INTRODUCTION
In Korea, interest in high-energy and high-protein feed is increasing to shorten the rearing period and decrease the production costs associated with Hanwoo steers [1–3]. In general, a difference exists in the supply of energy and protein according to the fattening stages of Hanwoo steers [3]. Recently, the Korean government decided to limit the crude protein content of the formula feed for early and late-fattening stages, to a maximum of 17% and 15%, respectively, to supply adequate protein for each fattening stage. The government plans to reduce the methane gas generation from Hanwoo steers by restricting the protein in the formula feeds [4]. In response, the need for raw material feed with a high protein content has increased.
Lupin has a high protein content [5], which varies from 32% to 40% depending on the processing method [6]. In addition, lupin contains tocopherol, lutein, α-carotene, β-carotene, and various polyphenols and is used as a raw material for food and livestock feed worldwide [7–9].
Previous studies [10,11] have reported that lupin-rich feeds improve digestibility, apparent metabolizable energy (AME), and average daily gain (ADG) in poultry. In swine feed, the supplementation level of lupin can be as high as 35%, owing to its excellent palatability [12]. However, the ADG is reported to decrease if the lupin supplementation level is >15% in swine feed [13]. Furthermore, lupin does not cause acidosis in beef cattle because of its low starch content [14]. The rumen by-pass protein and fiber contents of lupin were ≥ 30% and 39%, respectively. However, the digestibility of lupin is high (approximately 90%) because of its low lignin content (less than 1%) [15]. Lupin exerts a buffering effect on the rumen pH [16,17], and it has been reported that lupin has a highly effective protein degradability. Additionally, it has been shown that lupin promotes protein absorption because of its high content of β-conglutin, a protein that is easily decomposed as it lacks disulfide bonds [18,19]. Furthermore, previous studies [16] have reported that roughage intake was decreased in treatment fed lupin-supplemented formula feed, reducing feed costs.
A study [20] reviewed the shortening of the fattening period and the optimal nutritional level for Hanwoo steers. Lupin has been used as a high-energy, and high-protein source in various livestock feeds [21]; however, the optimal supplementation level of lupins in formula feed for beef cattle (especially Hanwoo steers) has not been previously determined. Thus, this study was conducted to determine the effects of the rumen fermentation characteristics of lupin flakes and supplementation with lupin flakes on the growth performance, blood metabolites, and carcass characteristics of Hanwoo steers.
MATERIALS AND METHODS
This study was approved by the Kangwon National University Animal Experimental Ethics Committee (approval number: KW-200820-2). Three Hanwoo cows (423.0 ± 44.8 kg), each implanted with a ruminal fistula, were used. The experimental materials (lupins) were divided into whole grains and flakes, crushed with a Wiley mill (Thomas Scientific, Swedesboro, NJ, USA) equipped with a 2 mm screen. Lupin flakes were supplied by Suni lupin (Seoul, Korea). The production conditions for lupin flakes were as follows: 100°C (chamber temperature), 3–4 mm (thickness). And the production yield were 100 tons/hour. The cows were raised in three pens (one cow per pen), and provided the formula feed and roughage twice daily at 09:00 and 18:00. The chemical compositions of the experimental diets are listed in Table 1. The cows had free access to water and mineral blocks. Other feeding management procedures were conducted according to the practices of the experimental farm.
A total of 40 early-fattening Hanwoo steers, each weighing 444.3 ± 32.9 kg, were randomly divided into the following four groups: control, T1, T2, and T3. They received unsupplemented, 3% lupin flake–supplemented, 6% lupin flake–supplemented, and 9% lupin flake–supplemented formula feeds, respectively. The steers were raised in eight pens (five steers per pen) and were given the formula feed and roughage three times daily at 08:30, 13:00, and 17:00. The ingredients and chemical composition of the experimental diets are listed in Table 2. The steers had free access to water and mineral blocks. Other feeding management procedures were conducted according to the practices of the experimental farm.
Contorl, 0% lupin flake-supplemented formula feed; T1, 3% lupin flake-supplemented formula feed; T2, 6% lupin flake-supplemented formula feed; T3, 9% lupin flake-supplemented formula feed.
