INTRODUCTION
Equine influenza virus (EIV) and equine herpesvirus (EHV) are the major causes of contagious respiratory diseases in horses. EIV belongs to the family Orthomyxoviridae family, and the Influenza A genus. EIV-induced respiratory diseases in horses are characterized by anorexia, cough, nasal discharge, pyrexia, secondary bacterial pneumonia, and infections [1,2]. Two distinct subtypes of EIV, H7N7, and H3N8 have been reported in horses; however, the H7N7 subtype has not been isolated since the late 1970s [3,4]. In naïve and unvaccinated horses, clinical signs and symptoms start to appear 48 h after EIV infections [5]. EHV is an alpha-herpesvirus of the Herpesviridae family that induces symptoms of upper respiratory diseases similar to those induced by EI; however, it also causes abortion in mares and neurological disorders, such as EHV myeloencephalopathy, in foals [6]. These viruses spread quickly, especially in naïve populations, leading to the implementation of movement restrictions for horses and disruption of equestrian events [7]. Additionally, because of their high morbidity, infections with these viruses require strict prevention protocols including disease surveillance, quarantine of affected horses, and regular vaccination programs, which ultimately cause enormous financial losses in the equine industry [5].
Various EIV and EHV vaccines are available worldwide, including inactivated, subunit, recombinant virus-vectored, and modified live vaccines. In Korea, the recombinant canarypox vectored EIV (ProteqFlu®, Boehringer Ingelheim, Ingelheim am Rhein, Germany) and inactivated EHV vaccines (Pneumabort-K®+1b, Zoetis, Parsippany, NJ, USA) are officially used [8]. The ProteqFlu® vaccine contains two recombinant canarypox viruses expressing the hemagglutinin (HA) gene from the equine influenza virus strains A/eq/Ohio/03 (H3N8) and A/eq/Richmond/1/07 (H3N8), adjuvanted with carbomer 974P [9]. According to the manufacturer’s protocol, 6-month-old foals received two initial primary vaccinations (V1 and V2) at intervals of 4-6 weeks followed by a second dose (V3) 5 months later. Boosters shots (V4) are administered to horses every 6 months [10]. The Pneumabort-K®+1b vaccine is administered to horses for protection against EHV in a manner similar to the EIV vaccine. The horses receive the first vaccination (V1) after weaning, followed by a V2 3-4 weeks later, and V3 6 months after the second dose, and are given booster vaccination annually [11]. Each vaccine is administered individually because there is no information regarding the immune responses induced by concurrent vaccination with the ProteqFlu EIV and Pneumabort-K®+1b EHV vaccines in horses.
However, there are several reports of concurrent vaccinations against EIV and EHV to simplify horse management and minimize veterinary expenses in countries where multivalent vaccines are not available [12,13]. The concurrent vaccination means that administering different vaccines on the same day, but not combined in the same syringe. It not only decreases veterinary expenses but also reduces the stressful environment for foals by limiting handling and restraint [7]. Nevertheless, according to previous studies, the efficacy of concurrent administration of EIV and EHV vaccines in horses remains controversial. Ohta et al. [13] compared the EIV-specific antibody responses between concurrent and consecutive administrations of an inactivated EIV vaccine and a live EHV-1 vaccine in thoroughbred horses. They showed that concurrent EIV and EHV vaccination induced lower immune responses against EIV compared to consecutive vaccinations. In contrast, Gildea et al. [12] evaluated the induction of EIV-specific antibody response following concurrent and consecutive vaccinations of inactivated EIV and bivalent EHV-1/4 EHV vaccines in thoroughbred horses and reported that concurrent EIV and EHV-1/4 vaccination increased humoral immune responses against EIV. However, there have been no reports of concurrent administration of recombinant canarypox EIV and inactivated EHV vaccines in horses.
Therefore, in this study, we aimed to compare the EIV-specific immune responses induced by concurrent administrations of a recombinant canarypox EIV vaccine and an inactivated bivalent EHV vaccine with those induced by a single recombinant canarypox EIV vaccine in experimental horse and mouse models.
