INTRODUCTION
5-Hydroxytryptamine (5-HT), a monoamine, as a local regulator in the mammary gland is a chemical signal produced by the mammary epithelium cell that inhibits prolactin (PRL)-induced β-casein gene expression in mammary epithelial cells during lactation[1,2].5-HT could regulate the tight junctions of mammary epithelial cell, stimulate mammary gland release of parathyroid hormone related protein that affects calcium regulation through activation of the Hedgehog signaling pathway [3] and reduce blood flow to the mammary glands by constricting blood vessels [4]. In vitro research on bovine mammary epithelial cells showed 5-HT reduced milk synthesis, while blocking 5-HT receptors (5-HTR) increased milk synthesis [5]. In vivo studies of dairy cows have shown that 5-HT can regulate milk calcium levels and improve energy metabolism in cows [6,7]. In addition, 5-HT plays an important role in lactation regulation during lactation and mammary degeneration during the dry period in dairy cows.
5-HT is well known for its function in the mammary gland to increase apoptosis and reduce milk protein gene expression [8]. During the weaning period, the lack of sucking can lead to a large amount of milk being retained in the mammary glands [9]. In the early stages of milk stasis, lactation of the mammary epithelium is inhibited and mammary epithelial cells begin to shed and enter apoptosis, a process that can be reversed by re-sucking. Prolonged lack of suckling can lead to irreversible degeneration through the breakdown of the mammary epithelial structure and massive remodeling of the mammary gland tissue [10]. 5-HT plays a role in promoting apoptosis in mammary epithelial cells during the initial and reversible stages of mammary gland degeneration. Researchers have demonstrated that 5-HT inhibits milk protein synthesis and causes epithelial cell apoptosis in the mammary gland of lactating and dry cows [11]. A study in mice showed that 5-HT promotes apoptosis in mammary epithelial cells and disrupts the tight junctions between cells in a reversible manner, resulting in the shedding of mammary epithelial cells [12]. Data from these studies suggest that 5-HT can promote the apoptosis of mammary epithelial cells and play an important role in the process of mammary gland degeneration.
However, studies in other tissues have shown that 5-HT can effectively promote cell division, such as nerves [13] and blood vessels [14]. Increased cell proliferation was found in the liver of cows following injection of 5-hydroxytryptophan, a precursor of 5-HT synthesis [15]. In mice, 5-HT is necessary for the maintenance of normal mammary gland morphology, viability and the proliferation of mammary epithelial cells [16]. It was found that after the inhibition of 5-HTR in breast tumor cell lines, the viability of tumor cells was decreased [17]. 5-HT can also act as an antioxidant to reduce oxidative stress and improve the survival of β cells in lactating mice [18]. The prolongation of cellular lifespan by 5-HT as an antioxidant is via a non-receptor-mediate, as demonstrated by the studies of Azouzi S et al. on erythrocytes lacking the 5-HTR [19]. These studies suggested that 5-HT could promote cell proliferation and viability, which contradicts the promotion of apoptosis by 5-HT in the mammary gland. A recent study has shown that precursor of 5-HT inhibits apoptosis in goat mammary epithelial cells [20]. The effects of 5-HT on cell viability and apoptosis appear to remain to be investigated. However, previously published studies are limited to the effects of 5-HT on the apoptosis of bovine mammary not on cell viability.
The complex biological role of 5-HT is achieved through specific 5-HTR, which are divided into 7 classes or 14 subtypes [2]. Among them, 5-HTR subtypes 1B, 2A, 2B, 4, and 7 are expressed in the mammary gland of dairy cows [21]. Tryptophan hydroxylase (TPH) is the rate-limiting enzyme in 5-HT synthesis, which the main function of TPH is to convert L-tryptophan to 5-hydroxy-L-tryptophan [11]. The second enzyme involved in 5-HT synthesis is aromatic amino acid decarbonase, which converts 5-hydroxy-L-tryptophan into 5-HT [5]. Previous studies revealed that PRL could directly increase gene expression of TPH in mouse and cow mammary epithelial cells [5,11]. In lactating mice, a study showed that PRL induces 5-HT production in β cells via prolactin receptor-signal transducer and activator of transcription 5-tryptophan hydroxylase (PRLR-STAT5-TPH1) axis [18].Thus, PRL may affect the process of 5-HT synthesis and utilization.