Concentrated feed contained the following percentage of ingredients: corn, 23.5%; cane molasses, 4.0%; cassava residue, 6.0%; wheat bran, 12.5%; corn gluten feed, 12.5%; soybean meal, 10.0%; rapeseed meal, 7.0%; coconut meal, 11%; palm kernel meal, 10%; animal fat, 0.3%, salt dehydrate, 0.7%; limestone, 1.9%; sodium bicarbonate, 0.5%, vitamin-mineral premix, 0.1%.
Ruminal fluid was collected from the ruminal fistula of Hanwoo cows before feeding in the morning and filtered through four layers of cheese cloth, before being stored at 39°C in a thermos flask. After that, O2-free CO2 gas was infused for 30 s to eliminate air. The collected ruminal fluid was transferred to the laboratory and allowed to stand for 1 h in an incubator at 39°C to eliminate feed particles, and was used as an inoculum for in vitro incubation.
In vitro cultures were established by adding 400 mL of rumen inoculum to 1,596 mL of previously prepared artificial saliva (i.e., buffer solutions A: 1,330 mL and B 266 mL) following McDougall’s method [22]. Table 3 shows the composition of buffer solutions A and B.
| Item | g/L |
|---|---|
| Buffer solution A | |
| KH2PO4 | 10.0 |
| MgSO4·7H2O | 0.5 |
| NaCl | 0.5 |
| CaCl2·2H2O | 0.1 |
| Urea (regent grade) | 0.5 |
| Buffer solution B | |
| Na2CO3 | 15.0 |
| Na2S·9H2O | 1.0 |
In total, 70 mL of the prepared in vitro culture solution was placed in a 100 mL bottle (4 oz glass bottle) containing 2 g of ground lupin grains and lupin flakes, and O2-free CO2 gas was infused for 5 s to eliminate air from the culture bottle. The bottles were then incubated for 0, 3, 6, 9, 12, 24, and 48 h in a shaking incubator (HB- 201SLI, Hanbaek Scientific, Bucheon, Korea) at 39°C.
The in vitro ruminal pH was measured using a pH meter (Corning 445, Corning, NY, USA) in a 100 mL bottle for each incubation time. To analyze ammonia concentration, after centrifugation (3,000×g, 15 min, 4°C) of 10 mL of rumen fluid collected at each incubation time, 5 mL of the supernatant and 0.05 mL of HgCl2 were mixed and centrifuged again (3,000×g, 15 min, 4°C). One milliliter of the supernatant was collected, and the ammonia concentration was analyzed according to the method of Chaney and Marbach [23].
To analyze the volatile fatty acid (VFA) concentration, 10 mL of the culture solution was collected in a 100 mL bottle for each incubation time. This was followed by the addition of 1 mL of 20% HPO3 and 0.5 mL of saturated HgCl2, and centrifugation at 1,250×g for 15 min at 4°C. The supernatant was then collected, and the VFA concentration was measured by gas chromatography (6890N, Agilent Technologies, Santa Clara, CA, USA).
Experiments were performed by collecting nylon bags at 0, 3, 6, 9, 12, 24, and 48 h after a 14 d feed adaptation period in Hanwoo equipped with rumen fistulas. In detail, five grams of sample was placed in a nylon bag (ANKOM 5 × 10 concentrate bags), and were inserted into the rumen via a fistula before feeding in the morning. The nylon bag was washed until the water ran clear and dried in a 70°C forced air drying oven for 72 h. The dried samples were weighed and used to calculate dry matter disappearance rates. The samples were ground to a particle size of 1.0 mm, and their chemical composition was analyzed to determine the disappearance of crude protein, neutral detergent fiber (NDF) and acid detergent fiber (ADF).
ADG was calculated by measuring body weight at the beginning and end of the experimental period. Feed intake was calculated by measuring the quantity of residual feed before feeding in the morning. The feed conversion ratio (FCR) was calculated based on dry matter intake (DMI) and ADG values.