MATERIALS AND METHODS
Twelve mixed-breed horses were used in this study and were randomly divided into two groups. The range of age was between 2 and 23 years old. The horses were vaccinated against EIV every six months after the two initial doses of primary vaccination and had no EHV vaccination history. All horse experiments were performed according to the guidelines of the approved Institutional Animal Care and Use Committee (IACUC) protocol (protocol number 2021-0035) from Jeju National University (JNU).
Fifteen female BALB/c mice (Samtako BioKorea, Osan, Korea) were used in this study and were divided into three groups. The mice were 7-weeks old at the time of prime immunization and maintained at the Jeju National University Animal Facility. All mouse experiments were performed in accordance with the guidelines of the approved IACUC protocol (protocol number 2021-0051). Mice were acclimatized and randomly divided into three groups before the start of the study, as per the experimental design.
The recombinant canarypox EIV vaccine ProteqFlu (Boehringer Ingelheim) and inactivated bivalent EHV vaccine Pneumabort-K+1B (Zoetis) were used in this study. The EIV vaccine contains two recombinant canarypox viruses expressing the HA genes of equine influenza virus strains A/eq/Ohio/03 (H3N8) (American strain, Florida clade 1) and A/eq/Richmond/1/07 (H3N8) (American strain, Florida clade 2). The EHV vaccine contains EHV-1 strains 1p and 1b.
Five horses were immunized intramuscularly with the EIV vaccine (1 mg), whereas seven horses were concurrently immunized with EIV and EHV vaccines (1 mg each) through the same administration route. Blood samples were collected on the day of immunization (day 0) and days 7, 14, 28, and 140 post-immunization (Fig. 1A). During immunization, one horse in each group experienced adverse effects, including swelling at the injection site, but recovered within a few days. The sera were isolated from the blood samples by centrifugation and stored at −20°C until further analysis.
Each group (n=5) of the mouse model was immunized intramuscularly with phosphate-buffered saline (PBS) (100 μL), EIV (10 μg), or EIV+EHV vaccine combinations (10 μg each) three times (prime, first and second boost) with 2 weeks intervals (Fig. 1B). Blood samples were collected 1 week after each booster immunization, and sera were separated by centrifugation. At 4 weeks after the last immunization, naïve and immunized mice were challenged intranasally with an A/eq/Miami/1/63 virus (H3N8) and sacrificed 5 days later to evaluate the EIV-specific immune responses [14].
Heparinized blood samples (50 mL) were collected from each horse via jugular venipuncture. PBMCs were isolated by density centrifugation (400×g, 30 min, 4°C) using Ficoll Histopaque-1077 (Sigma-Aldrich, St. Louis, MO, USA) and washed in sterile PBS before cell counting. PBMCs (5 × 106 cells/well) from each horse were seeded into a 6-well plate and cultured in RPMI-1640 medium (Sigma-Aldrich) supplemented with 10% complement-inactivated fetal bovine serum (FBS) and 1× antibiotic-antimycotic (Gibco BRL, Thermo Fisher Scientific, Waltham, MA, USA). PBMCs were stimulated with or without 10 μg of the EIV vaccine and incubated for 18 h at 37°C and 5% CO2. After incubation, the cells were harvested using a cell scraper and then transferred to a 1.5 mL tube for RNA extraction.
Total RNA was extracted from cultured PBMCs using an RNA extraction kit (iNtRON Biotechnology, Seongnam, Korea). The concentration and purity of each RNA sample were determined using a DS-11 spectrophotometer (DeNovix, Wilmington, DE, USA). For complementary DNA (cDNA) preparation, 1 μg of the RNA sample was used to synthesize cDNA using a cDNA synthesis kit (iNtRON Biotechnology). The concentration and quality of the synthesized cDNA were also measured by spectrophotometry as described above and diluted to an appropriate concentration for subsequent PCR analyses. Primer information for interferon-gamma (IFN-γ) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) has been published previously [15]. All samples were measured in triplicate using qPCR master mix reagents (iNtRON Biotechnology) and a Thermal Cycler Dice Real-Time System II (Takara Bio, Kusatsu, Shiga, Japan). The thermal profile consisted of an initial hold at 75°C for 5 min, followed by a single denaturation step at 95°C for 10 min, and then 40 cycles at 95°C for 15 s and 60°C for 60 s. Data analysis was performed by normalizing the IFN-γ amplification Ct values to the corresponding endogenous control (GAPDH, reference Ct values).
