INTRODUCTION
Intramuscular fat deposition is an important characteristic of meat quality in ruminants because of its positive correlation with meat flavor. The formation of intramuscular fat is a very complex process affected and regulated by multiple factors which are directly or indirectly involved in cell proliferation, differentiation and fatty acid metabolism [1]. Many of the important aspects of fat synthesis in beef cattle occurs at the molecular level. One of the latest targets of nutrigenomic research is the correlation of nutritional factors with the alteration of its metabolism [2].
In Korea, the conventional feeding stipulates a restricted feeding of concentrates but a steady increase from 50% to 90% in the provided concentrate to forage ratio to amply allocate energy to promote deposition of fat during the 17-month of fattening phase [3]. Forages are usually supplied ad libitum during the growing stage (6–11 months) and are slowly decreased from 30% to 10% of total dry matter (DM) intake from the early (12–20 months) to late fattening (21–29 months) stages, respectively [4]. In concentrate feeds, the amount of total digestible nutrient (TDN) can be gradually increased to up to 74% DM, and crude protein (CP) can be reduced from 16% to 12% DM [5]. However, some commercial farms have been modifying their fattening stage by supplying concentrate feeds with a CP content higher than that in conventional feeds during the entire fattening stage. These different practices that changes the supplied CP in the diet might affect the amino acid profile of the feed and affect the mechanism of fat deposition in Hanwoo beef cattle.
Amino acids are building blocks of proteins and alternative sources of energy. They are essential for various physiological functions, which includes antioxidant activity, stress reducing properties, immunomodulation and indirect improvement in meat quality [6]. However, recent researches have also recognized the function of amino acids as signal transducers in major metabolic pathways [7,8]. Lysine is a promising amino acid. Although reports are inconsistent, amino acids are reported to induce lipid accumulation in vivo and in vitro [9,10]. The mechanisms involved in enhancing adipocyte differentiation through decreasing level of lysine are still not fully understood. One of the potential pathways that can lead to increased adipogenesis is through increased preadipocyte commitment via Zinc finger protein 423 (Zfp423). Zfp423 was recently identified as a critical marker of committed preadipocytes and a key initiator of adipocyte differentiation [11]. We hypothesized that low lysine levels may differentially regulate the Zfp423 signaling pathway to increase preadipocyte commitment of bovine stromal vascular cells (SVC) resulting in increased adipocyte differentiation.
MATERIALS AND METHODS
Bovine intramuscular adipose tissue was collected directly at the cervical longissimus dorsi muscle of Hanwoo beef cattle from Dodram Slaughterhouse, Anseong, Korea. The samples were immediately delivered to the Applied Biochemistry Laboratory at Hankyong National University in a sterile phosphate-buffered saline (PBS) supplemented with Amphotericin B (2 ng/mL) and gentamycin (0.2 mg/mL). The dissected adipose tissues were then finely minced and incubated in Dulbecco’s Modified Essential Medium (DMEM) containing collagenase type 1 (2 mg/mL) and bovine serum albumin (4 mg/mL). Digestion was performed for 50 min in a water bath set at 37°C with gentle shaking. After digestion, 5 mL of DMEM were added to stop the digestion process. The digested adipose tissues were then filtered through a 100-µm nylon cell strainer, and the suspension was centrifuged at 700×g for 10 min. The supernatant and lipid layer were removed by suction. The collected pellet was washed three times with DMEM. The cells were resuspended and incubated in the growing media composed of DMEM with 10% bovine calf serum (BCS), 100 U/mL penicillin and 100 µg/mL streptomycin at 37°C under a humidified atmosphere and 5% CO2.
To determine the effect of different lysine concentration on the proliferation of isolated SVC, a cell proliferation assay was performed using Cell Counting kit-8 (CCK-8, Dojindo, Kumamoto, Japan) following the manufacturer’s instruction. Briefly, the cells were seeded at a density of 1 × 104 cells per well and grown in DMEM BCS media supplemented with increasing levels of lysine (0 to 300 µg/mL) for 24 and 48 h. After incubation, the media were removed and replaced with new DMEM with 10 µL of CCK-8 reagent was added per well. The plates were then incubated at 37°C for 2h. Absorbance at 450 nm was measured using an ELISA plate reader (Tecan, Switzerland).