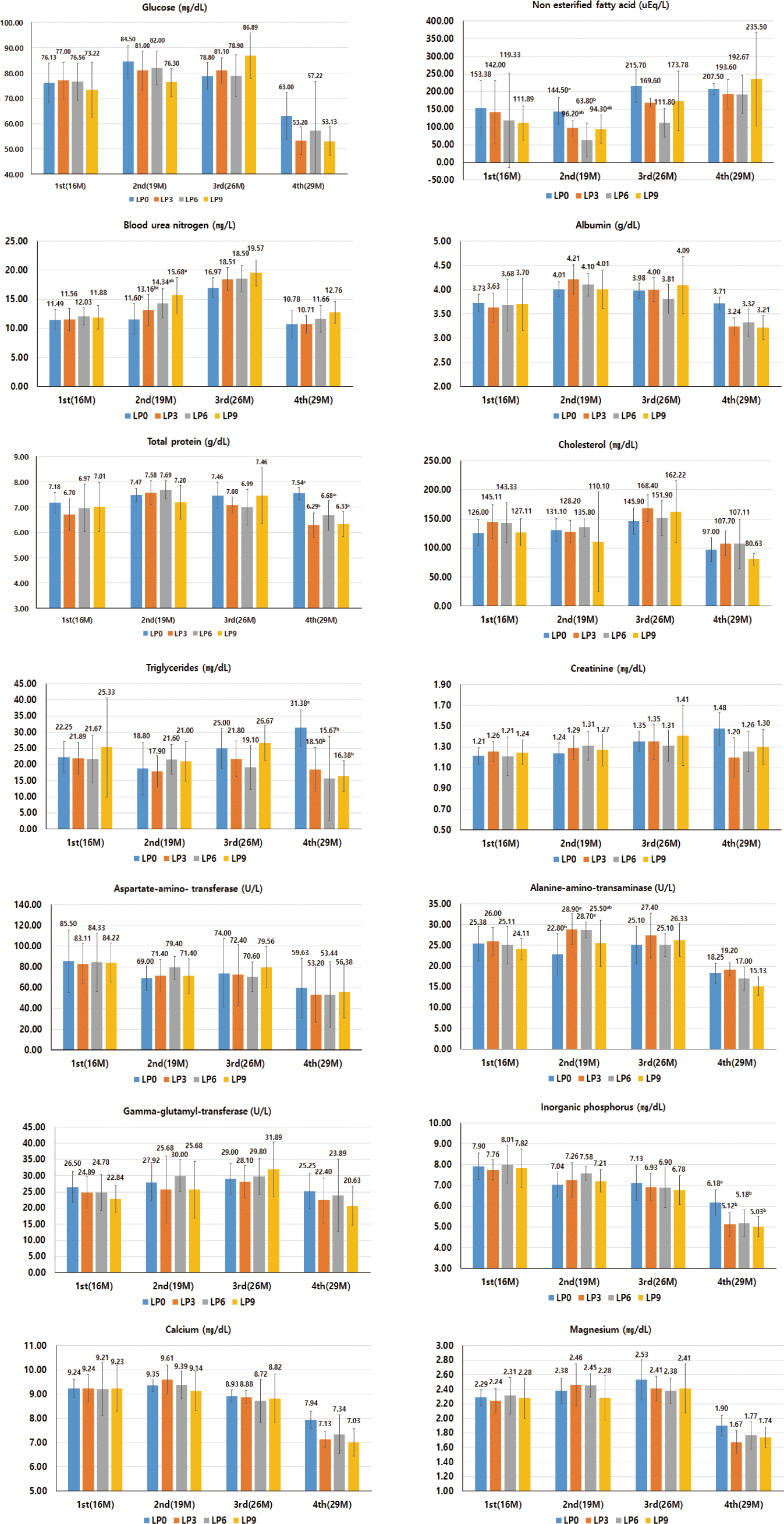
Blood samples were collected from the jugular vein of the steers before feeding in the morning, using a 10 mL vacutainer (Becton Dickinson, Franklin Lakes, NJ, USA). To analyze the metabolites in the blood, heparinized blood samples were centrifuged at 2,000×g for 15 min to separate the plasma. Plasma metabolites were analyzed using an automatic blood analyzer (Hitachi 7020, Hitachi, Tokyo, Japan). The following plasma parameters were analyzed: glucose (GLU), non-esterified fatty acid (NEFA), blood urea nitrogen (BUN), albumin (ALB), total protein (TP), cholesterol (CHOL), triglyceride (TG), creatinine (CREA), calcium (Ca), inorganic phosphorus (IP), and magnesium (Mg).