To measure the EIV-specific IgG levels in the serum, serially diluted sera were added to A/eq/Miami/1/63 (H3N8)-coated ELISA plates (400 ng/well) after blocking. Horseradish peroxidase (HRP)-labeled anti-horse IgG and HRP-labeled anti-mouse IgG secondary antibodies were used to detect EIV-specific IgG in equine and murine sera, respectively. After the addition of 3,3’,5,5’-Tetramethylbenzidine substrate solution, the reaction was then stopped by a 0.16 M sulfuric acid stop solution. Optical density was measured at 450 nm using a plate reader.
Ten microliters of equine and murine sera and 30 μl of the receptor-destroying enzyme (Denka Seiken, Chuo, Tokyo, Japan) were mixed and incubated at 37°C for 18 h and then inactivated by heating at 56°C for 30 min. The sera were then serially diluted (final volume of 25 μL) and incubated with eight hemagglutination units of A/eq/Miami/1/63 (H3N8) virus (final volume of 25 μL) in U-bottom plates for 30 min. Fifty microliters of 0.5% chicken red blood cells were added to the plates, and HAI titers were determined after 40 min [14].
RESULTS
To evaluate the effects of concurrent EIV and EHV vaccination on EIV-specific serum IgG levels in vivo, we immunized mixed-breed horses and BALB/c mice with EIV vaccine alone or in combination with EHV, intramuscularly. After immunization, sera were collected and the EIV-specific serum IgG antibodies were quantified by ELISA.
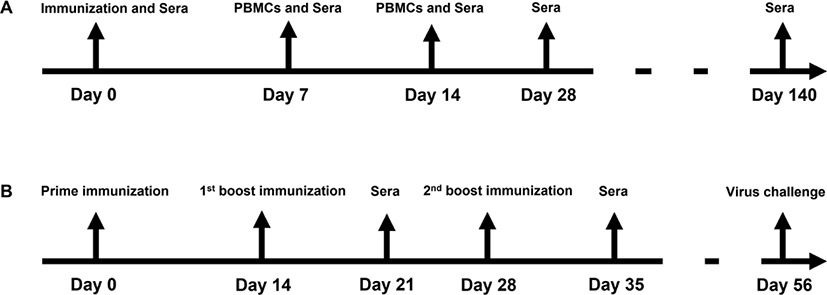
In horses, both the EIV and the EIV+EHV vaccinated groups showed similar mean serum IgG levels on the day of vaccination and for 14 days after (Figs. 2A, 2B and 2C). However, at days 28 to 140 post-vaccination, the concurrently vaccinated group exhibited higher mean serum IgG levels than the EIV only group (Figs. 2D and 2E), despite no significant differences between the groups (p < 0.05).
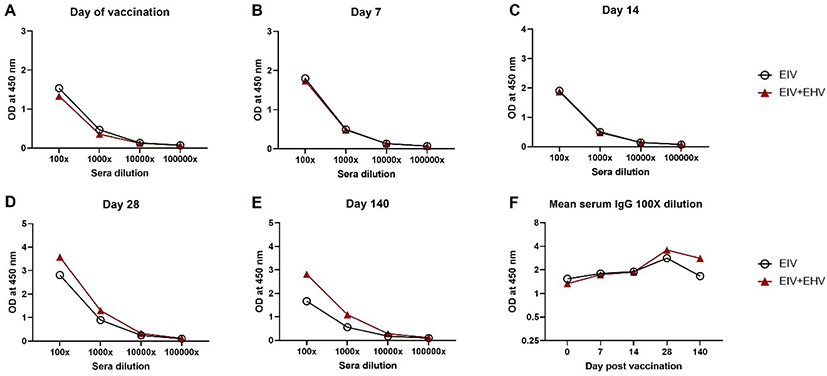
In mice, after prime immunization, both the EIV only and the EIV+EHV concurrently vaccinated groups showed similar serum IgG patterns at day 21 post-vaccination (Fig. 3A). However, the EIV+EHV group had higher serum IgG levels at day 35 post-vaccination and after the virus challenge (Figs. 3B and 3C). No significant differences were observed between the groups (p < 0.05). These results suggest that concurrent EIV and EHV vaccination does not influence the EIV-specific serum IgG levels.