Mammalian target of rapamycin (mTOR) is an evolutionarily conserved serine/threonine kinase that regulates cell proliferation, cell viability and growth rates in response to a variety of stimuli including hormones (e.g. insulin, 5-HT), growth factors (e.g. insulin-like growth factors), nutrients (amino acids), energy status and oxygen levels [22–24]. As an important signaling pathway during mammary gland development, lactation, mTOR signaling pathway has received considerable attention in the mammary gland [25]. mTOR can form 2 different complexes, mTOR complex 1 (mTORC1) and 2 (mTORC2), where mTORC1 is associated with intra- and extracellular signaling and cell viability [22]. The two principal effectors of MTORC1 signaling on protein synthesis are the eukaryotic translation initiation factor 4E-binding proteins (4EBP) and the ribosomal protein S6 kinase (S6K) [26]. In addition, the mTOR pathway also affects downstream eukaryotic elongation factors (EEF), which control the translation of mRNA [27]. However, the relationship between 5-HT and mTOR has been less studied in the dairy mammary gland, and it would be interesting to investigate whether 5-HT can promote the viability or apoptosis of mammary epithelial cells and the relationship with the mTOR pathway. In the mammary gland, janus kinase 2 (JAK2) and signal transducer and activator of transcription 5 (STAT5) are major molecules that mediate PRL induction of target gene expression [28]. Previous study found that PRL could increase cell viability and the secretion of milk protein through the Jak2/STAT5 signaling pathway [29,30]. Jak2/STAT5-induced TPH expression which the rate-limiting enzyme for the synthesis of serotonin is mediated by PRL [31]. Besides, 5-HT is an essential hormone that involved in the neuroendocrine regulation of PRL [32]. However, the effect of 5-HT combined with PRL on mammary cells has rarely been investigated.
Therefore, the aim of our study was to investigate the effect of 5-HT on mammary epithelial cell viability of dairy cows and its mechanism, and to investigate whether PRL and 5-HT have a reciprocal relationship. It is hoped that this research will contribute to an understanding of the effect of 5-HT on the viability of bovine mammary epithelial cells.
MATERIALS AND METHODS
Mammary gland tissue of Holstein cows during late lactation was collected from the slaughterhouse. After cows were slaughtered, mammary tissue samples were cut into small pieces (1 cm2) and immediately frozen in liquid nitrogen and stored at −80°C for future analysis.
Bovine mammary alveolar cell-T (MAC-T) cell line were a gift from Dr. Prof. Mark Daniel Hanigan (Department of Dairy Science, Virginia Tech University). MAC-T cells were cultured in Dulbecco’s Modified Eagle Medium: Nutrient Mixture F-12 (DMEM/F-12) High Glucose media supplemented with 10% fetal calf serum (Gibco, Waltham, MA, USA). Cells were grown in 25 cm2 culture flasks (Corning, NY, USA). When the cell density reaches 85%–90%, the cells were transferred to a 96-well plate (Corning, NY, USA) for culture with 103 cells/well and 105 cells/well for a 6-well plate (Corning, NY, USA). Six compound holes were set for each treatment. MAC-T cells were left overnight to adhere in full medium and then starved in serum-free medium for 24h prior to the supplementation of 5-HT and PRL to synchronize the cells. Cell number was measured by the trypan blue assay in a hemocytometer. The dose of 5-HT (Tocris, Bristol, UK) supplement in culture medium was 0 μM, 2 μM (0.42 μg/mL), 20 μM (4.2 μg/mL), or 200 μM (42 μg/mL) based on a previous study [5]. MAC-T cells with different 5-HT concentrations were all cultured for 12, 24, 48 or 72 hours. To detect the combined effect of 5-HT and PRL (Sigma, St. Louis, MO, USA) on MAC-T cells, cells were treated with 5-HT (2, 20 or 200 μM) and PRL (25, 50 or 100 ng/mL) in DMEM containing 10% fetal bovine serum (FBS) for 24 h. The amount of PRL addition is based on a previous study and was adjusted to explore the optimum concentration [5].