Bovine SVC were cultured in DMEM supplemented with 10% BCS until they reached 80% to 90% confluence. Differentiation media with different concentrations of lysine and containing 10% fetal bovine serum (FBS), 1 μM dexamethasone (DEX, Sigma-Aldrich, St. Louis, MO, USA), 0.5 mM isobutyl methylxanthine (IBMX, Sigma-Aldrich), and 10 μg/mL insulin (INS, Sigma-Aldrich) were used. After 2 days, the media was replaced by DMEM supplemented with 10% FBS and 10 μg/mL insulin. The cells were maintained in DMEM supplemented with FBS until day 10.
Oil Red-O (ORO) staining was used to determine the extent of adipocyte differentiation at day 10. Differentiated bovine SVC were fixed in 10% formalin for 1 hour, washed with distilled water, and stained with 0.6% Oil Red-O solution (Sigma Aldrich) in the dark for 15 min. After staining, the cells were washed with water and coated with glycerol for imaging. Differentiation was observed by microscopy, relative lipid content was measured by isopropanol elution and optical density was evaluated at 520 nm.
To determine preadipocyte commitment, bovine SVC were grown in six-well plates with DMEM containing 10% BCS and different concentration of lysine (0, 37.5, 75, 150 and 300 µg/mL) for 48 h. After 48 h, total RNA was isolated from the bovine SVC using RNAiso Plus (TaKaRa Bio, Kusats, Japan). The concentration and purity of RNA was determined at an absorbance of 260 nm and 280 nm, respectively using a Nanodrop 1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA). Reverse transcription was conducted with Maxime RT Premix Kit (iNtRON Biotechnology, Seongnam, Korea) following the manufacturer’s recommendations.
For the evaluation of adipocyte differentiation, total RNA was isolated using the previously described procedure using bovine SVC differentiated for 10 days. PCR consisted of 5 min denaturation process at 95°C, gene amplification for 32 cycles: 40s at 95°C, primer attachment for 40 seconds (annealing temperature 56°C–68°C), and extension at 72°C for 1 min. The following oligonucleotide primers were used in RT-PCR: Zfp423 forward: GGA TTC CTC CGT GAC AGC A; Zfp423 reverse: TCG TCC TCA TTC CTC TCC TCT; Pref1 forward: AAG CAC CGG CAG ACA AGA; Pref1 reverse: CAG AGG AAG GAG TCG TCA GTA; peroxisome proliferator-activated receptor-gamma (PPARγ) forward: CCG CTT CCA GGG GTG TCA GT; PPARγ reverse: GGA TAT GAG GAC CCA TCC T; CCAAT enhancer binding protein alpha (C/EBP α) forward: ACT AAC CAG TGA CAA TGA CC; C/EBP α reverse: CTT GAC CAG GGA GCT CTC G; sterol regulatory element binding transcription factor 1c (SREBP-1c) forward: GAC GCT CTT ACA TCA ATG AC; SREBP-1c reverse: TTC GGC CAT TTG CTT TTG TG; fatty acid binding protein 4 (FABP4) forward: ACT TAG ATG AAG GTG CTC TG ; FABP4 reverse; CCT CAG GAC TAA ACA ACT TAT G; stearoyl-CoA desaturase (SCD) forward: CCT GTG GAG TCA CCG AAC C; SCD reverse: CCT TGG ATA CTT TCT TCC GGT C; and β-actin forward: GGC CAT GGA TGA TGA TAT TGC; β-actin reverse: ACG CCG CCT TTC ACA T.
SVC were grown in six-well plates and was allowed to differentiate for 10 days with different levels of lysine. Protein extraction was performed on day 10 by adding protein extraction solution (iNtRON Biotechnology). The protein samples were purified by centrifugation at 15,000 ×g for 15min at 4°C and the protein content of the supernatant was measured using a modified Bradford assay. Prepared protein samples were allowed to separate by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes. Blocking was performed with 5% skimmed milk and the samples were incubated with the following primary antibodies (Cell Signaling Technology, Danvers, MA, USA): rabbit polyclonal anti-PPARγ, rabbit polyclonal anti-C/EBPα, polyclonal anti-SREBP-1c, rabbit polyclonal anti-FABP4, mouse monoclonal anti-SCD, and mouse monoclonal anti-β-actin. Proteins were detected by incubating the nitrocellulose membranes with secondary antibodies conjugated with horseradish peroxidase followed by a treatment with enhanced chemiluminescence (AB Frontier, Seoul, Korea). The specific proteins of concern were evaluated using radiographic film exposure.