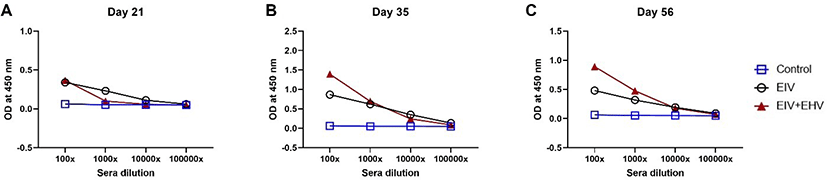
In horses, compared with the EIV vaccinated group, the concurrently vaccinated group showed higher mean HI titers when measured on the day of vaccination and at day 7 post-vaccination. Both the EIV and concurrently vaccinated groups showed similar levels of HI titers at day 14 post-vaccination, which were the highest values observed during the study. Surprisingly, the concurrently vaccinated group showed sustained levels of HI titers at day 28 post-vaccination, whereas the EIV only group showed decreased HI titers. At day 140 post-vaccination, both groups showed the lowest levels of HI titers observed during the experiment. There were no significant differences in the HI titers between the two groups during the experiment (p < 0.05; Fig. 4).
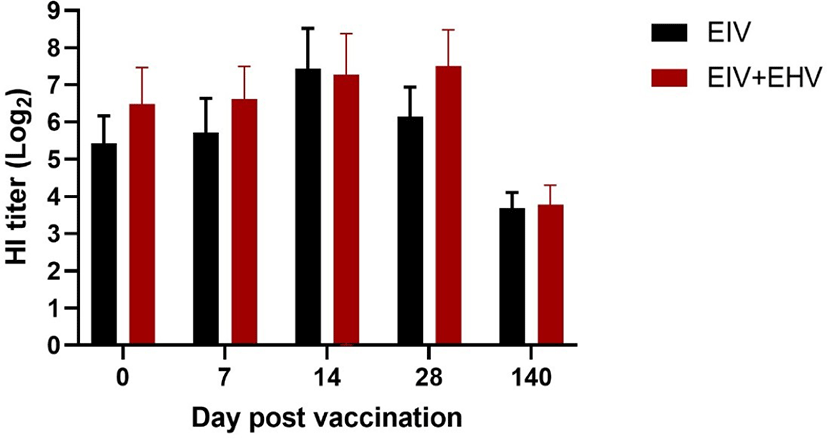
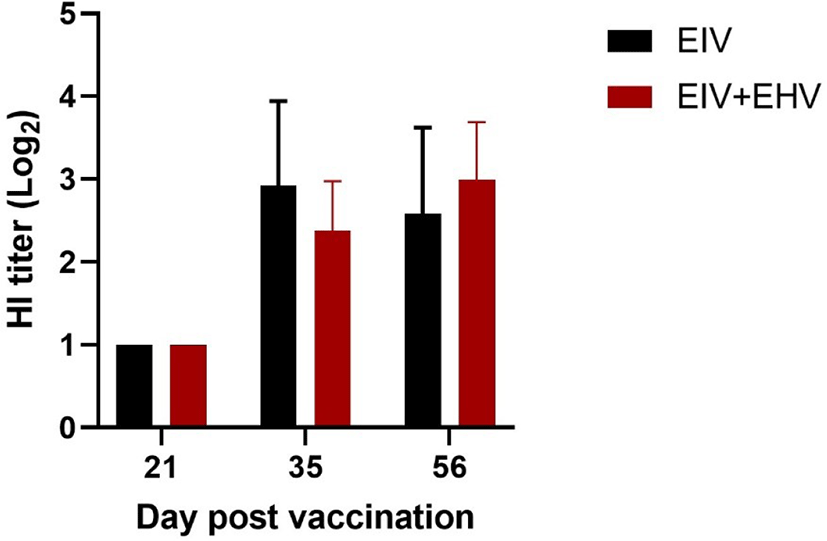
In mice, both groups showed a similar mean HI titer against EIV measured at day 21 post-vaccination. At day 35 post-vaccination, although both groups showed increased HI titers, the EIV vaccinated group showed higher HI titers than the concurrently vaccinated group. However, despite was no significant differences in HI titers between the groups at days 35 and 56 post-vaccination (p < 0.05), the concurrently vaccinated group showed higher HI titers than the EIV vaccinated group after the virus challenge at day 56 (Fig. 5). These results indicate that concurrent EIV and EHV and single EIV vaccinations induce similar levels of HI titers against EIV.