All 5-HTR antagonist were purchased from Tocris and administered in the recommended dosage according to the instructions. MAC-T cells were treated with SB204741 (0.1 µM), ritanserin (1.0 µM), SB204070 (0.0001 µM), Pimozide (0.1 µM), or SB224289 (1.0 µM), which are competitive antagonists of 5-HTR 2B, 2A, 4, 7 and 1B, respectively, along with 20 µM 5-HT 24 hours to observe the effect on cell viability. The dose of each receptor antagonist was added according to the instructions. Cells were pretreated with the respective receptor antagonist for 1 h before 5-HT application.
Cell viability was determined using cell counting kit-8 (CCK-8) (A311-01, Vazyme, Nanjing, China). 100 μL of cell suspension was added to each well of the 96-well plate and 10μl of CCK8 was added after 12–72 h of incubation. After incubation at 37°C; for 2 hours, optical density was measured at 450 nm using a plate reader (168-1130, Bio-Rad, Hercules, CA, USA).
Total RNA extraction from bovine mammary tissue and cells was performed with a Total RNA Extraction Kit (DP419, TIANGEN, Beijing, China) according to the manufacturer’s protocol. In short, 50–100 mg of bovine mammary tissue was added to each milliliter of lysate and homogenized using a homogenizer (Tissuelyser-24, Jingxin, Henan, China). For cells, the lysate was added to the culture plate (10 cm2/mL). 200 μL of chloroform was added followed by centrifugation. Collecting the RNA solution after passing through the solid adsorption column. RNA quality was analyzed by 1% agarose gel electrophoresis. The RNA sample purity and concentrations were determined by OD260/OD280 using a NanoDrop ND-1000 instrument. Then, cDNA was synthesized by PrimeScript RT reagent Kit (RR047A, Takara Bio, Kusatsu, Japan). The reverse transcription was incubated at 37°C for 15 min, 85°C for 5 sec, and stored at 4°C min. Polymerase chain reaction (PCR) primer sequences are provided in Table 1. PCR was performed with TaKaRa Ex Taq (RR001A, Takara Bio). The PCR reaction was run for 40 cycles as follows: denaturation at 94°C for 30 sec, annealing at 55°C–60°C for 30 sec and extension at 72°C for 1min, with an initial denaturation at 94°C for 2 min and final extension at 72°C for 10 min. The PCR products were electrophoresed on agarose gel and visualized with ethidium bromide. DL2,000 DNA Marker (3427A, Takara Bio) and 100 bp DNA Ladder (3422A, Takara Bio) were use as DNA ladders. All PCR assays were performed in triplicates in three independent experiments.