All the data are presented as mean ± SD. Statistical differences between the control and treatment group for cell proliferation, relative lipid accumulation, and mRNA and protein expression were analyzed by one-way analysis of variance (ANOVA) with Turkey’s honestly significant difference (HSD) post-hoc test (SPSS v 23). Values were considered statistically significant at p < 0.05. All presented data are representative of at least three independent tests (n ≥ 3).
RESULTS AND DISCUSSION
Intramuscular fat deposition is one of the most important qualities of meat and can translate into a massive economic benefit for the beef industry. Specific feeding strategies have been developed in Korea to improve the quality of Hanwoo beef. Changes in the CP content of the diet have been the focus of several studies that aimed to promote intramuscular fat deposition but which yielded conflicting results [3,12,]. The effects of dietary protein and amino acid levels on the transcriptional expression of genes responsible for fatty acid biosynthesis during the fattening stage of Hanwoo beef cattle remain unclear. However, recent studies have shown that fibroblasts and adipocytes originate from common progenitor cells that are mainly located in the stromal vascular fraction of the skeletal muscles [13–15]. SVC isolated from skeletal tissues are composed of a diverse population of cells. These cells can easily be grown in vitro and with the proper stimulation, have great potential to differentiate into different cell lineages. In beef cattle, the regulation of the mechanisms involved in the early commitment of intramuscular SVC to the adipogenic lineage are still unclear. In this study, we demonstrated that decreasing the level of lysine in the media increased the preadipocyte commitment, differentiation and lipid accumulation in Hanwoo intramuscular SVC.
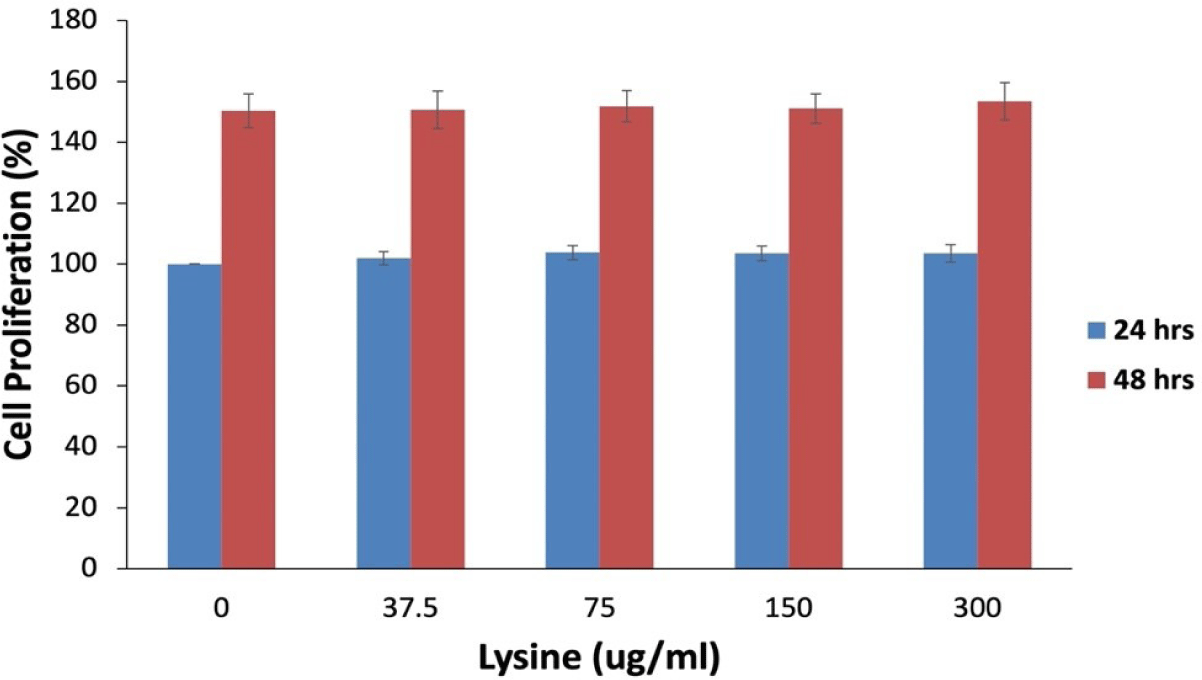
To determine the potential impact of lysine on the viability of SVC from Hanwoo beef cattle, isolated SVC were grown in DMEM BCS media containing 0 to 300 µg/mL of lysine. The effect of lysine levels on the proliferation of SVC is shown in Fig. 1. The lysine concentration of up to 300 µg/mL had no significant effect on the proliferation of Hanwoo SVC. Thus, lowering or increasing the lysine level had no detrimental effect on the proliferation of Hanwoo SVC.
