now providing the global meat industry with an unpresented opportunity to use this process, cost effectively, to remove more blood from carcasses (beef, lamb and bison), optimize pH decline, and facilitate chilling early PM in a manner that enhances meat quality (color and tenderness) and has a positive impact on product shelf life and food safety. The rinse solution uses widely recognized and approved food grade ingredients that represent substrates the muscle metabolizes for normal energy production, thus resulting in no detectable differences between conventionally chilled carcasses and carcasses that are vascularly rinsed using the RCT process. Since the carcasses are vascularly rinsed at no more than 10% of the carcass weight with a cold isotonic solution and the solution is allowed to freely drain, no labelling is required (other than liver, heart, and kidneys) in any country that Rinse & Chill® is currently being used in (US, Canada, and Australia) based on a difference of than 0.5% in moisture fat-free compared to non-rinsed carcasses. The process has boosted the meat industry’s profitability and productivity by improving dressing percentage and boning yields. With a continuously increasing demand for this technology, industry implementation has expanded over the past several years. Such growth will continue particularly with engineering achievements that enable accommodating larger capacity harvest plants.