The bovine mammary tissue samples were washed with phosphate-buffered saline (PBS), then 100 mg of mammary tissue was added to each milliliter of RIPA Lysis Buffer (P0013D, Beyotime Biotechnology, Shanghai, China) with 1% w/v PMSF (ST506, Beyotime) and homogenized using a homogenizer (Tissuelyser-24, Jingxin). 100 μl of RIPA Lysis Buffer (P0013D, Beyotime) was added to each 6-well cell culture plate. The cells are scrapped by cell scrapers from the bottom of the plate and transferred to a 1.5 ml centrifuge tube. The lysates of cells and mammary tissue were sonicated by the ultrasonic cell disruption system (Sonics vCX130, 130w). Samples in ice water bath were ultrasonically crushed for 5s and cooled for 10s, each sample was repeated 5 times. BCA Protein Assay Kit (P0012, Beyotime) was used to determine the protein concentration. A standard curve was established based on known bovine serum albumin (provided by BCA Protein Assay Kit) concentrations. The mass of protein loaded per lane was 40 µg with loading volume of 20 μL. Protein samples are prepared in amounts that can be used for 10 times polyacrylamide gel electrophoresis, and the required loading volume for each sample is calculated based on the protein concentration. Then added 40 µL of 5x SDS (P0015L, Beyotime) loading buffer and the protein sample solution was replenished to 200 µL with RIPA lysate (containing 1% PMSF). Protein samples were denatured completely by a metal bath at 100°C for 10 minutes. The denatured protein samples were centrifuged and stored at −80°C. The proteins were subjected to 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis, and then transferred onto a Polyvinylidene fluoride (PVDF) membrane. The membranes were blocked with WB blocking buffer (P0023B, Beyotime) for 2 hours at room temperature. Antibodies used for immunoblotting were anti-mTOR (1:1000, Rabbit monoclonal. 2983S, Cell Signaling Technology, Danvers, MA, USA), anti-EEF2 (1:1000, Rabbit polyclonal. 2332S, Cell Signaling Technology), anti-4EBP1 (1:1000, Rabbit polyclonal. ab2606, Abcam, Cambridge, UK), anti-S6K1 (1:1000, Rabbit monoclonal. ab32359, Abcam), anti-JAK2 (1:1000, Rabbit monoclonal. 3230S, Cell Signaling Technology), anti-STAT5 (1:1000, Rabbit monoclonal. 94205T, Cell Signaling Technology), and HRP-labeled Goat Anti-Rabbit IgG (H+L) (1:1000, A0208, Beyotime). The protein bands were visualized using Ultra High Sensitivity ECL Kit with Luminol Substrate (P0018S, Beyotime). The chemiluminescence of bands of interest was detected with FUSION Fx imaging system (Vilber Lourmat, Marne-la-Vallée, France). The band density was quantified with Image J software (v1.53c, National Institutes of Health, Bethesda, MD, USA). The results are representative of three independent experiments that were used to determine the statistical significance.