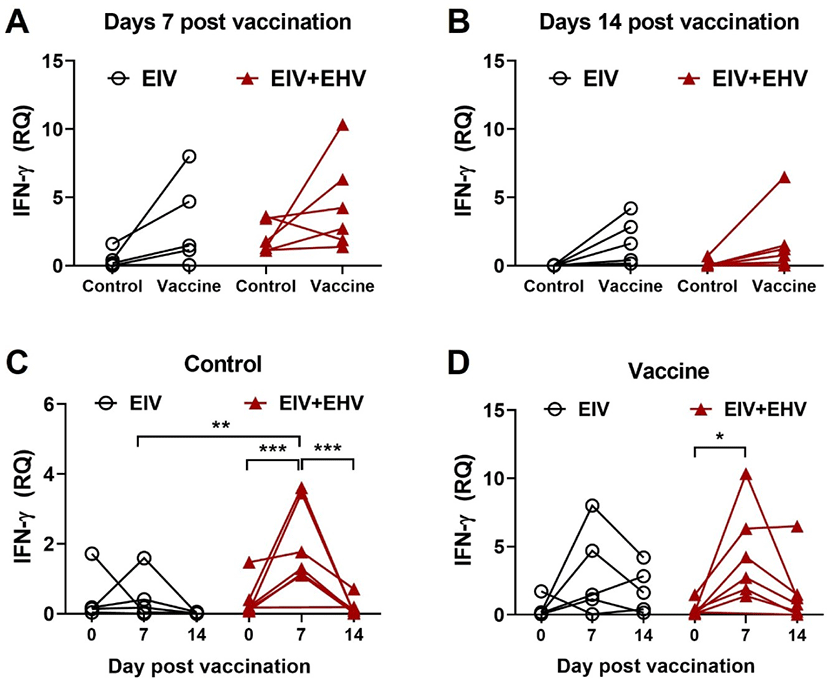
To investigate the cellular immune responses induced by concurrent vaccination ex vivo, equine PBMCs were isolated from the blood and cultured alone or with the EIV vaccine (10 μg). After 18 h of culture, the cells were harvested to determine IFN-γ expression levels by qPCR. Both single and concurrent vaccinations resulted in elevated levels of IFN-γ at day 7 post-vaccination (Fig. 6A). However, IFN-γ levels declined at day 14 post-vaccination in both groups (Fig. 6B). Surprisingly, only concurrent vaccination resulted in a significantly increased IFN-γ production at day 7 post-vaccination compared with day 0 in the PBMCs stimulated with or without the EIV vaccine (p < 0.05; Figs. 6C and 6D). These data demonstrate that concurrent vaccination against EIV and EHV is a good approach for enhancing EIV-specific cellular responses.
DISCUSSION
In this study, we compared the EIV-specific humoral and cell-mediated immune responses between a group that was concurrently vaccinated with recombinant canarypox EIV and inactivated bivalent EHV, and a group that was vaccinated with EIV, in horses and mice, to investigate the feasibility of the concurrent immunization protocol for EIV and EHV vaccines.
The mean serum IgG level induced by EIV vaccinations in our horses was similar to that of concurrent EIV+EHV vaccination at days 7 and 14 post-vaccination. This result contradicts that of Gildea et al. [12], in which horses immunized with EIV and EHV on the same day had significantly higher antibody levels 2 weeks post-vaccination than those immunized with EIV alone. In addition, our results also contradicted those of Allkofer et al. [7], where the mean antibody titer of separately vaccinated horses was significantly higher than that of concurrently vaccinated horses at 2 weeks post-vaccination. These differences are probably attributable to the types of vaccines and adjuvants, influenza vaccine strains, and variations in horse species in the experiments. In contrast, our results showed that the serum IgG level was higher in the EIV+EHV vaccinated group than in the EIV vaccinated group when measured at days 28 and 140 post-vaccination, although there were no significant differences (p < 0.05), which indicates that the concurrent EIV+EHV vaccination does not negatively impact the humoral response against EIV. However, further investigations on a larger population are required to validate our results.