All steers were slaughtered at a local slaughterhouse. Carcass yield (carcass weight, back-fat thickness, and rib-eye area) and quality grades (marbling score, meat color, fat color, texture, and maturity) were examined according to the criteria of the Korean carcass grading system [24]. Each carcass was chilled for 24 h, and the weight of the cold carcass was measured. Next, the left side of each carcass was cut between the thirteenth rib and the first lumbar vertebrae, and used to determine the quality grade.
The quality grade was determined by assessing the degree of marbling and firmness in the cut surface of the rib-eye based on the maturity, meat color, and fat color of the carcass. According to the Korean beef quality grading system, quality grades were evaluated as follows: 1++ (excellent quality), 1+, 1, 2, and 3 (low quality). The rib-eye area was measured from the longissimus muscle at the thirteenth rib. Back-fat thickness was measured at the thirteenth rib. The yield index was calculated as follows: [(11.06398–1.25149 × back-fat thickness (mm)] + [0.28293 × rib-eye area (cm2)] + [0.56781 × carcass weight (kg)] / [carcass weight (kg) ×100]. Yield grades were classified according to the Korean beef yield grading system as A (high yield), B, and C (low yield) based on the yield index (grade A ≥ 67.20, grade 67.20 > B ≥ 63.30, and grade C < 63.30). The score was determined using a color standard [24]. The texture and maturity scores were calculated using the KIAPQE reference index [24]. The marbling scores were graded on a scale of 1 to 9, with higher numbers indicating better quality (1 = devoid, 9 = abundant). Additional scores included those for meat color (1 = bright red, 7 = dark red), fat color (1 = creamy white, 7 = yellowish), and texture (1 = soft, 3 = firm).
Statistical analyses to obtain the average values and standard differences were performed using the IBM SPSS (Statistical Package for the Social Sciences, SPSS Inc. Chicago, IL, USA) software. Student’s t-test and one-way analysis of variance were used to calculate the average value for each treatment group. One-way ANOVA was performed using Duncan’s multiple range test [25]. Statistical significance was set at p < 0.05.
RESULTS
Table 4 shows the changes in pH and ammonia concentration in the in vitro rumen fluid of lupin grain and lupin flake. The pH was lower in lupin flake than in lupin grain at all incubation times, except at hour 3 (p < 0.05). The ammonia concentration was higher in lupin flake than in lupin grain at 24 and 48 h of incubation (p < 0.05).
Table 5 shows the changes in the concentration of VFAs in the in vitro rumen fluid of lupin grain and lupin flake. Acetate concentrations were higher in lupin flake than lupin grain after 24 h of incubation (p < 0.05). Concentrations of propionate, butyrate, and total VFA were higher in lupin flake than in lupin grain after 12 h of culture (p < 0.05).
Table 6 shows the changes in the disappearance rates of dry matter, crude protein, NDF, and ADF of lupin grain and lupin flake in the rumen. The disappearance rates of dry matter were slightly, but not significantly, higher in lupin flake than in lupin grain at all incubation times. Furthermore, the disappearance rates of crude protein were higher in lupin flake than in lupin grain at hours 9 and 12 (p < 0.05). The disappearance rates of NDF were higher in lupin flake than in lupin grain until hour 9 (p < 0.05). The rates of ADF were also slightly, but not significantly, higher in lupin flake than in lupin grain.
Table 7 shows the effects of lupin flake-supplemented formula feed on the growth performance of Hanwoo steers. Lupin flake supplementation did not affect the ADG of Hanwoo steers. Formula feed and total digestible nutrient (TDN) intake were higher in lupin flake-supplemented groups than in the control group (p < 0.05). Rice straw and DMI were lower in the lupin flake-supplemented groups than in the control group (p < 0.05). The FCR was slightly, but not significantly, lower in the T2 and T3 groups than in the control group.