The data were analyzed as a completely randomized design using one-way ANOVA of SAS 8.2 (SAS Institute, Cary, NC, USA). When ANOVA results showed significant differences, the means were compared using Duncan’s Multiple Range test for multiple comparisons. Significance was declared at p < 0.05. The time and 5-HT concentration effect analyses were performed using SPSS (Two-way ANOVA, IBM SPSS Statistics 25, Chicago, IL, USA).
RESULTS
The morphology and cell growth curve of cells were shown in Figs. 1A and 1B. MAC-T cells show a typical epithelial morphology: “cobblestone” structure, consistent with epithelial cells morphology (Fig 1A). The growth curve of cells is obvious S-shape, with normal cell division and viability characteristics (Fig. 1B). The PCR analysis of cytokeratin-18 and casein gene CSN2 and CSN1S1 between bovine mammary tissue and MAC-T cells showed that MAC-T cells have a similar expression pattern to bovine mammary tissue (Figs. 1C, 1D, and 1E). In addition, we detected gene expression of 5-HTR in MAC-T cells, demonstrating that the identified mammary 5-HTR in dairy cows, could be expressed in MAC-T cells.
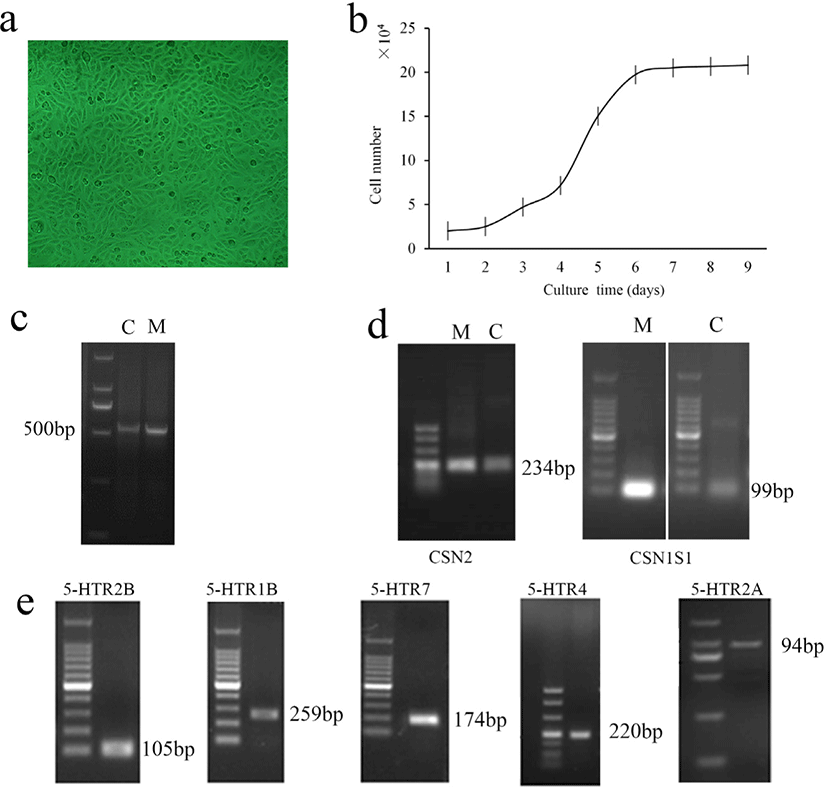
The effects of times and 5-HT concentrations on the viability of MAC-T cells are shown in Fig. 2. Two-way ANOVA showed that 5-HT concentration (p = 0.026) and time (p < 0.001) significantly affected cell viability, but there was no interaction between 5-HT concentration × time (p = 0.103). We found that when incubated MAC-T cells with 5-HT in different concentrations for 12 or 24 hours, the cell viability significantly increased in the 20 μM group (p < 0.05). At 48 hours, 5-HT addition had little effect on cell viability (p > 0.05). At 72 hours, the addition of 200 μM 5-HT significantly reduced cell viability compared to the control group (p < 0.05).
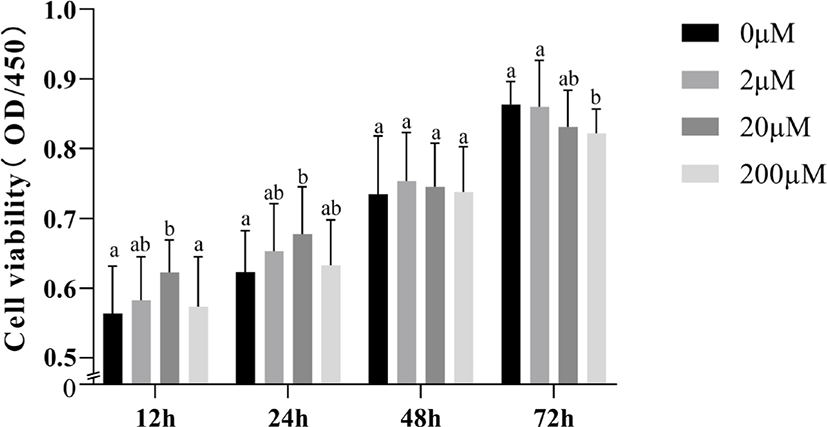
To demonstrate whether the promotion of cell viability by 5-HT interacts with PRL, we examined the effect of simultaneous addition of 5-HT and PRL on cell viability (Fig. 3). With the exception of the 200 μM 5-HT group (p > 0.05), we found a significant increase in cell viability when incubated with 2 μM or 20 μM 5-HT alone for 24 h (p < 0.05; Fig. 3A) When incubated with PRL (20 ng/mL) alone for 24 h, the increase was not significant (p > 0.05). Supplementation of 50 or 100 ng/mL PRL alone could increase cell viability (p < 0.05). When MAC-T cells were incubated with 5-HT (20 μM or 200 μM) simultaneously with PRL (50 ng/mL) for 24 h, cell viability was significantly enhanced compared to the 5-HT or PRL addition groups alone (p < 0.05). In addition, we also investigated the role of 5-HT and PRL on mTOR protein expression in MAC-T cells, the results showed that the level of mTOR protein was significantly increased by addition of 20 μM or 200 μM 5-HT simultaneously with 25 ng/mL (Fig. 3B), 50 ng/mg (Fig. 3C), or 100 ng/mg (Fig. 3D) of PRL (p < 0.05).