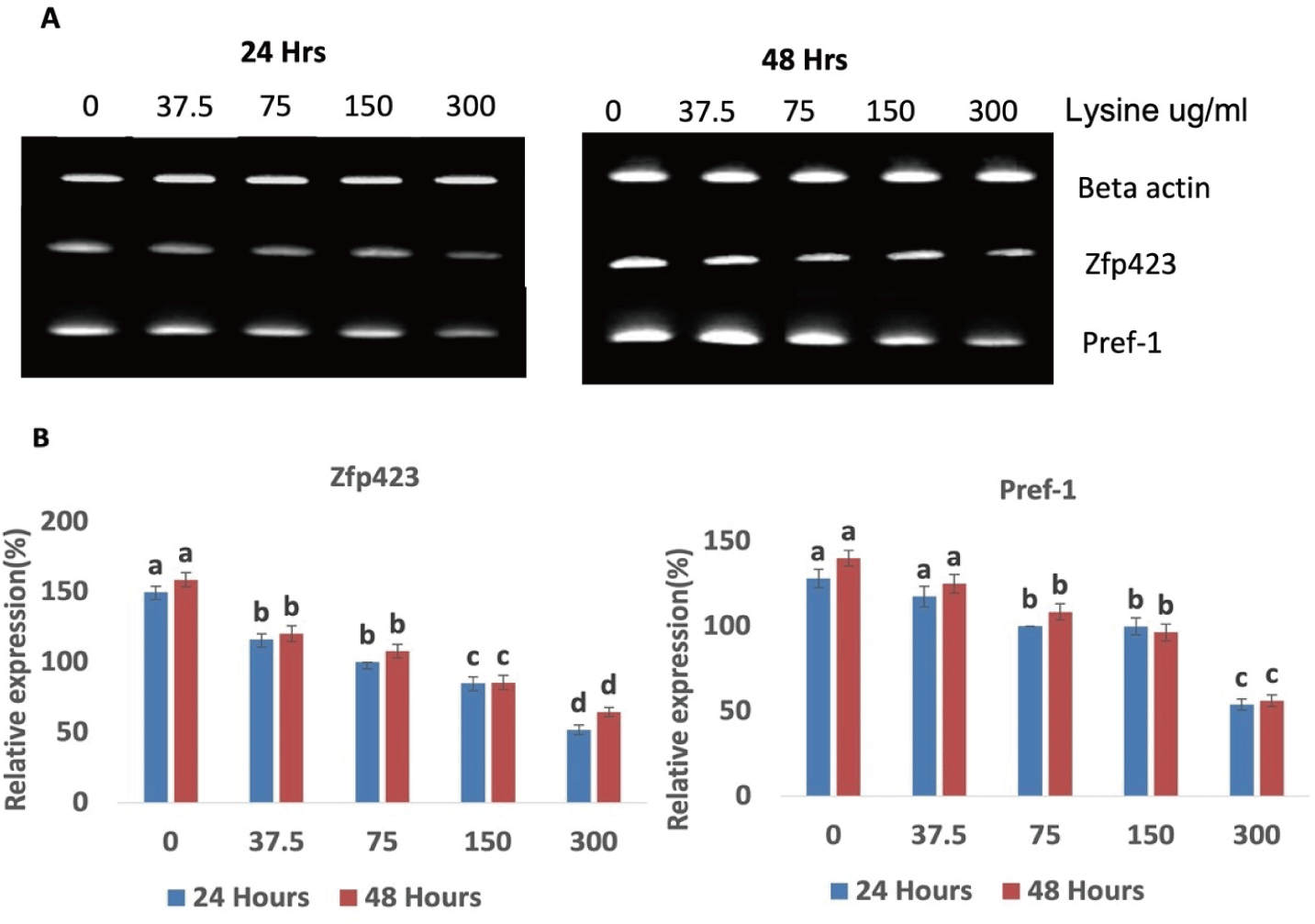
The effect of lysine levels on preadipocyte determination was evaluated. A lower level of lysine in the media resulted in significantly higher expression of Zfp423 and Pref-1 in Hanwoo SVC after 24 and 48 h of incubation (Fig. 2). This finding indicates that preadipocyte commitment is negatively correlated with lysine levels. Zinc finger protein 423 (Zfp423) was recently identified as a key transcriptional factor that induces PPARg expression and is crucial for the early commitment of progenitor cells to adipogenesis [11]. Preadipocyte factor-1 (Pref-1) is highly expressed in preadipocytes but decreases during differentiation and is absent in mature adipocytes. Thus, it is used as a common preadipocyte marker [16–18]. Our results showed that exposure of intramuscular SVC to different concentrations of lysine significantly affected the expression of these two transcriptional markers. Decreasing the concentration of lysine in the growth media significantly increased the expression of the pre-adipocyte commitment marker Zfp423 and preadipocyte determination marker Pref-1 (Figs. 2A and 2B). These data suggests that low lysine levels resulted in increased commitment of intramuscular SVC to the adipogenic lineage by the upregulation of Zfp423. This was confirmed by the increased expression of Pref-1 which is highly expressed in preadipocytes. The findings agree with those of Jing et al. [19], who observed a transient rise in Pref-1 levels in mesenchymal stem cells during the commitment stage. Correlating the adipocyte determination and proliferation data indicates that although lysine levels did not significantly affect the proliferation of Hanwoo SVC, the preadipocyte determination data showed that lower levels of lysine significantly increased the commitment of Hanwoo SVC to the adipogenic lineage, which can potentially be stimulated to become mature adipocytes.