In mice, the pattern of serum IgG levels in both groups was similar to that obtained in the horse groups in the present study. Additionally, the serum IgG levels were similar between both groups at day 21 post-vaccination. Concurrently vaccinated mice had higher serum IgG levels than EIV vaccinated mice at days 35 and 56 post-vaccination; however, there was no significant difference between the two groups (p < 0.05). Our results are consistent with those of previous studies in which BALB/c mice have been used as an experimental animal model to study immune responses against the H3N8 EIV, showing that the first and second boost immunizations significantly increased EIV-specific serum IgG levels [16,17]. However, while Pavulraj et al. [17] showed that the IgG antibody response continued to increase even after the H3N8 virus challenge [17], Kumar et al. [16] showed that the IgG antibody response at day 5 post EIV challenge was lower than that measured after the second booster vaccination [16], which is consistent with our results. These different results might be due to the differences in the intervals between each booster immunization and virus challenges. Further studies are required to evaluate the humoral responses.
Cell-mediated immunity (CMI) plays an important role in the protection against many viral diseases. It contributes to the clearance of the virus and the establishment of long-term immunity after viral infections. IFN-γ is a key cytokine in CMI and induces antiviral responses by promoting viral peptide presentation by antigen-presenting cells, lymphocyte recruitment, and development of T helper cells [18,19]. Virus-specific IFN-γ production in equine PBMCs has been used as a marker to evaluate cell-mediated immune responses in horses [9,18,20]. Following vaccinations with recombinant canarypox EIV, ISCOM-based EIV, and inactivated EIV vaccines a significant increase in IFN-γ gene expression has been reported in unvaccinated naïve horses [21]. This increase peaked at day 7 post-vaccination in all horses regardless of the type of vaccine, and there was no significant difference among horses vaccinated with either vaccine [21]. In addition, in our study, concurrent vaccination with EIV and EHV resulted in a significant increase in IFN-γ gene expression at day 7 post-vaccination (p < 0.05), whereas single EIV vaccination did not, even at day 14 post-vaccination, in horses aged between 2–23 years. This result is consistent with that of a previous study showing that recombinant canarypox EIV vaccination was effective in promoting IFN-γ gene expression in naïve horses but had a limited effect on old horses with a previous history of EIV vaccination [22]. Therefore, we speculate that concurrent EIV and EHV vaccines may induce EIV-specific IFN-γ responses in old horses.
Additionally, in our study, the levels of HI titers in both the EIV only and the EIV+EHV vaccinated groups peaked at day 28 following vaccination and declined until the next booster vaccination, and there were no significant differences in the HI titers between the groups at any measured time point (p < 0.05). Our results are consistent with those observed in a previous study in which HI titers increased after booster vaccination, peaked after 1 month, and then declined until the next booster vaccination in horses immunized with the recombinant canarypox EIV vaccine [23]. Gildea et al. [12] suggested that the concurrent administration of two carbopol-adjuvanted EIV+EHV vaccines might increase the humoral response against EIV. However, we did not observe a synergistic effect on HI titers following the administration of carbomer adjuvanted EIV and oil adjuvanted EHV vaccines.
In the present study, serum HI titer increased progressively after the first and second booster immunization (on days 21 and 35 post-prime immunization) but was not affected by the EIV challenge in either mouse group. In contrast, in previous investigations, a distinct increase in HI titer was observed following challenges with EIV in mice vaccinated with EIV as well as a more than a 5–6 fold increase in antibody titers at day 5 post-challenge [16,17]. We speculate that in our study, HI titers failed to increase due to an insufficient viral concentration during the challenge or differences in the viral strains [A/eq/Ohio/03 (H3N8), A/eq/Richmond/1/07 (H3N8)] included in the vaccine, and [A/eq/Miami/1/63 (H3N8)] used in the challenge.
Our study had several limitations, such as a limited population (12 horses and 10 mice). Moreover, an additional analysis should be performed using weanlings seronegative for EIV or horses that have not been previously vaccinated against EIV. Therefore, further studies using a larger population and viral strains that are included in the EIV vaccine in our study should be performed to obtain more reliable data. Despite these limitations, our study provides valuable information on humoral and cellular immune responses induced by concurrent EIV and EHV vaccination which can be applied to develop a combined EIV and EHV vaccination protocol.