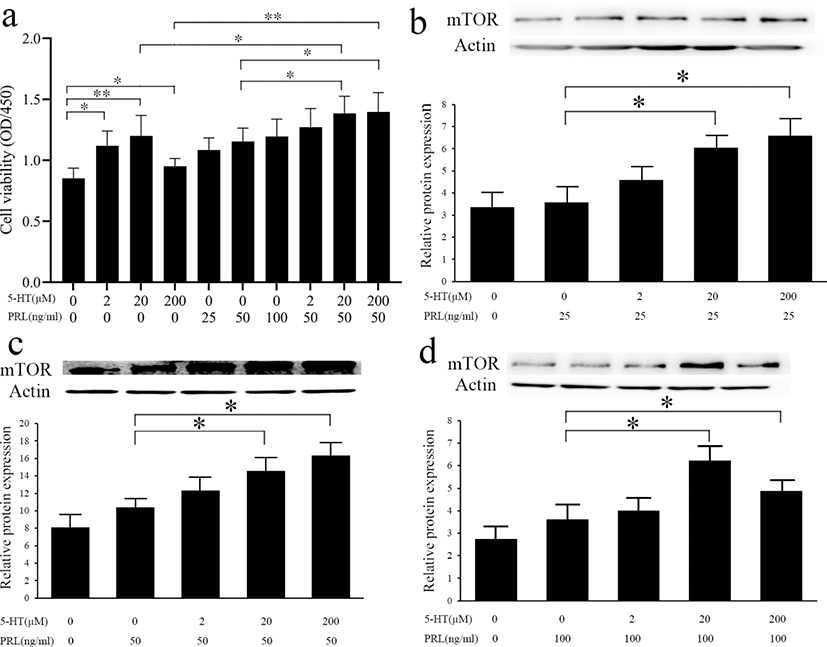
5-HT plays regulatory role by binding to its’ receptors. Different types of 5-HTR have different distribution and functions in the mammary gland. To determine by which receptor 5-HT promotes the viability of MAC-T cells, we blocked different receptors of 5-HT using five inhibitors, SB224289 (antagonist of 5-HT1B receptor), SB204070 (antagonist of 5-HT4 receptor), Ritanserin (antagonist of 5-HT2 receptors, particularly 5-HT2A receptor), SB204741 (antagonist of 5-HT2B receptor), and Pimozide (an effective antagonist of various types of 5-HTR, particularly 5-HT 7 receptor). From the results of cell viability (Fig. 4), the addition of two antagonists, SB224289 or SB204070, inhibited the promotion of cell viability by 5-HT (p < 0.05) while the other antagonists had no significant effect on cell viability (p > 0.05).
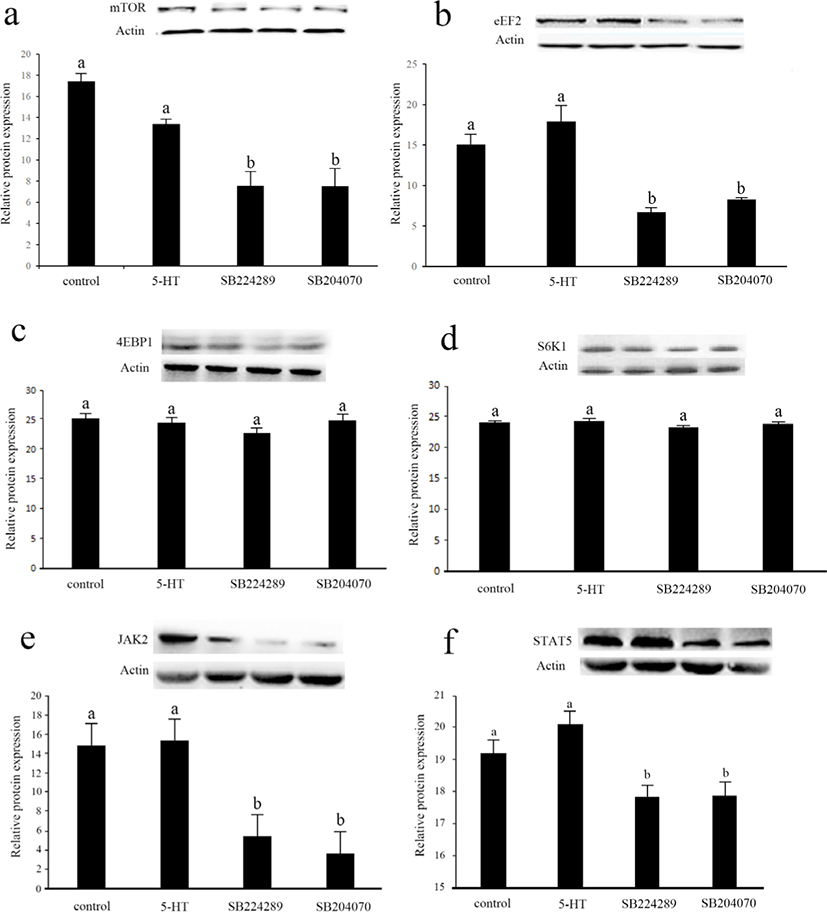
In order to further investigate the mechanism of 5-HT on cell viability, we measured key proteins in two important signaling pathways that regulate cell viability, mTOR and JAK2-STAT5 (Fig. 5). Interestingly, there was no significant difference in protein expression in these signaling pathways between the control group and 5-HT group (p > 0.05). The protein expression of mTOR, EEF2, JAK2 and STAT5 was significantly down-regulated after the culture medium was supplemented with antagonists of 5-HT 1B or 4 receptors, respectively (p < 0.05). The protein expressions of 4EBP1 and S6K1 were not significantly different among the groups (p > 0.05).