We performed a 10-day differentiation induction experiment in Hanwoo beef cattle SVC cultured in DMEM media with increasing levels of lysine (0, 37.5, 75, 150 and 300 µg/mL) and the level of intracellular lipid concentration was determined using Oil Red-O Staining. As the level of lysine in the differentiation media decreased, the intracellular lipid accumulation is significantly increased (Figs. 3A and 3B). After 10 days of differentiation, Hanwoo SVC grown without lysine significantly accumulated lipid droplets (Fig. 3A). Quantification of relative lipid accumulation obtained by measuring the absorbance at 520 nm after the extraction of the triglycerides stained with Oil Red-O provided confirmation (Fig. 3B).
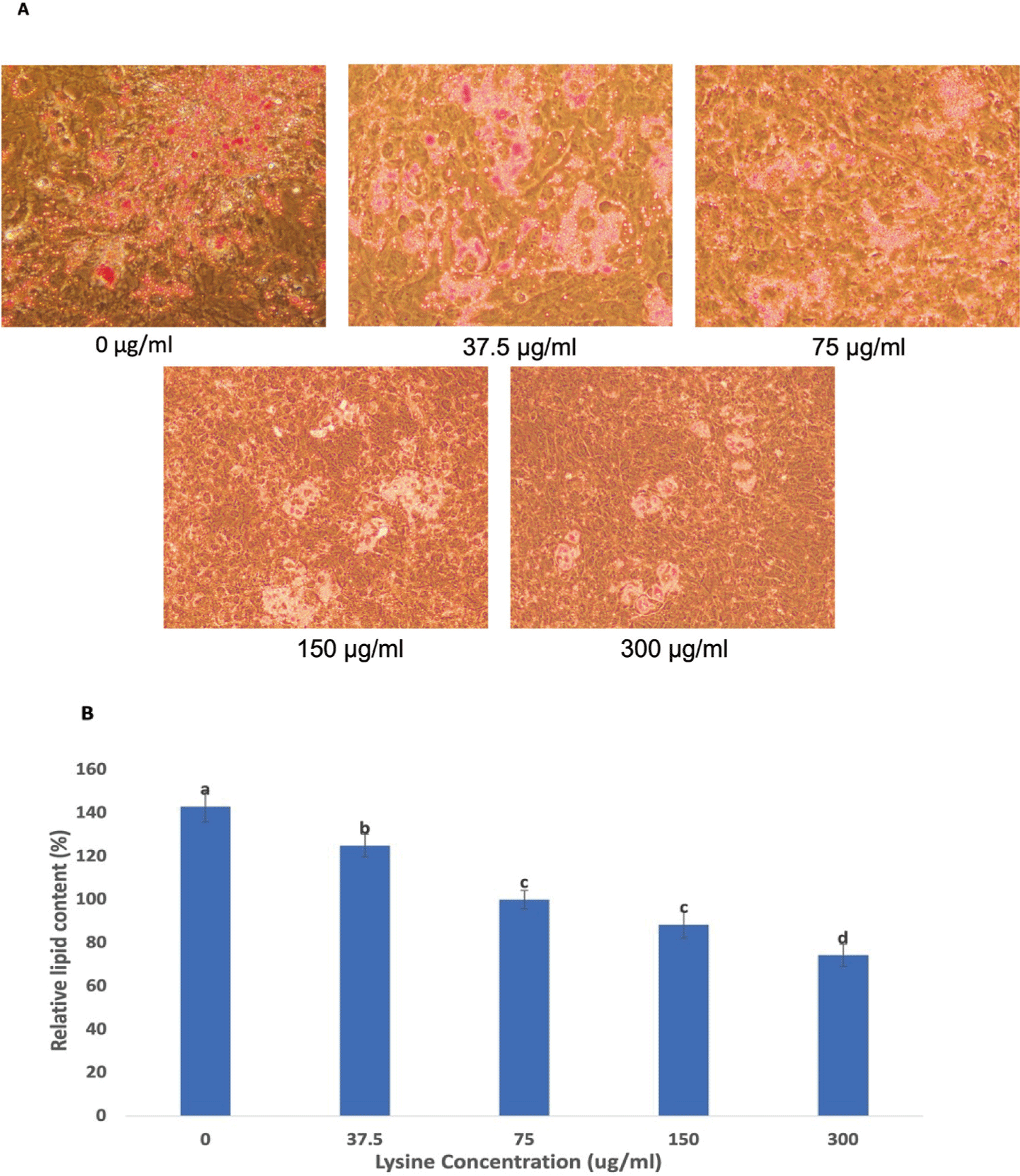
Several studies have previously evaluated the potential effects of lysine on the regulation of lipid metabolism in animals. Goda et al. [9] reported that triglycerides accumulate more in the muscle of rats fed with dietary lysine restriction. Katsuma et al. [20] conveyed that a lysine-deficient diet can increase the lipid content in the skeletal muscle of pigs. Beloor et al. [10] also showed that insufficient supply of lysine causes an increase in the adipogenic potential of bovine preadipocytes. These studies highlight the possibility that lysine plays a significant role in the control of lipid metabolism. Our differentiation data mirrored the claim of these researchers that lysine restriction increases triglyceride accumulation. Oil Red-O staining classifies adipocytes from other cells and has been used as a method to quantify and assess changes in the degree of adipogenesis in cell culture [21]. Oil Red-O staining revealed that a decrease in lysine concentration resulted in increased adipocyte differentiation and relative lipid content (Figs. 3A and 3B). These