Fig. 1 shows the effects of lupin flake-supplemented formula feed on the blood metabolites of Hanwoo steers. The plasma NEFA and alanine aminotransferase (ALT) concentrations were lower in the T2 group than in the control group at 19 months of age (p < 0.05), and the plasma BUN concentration increased with the supplementation level of lupin flake at 19 months of age (p < 0.05). The plasma TP concentrations were lower in the T1 and T3 groups than at 29 months of age (p < 0.05). The triglyceride and IP concentrations were lower in the lupin flake-supplemented groups than at 29 months of age (p < 0.05). Plasma glucose, NEFA, BUN, and ALT concentrations were not significantly different between the treatments at 29 months.
Table 8 shows the effects of lupin flake-supplemented formula feed on the carcass characteristics of Hanwoo steers. The yield index showed a tendency to improve in the T1 and T2 groups compared to the control groups, and the incidence rate of yield grade A tended to improve in the T1 and T2 groups compared to the control groups. The marbling score was slightly, but not significantly, higher in the lupin flake-supplemented groups than in the control group. The incidence rate of meat quality 1++ grade was higher in the lupin flake-supplemented groups than in the control group, and the incidence rate of meat quality 1+ grade or higher was the highest in the T2 group. The highest carcass auction price was found in the 6% lupin flake-supplemented group.
Area was measured from longissimus muscle taken at 13th rib and back-fat thickness was also measured at 13th rib; Yield index was calculated using the following equation: 68.184 - (0.625 × back-fat thickness (mm)) + (0.130 × rib eye area (cm2)) − (0.024 × dressed weight amount (kg)); Carcass yield grades from C (low yield) to A (high yield).
DISCUSSION
Flaking is a processing method that increases the digestibility of grains through physical shape change and gelatinization of grains, and is used for processing grains such as corn, lupin, sorghum, barley, and wheat [26]. The rumen pH is lowered because of the influence of VFAs and organic acids produced during the fermentation of feed by microorganisms [27]. Cho et al. [28] reported similar results, showing that the in vitro rumen pH of processed lupin was lower, whereas the ammonia concentration was higher than that of lupin grain. Therefore, similar to previous studies [28], it is considered that rumen pH is decreased, but ammonia concentration is increased by flaking lupin in this study.
Nowak and Wylegala [19] reported similar results, showing that the acetate concentration of in vitro rumen fluid was higher in processed (e.g., heated, ground, etc.) lupin than in lupin grains. In addition, the increase in digestibility because of gelatinization following flaking is also considered to be the cause of acetate concentration in the rumen fluid of those with the lupin flake diet. Nonstructural carbohydrates, such as starch, are converted to lactic acid and propionate by rumen microbes thereby affecting rumen pH [29,30]. Propionate is absorbed through the rumen wall and used as an energy source after converting GLU in the liver [31]. In this study, propionate and butyrate concentrations were higher in lupin flake than in lupin grain, which is considered to be the because of the increase in digestibility from the flaking treatment (Table 5).
Similar to the results of this study, Zaman et al. [32] reported that the by-pass rate of lupin in the rumen was about 30%, and the disappearance rates of dry matter of lupin grain and lupin flake after 24 h of fermentation were 69.98 and 71.46%, respectively. In addition, in a previous study [28] and in this study, the higher disappearance rate of lupin flake compared to lupin grain is considered to be because of the flaking treatment. Moreover, in this study, the disappearance rates of crude protein and NDF were higher in lupin flake than in lupin grain. This is related to the phenomenon of increased disappearance of dry matter because of the flaking treatment for lupin.