DISCUSSION
Previous studies have shown that 5-HTR subtypes 1B, 2A, 2B, 4, and 7 are expressed in the mammary gland of dairy cows [21]. We, therefore, assayed gene expression of these receptors to demonstrate that 5-HT utilization by the MAC-T cells is consistent with that in the mammary gland of dairy cows. We found that these 5-HTR were expressed in MAC-T cells. In addition, our results indicated that MAC-T can serve as a cell model to study 5-HT effects in mammary epithelial cells in dairy cows.
In contrast to studies in which 5-HT promotes apoptosis, our results suggested that short-term medium doses of 5-HT promote cell viability of mammary epithelial cells [11,12]. Our result is in agreement with the findings of Laporta et al. [16] in mouse mammary glands, which demonstrated that 5-HT is necessary to maintain mammary cell activity and normal mammary gland development and function, rather than causing apoptosis [16]. A previous study found that the effect of 5-HT on intercellular tight junctions was concentration-dependent, with moderate concentrations of 5-HT promoting the formation of tight junctions and high concentrations of 5-HT causing disassembly of tight junctions [33]. Ducy [34] pointed out that 5-HT can perform completely different functions in different environments, and that 5-HT secreted by different organs can have different effects. Thus, we hypothesize that the effects of 5-HT on mammary epithelial cell viability and apoptosis are bidirectional, which may be dose dependent, with low concentrations of 5-HT increasing cell viability and high concentrations of 5-HT increasing apoptosis. Moreover, the bidirectional effects of 5-HT on cell viability and apoptosis involve different 5-HTR. In previous studies, 5-HT 2A [35], 5-HT 6 [36] and 5-HT 7 [16,37] receptors, were reported to promote apoptosis in different tissues, while 5-HT 1A [38], 3A [39], 2B [40] and 1D [41] receptors are associated with cell proliferation and carcinogenesis. However, in our study, the viability of the cells was only assessed by the CCK8 method. A more detailed analysis of the proliferation capacity of the cells using techniques such as flow cytometry would be useful to better investigate the effect and mechanism of 5-HT on cell viability and proliferation.
PRL is known to trigger mammary epithelial cell differentiation through activation of the Jak-Stat5 pathway to promote secretory activity during lactation [3]. It is commonly believed that 5-HT and PRL have opposite effects, 5-HT synthesis down-regulates milk protein gene expression, whereas PRL up-regulates milk protein gene expression [5]. However, our results showed that after being cultured with 5-HT and PRL the cell viability was higher than cultured with 5-HT or PRL alone, suggesting a potential interaction between 5-HT and PRL. This process would also involve alterations in the total mTOR signaling pathway, suggesting that the mTOR pathway may play an important role in cell viability induced by 5-HT and PRL, but the exact mechanism still needs to be further investigated.