data can be attributed to the higher preadipocyte determination in Hanwoo SVC grown with low levels of lysine. We observed significantly showed that increased expression of Zfp423 and Pref-1 which are excellent markers for preadipocyte determination. More pronounced preadipocyte commitment due to Zfp423 and Pref-1 upregulation then resulted in an increased level of adipocyte differentiation and relative lipid content which was confirmed by our Oil Red-O data.
To determine the effect of lysine on the possible intracellular mechanisms regulating the differentiation of Hanwoo SVC, the expression of major adipogenic transcription factors during differentiation weas monitored using RT-PCR and Western Blot analysis. The results of RT-PCR and western blot analyses are summarized in Figs. 4A and 4B, and Figs. 5A and 5B, respectively. In the study of Huang et al. [15] and Gupta et al. [11], they showed that Zfp423 is a critical regulator of adipogenesis in bovine SV cells and that upregulation of Zfp423 intensifies the expression of PPARγ and C/EBPa. This pattern was also observed in this study. As the lysine concentration decreased, the expression of Zfp423 in intramuscular SVC increased and in return upregulated the expressions of PPARγ and C/EBPa. Multiple studies have shown that PPARγ and C/EBPα are considered to be the major transcription factors that facilitate adipogenesis [22–24]. PPARγ stimulates and promotes C/EBPα expression and vice versa, which creates a positive-feedback reaction. Additionally, PPARγ and C/EBPα also stimulate the expression of genes that are involved in lipogenesis, lipolysis and insulin sensitivity [25,26]. FABP4 and SREBP-1c, which stimulates lipid accumulation in cells are both regulated by PPARγ and C/EBPα [27]. We observed that lowering the lysine level in the media significantly upregulated FABP4 and SREBP-1c expression.