Vicenti et al. [33] and Sami et al. [34] reported a result similar to the one in this study, suggesting that using lupin-supplemented formula feed reduced feed intake for the fattening period. Kwak and Kim [35] reported similar results, indicating that the supplementation of lupin up to 15% in the formula feed did not affect the growth performance of Hanwoo steers. Similarly, Ahn et al. [36] reported that high-energy formula feed did not affect the ADG of late-fattening Hanwoo steers. Meanwhile, previous studies [33,34,37] have reported that ADG decreased with lupin supplementation. Conversely, some previous studies [38, 39] have reported that ADG was increased by supplementation with lupin. Robinson and McNiven [40] reported that the contents of rumen degradable proteins in lupin were higher than those in soybean meal. The protein composition of lupin is inferior to that of soybean meal [33]. In addition, lupin did not markedly improve ADG, potentially because it does not contain glutamic acid or sulfur-containing amino acids, such as methionine and cystine [41]. Thus, in the results of this study, it is thought that the supplementation of lupin flake had no effect on the ADG of Hanwoo steers and the difference in energy level had no effect on the ADG of Hanwoo steers.
Lovati et al. [42] reported that γ-conglutin in lupin exerts plasma glucose-lowering effects in ruminants. In this study, the plasma glucose concentration showed a tendency to decrease with the supplementation of lupin flakes, but this indicated that lupin did not markedly affect the plasma glucose concentrations. This might have occurred because of variations in the concentrations of γ-conglutin, depending on the processing method and supplementation level of lupin [43].
In ruminants, it is known as an indicator of body energy levels [44]. It is known that the higher the concentration of NEFA in the blood, the greater the decomposition of body fat [45]. Lestingi et al. [46] reported that supplementation of lupin increased the blood NEFA concentration of beef cattle. However, in this study, only the NEFA concentration temporarily increased because of decreased energy intake in the early fattening period in the lupin flake-unsupplemented group; nevertheless, it is considered that the effect of lupin flakeis small on the plasma NEFA concentration of Hanwoo steers.
Plasma albumin accounts for 60% of the total plasma protein, whereas globulin-based proteins account for 40% of the total plasma protein. Plasma TP concentrations illustrate protein metabolism and systemic nutritional status [47]. Previous studies have reported that supplementation with lupin upregulated plasma TP concentrations in pigs [41]. Consistent with the findings of this study, Lestingi et al. [46,48] reported that supplementation with lupin did not affect plasma TP concentrations. In this study, the plasma TP concentrations decreased with the supplementation of lupin flakecompared to the lupin flake-unsupplemented group at 29 months of age. Therefore, we consider that the decrease in the plasma TP concentration according to the supplementation of lupin flakemay be related to the decrease in the concentration of plasma albumin.
Spielmann et al. [49] reported that proteins in lupin decreased the plasma concentration of triglycerides by downregulating the expression of the sterol regulatory element-binding protein-1c in the liver, similarly to the results of this study.
Because the blood mineral concentration is maintained at a constant level, compared to other blood metabolites, it has been reported that it is possible to diagnose the nutritional status of livestock even with a small change in concentration [50]. Plasma IP is an important component of cells and is involved in energy metabolism, muscle contraction, oxygen supply, maintenance of body fluid pH, and the activation of vitamins and enzymes [51]. In this study, the plasma IP concentration at 29 months of age was decreased by supplementation with lupin flakes, which is considered to be caused by the decrease in feed intake in the lupin flake-supplemented groups.
Jeon et al. [1] reported that high-energy formula feed had not effects on carcass weight, rib-eye area, back-fat thickness and meat quality. King [52] reported that lupin increased back-fat thickness in a dose-dependent manner. In contrast, supplementation with lupin flake did not significantly affect back-fat thickness in this study, which is similar to the findings of Kwak and Kim [35]. Dawson [53] reported that lupin flake supplementation did not affect the marbling score. In contrast, Vicenti et al. [33] reported that the combination of lupin and soybean increased the marbling score. In the present study, although the overall effect of lupin flake supplementation on the individual carcass characteristics of Hanwoo steers was small, we consider that the 6% lupin flake-supplemented formula feed had positive effects on the yield and quality grade of Hanwoo steers.
CONCLUSION
Overall, lupin flake had positive effects on ammonia and crude protein disappearance rates without negative effects on VFA concentrations in the rumen. In addition, we suggest that the supplementation of 6% lupin flake to formula feed had a positive effect on feed conversion ratio, yield grade and quality grade in Hanwoo steers.