The complexity of the effects of 5-HT in organisms is largely due to the variable nature of its receptors. Basically, 5-HT plays a variety of physiological roles in signaling through approximately 15 different receptors [42]. A large number of receptor genes, selective splicing of receptor transcripts, different combinations of receptor subunits and heterodimerization with non-5-HTR all result in a wide variety of 5-HTR functions [43]. In a related study in the mammary gland, mammary epithelial structure is altered in lactating mice lacking 5-HT 7 receptor, with reduced epithelial cell shedding in the degenerative phase of the mammary gland [44]. In mice, 5-HT 2B receptor increases proliferation of mammary epithelial cells via an extracellular-related kinase pathway [16]. In our study, moderate doses of 5-HT promoted cell viability mainly through 5-HT 1B and/or 4 receptors, while the results of immunoblotting indicated that activation of the 5-HT 1B and/or 4 subtypes of receptors was necessary to maintain normal function of the mTOR and JAK2-STAT5 pathways. 5-HTR 1B was found to mediate the proliferation of human pulmonary artery smooth muscle cells [45]. In a breast cancer-related study, 5-HTR1B was expressed mainly in the cytoplasm of breast cancer cells, while 5-HTR4 was expressed only in the nucleus of malignant and non-malignant cells [46]. 5-HT 4 receptor may be associated with proliferation in epithelial tissues, and regulation of proliferation by 5-HT 4 receptor was found in colonic epithelium and bronchial epithelium [47,48]. In concert with our study results, 5-HT 4 receptor may play a very important role in the cell viability of the epithelium. In bovine mammary epithelial cells, our results showed that 5-HT modulates cell viability via 5-HT 1B and/or 4 receptors. Furthermore, there is a connection between 5-HT 1B, 4 receptors and the mTOR pathway, but the promotion of cell viability by 5-HT seems to be less related to mTOR. Although these 5-HTR antagonists had no effect on cell viability in studies with other cells[49-53], further research is needed on MAC-T cells to exclude the effect of these antagonists on cellular viability. In order to further investigate the effect of 5-HT and its receptors on MAC-T cells viability and mTOR pathway, the use of different 5-HTR agonists could also be performed.
Activation of the mTOR pathway is essential for mammary gland development and lactation, and PRL also exerts a regulatory effect on the mammary gland directly through mTOR [54,55]. A previous study suggested that the treatment with SB-6995551, a 5-HT 5A receptor blocker, may affect the proliferative capacity of human breast tumour cell lines via the mTOR pathway [56]. Another study showed that the 5-HTR 1B antagonist, SB216641, acts by inhibiting the mTOR pathway in human endothelial cells [14]. Similar to previous studies, our study suggested that blockers of 5-HT 1B and/or 4 receptors may also attenuate the effects of 5-HT on cell viability by affecting mTOR pathway.
The JAK2-STAT5 pathway plays an important role in mammary epithelial cell viability and proliferation and PRL regulates mammary gland function through phosphorylation of JAK2 and its downstream STAT5 activation [57]. There are no available data on the relationship between 5-HT and the JAK2-STAT5 pathway. Our study is the first to investigate the relationship between the effects of 5-HT on mammary epithelial cell viability and the JAK2-STAT5 pathway. Interestingly, the application of 5-HT promotes cell viability, does not affect mTOR and the JAK2-STAT5 pathway. However, inhibition of 5-HT 1B and/or 4 receptors directly reduced protein expression of mTOR, JAK2 and STAT5, suggesting that some 5-HTR may be involved in mTOR and JAK2-STAT5 signaling. We therefore hypothesize that the mTOR and JAK2-STAT5 pathways may be prerequisites for the promotion of cell viability by 5-HT. Activation of 5-HT 1B and/or 4 receptors maintains the normal function of the mTOR and JAK2-STAT5 pathways, and thus the normal proliferative activity of mammary epithelial cells. Further research should be undertaken to investigate the effect of mTOR and JAK2-STAT5 pathways on promotion of cell viability by 5-HT.
In conclusion, our study suggested that 5-HT promotes the viability of MAC-T cells by 5-HTR 1B and/or 4. There is a connection between 5-HT 1B, 4 receptors and the mTOR pathway, but the promotion of cell viability by 5-HT seems to be less related to mTOR. After cultured with 5-HT and PRL the cell viability was higher than cultured with 5-HT or PRL alone, suggesting a potential interaction between 5-HT and PRL.