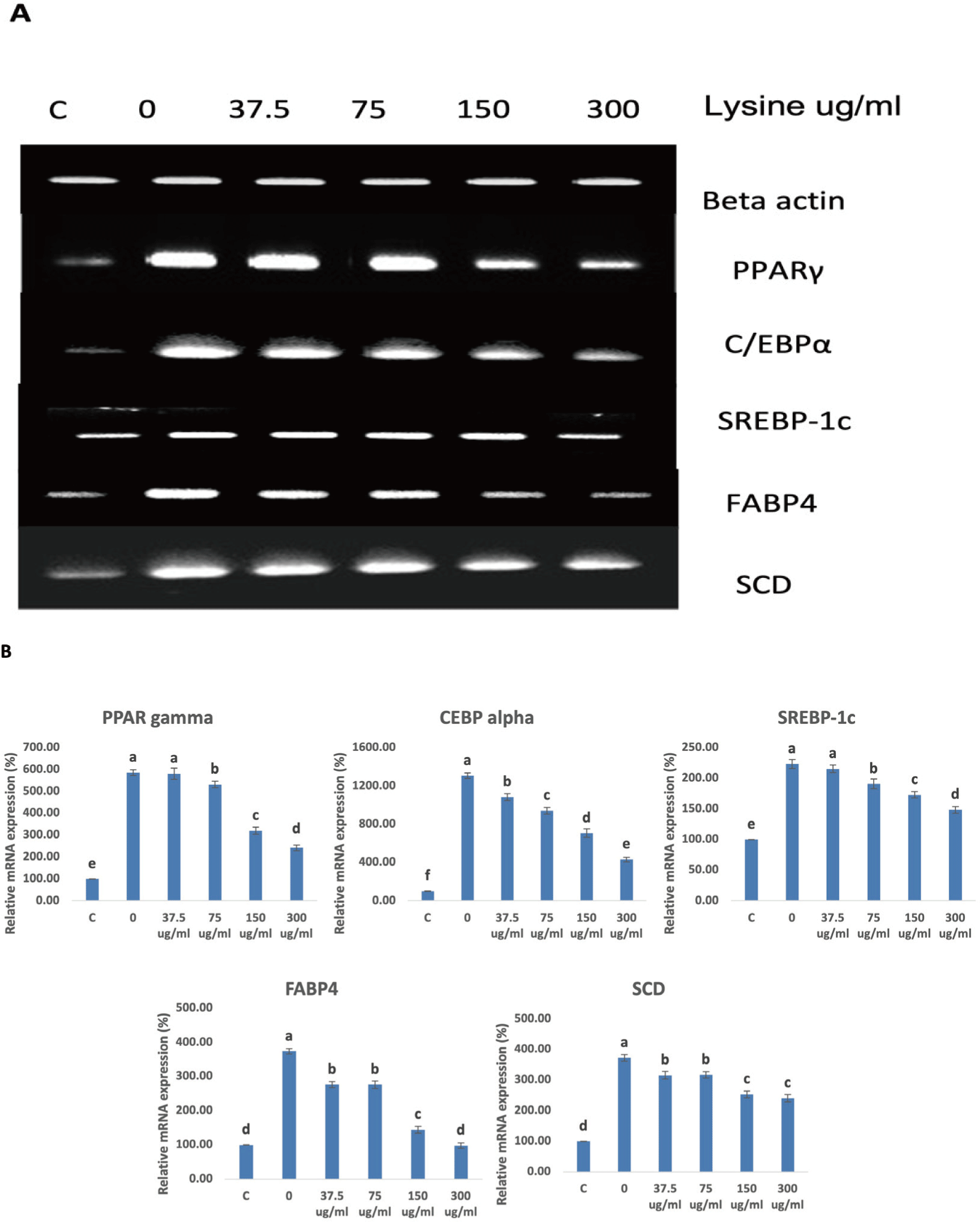
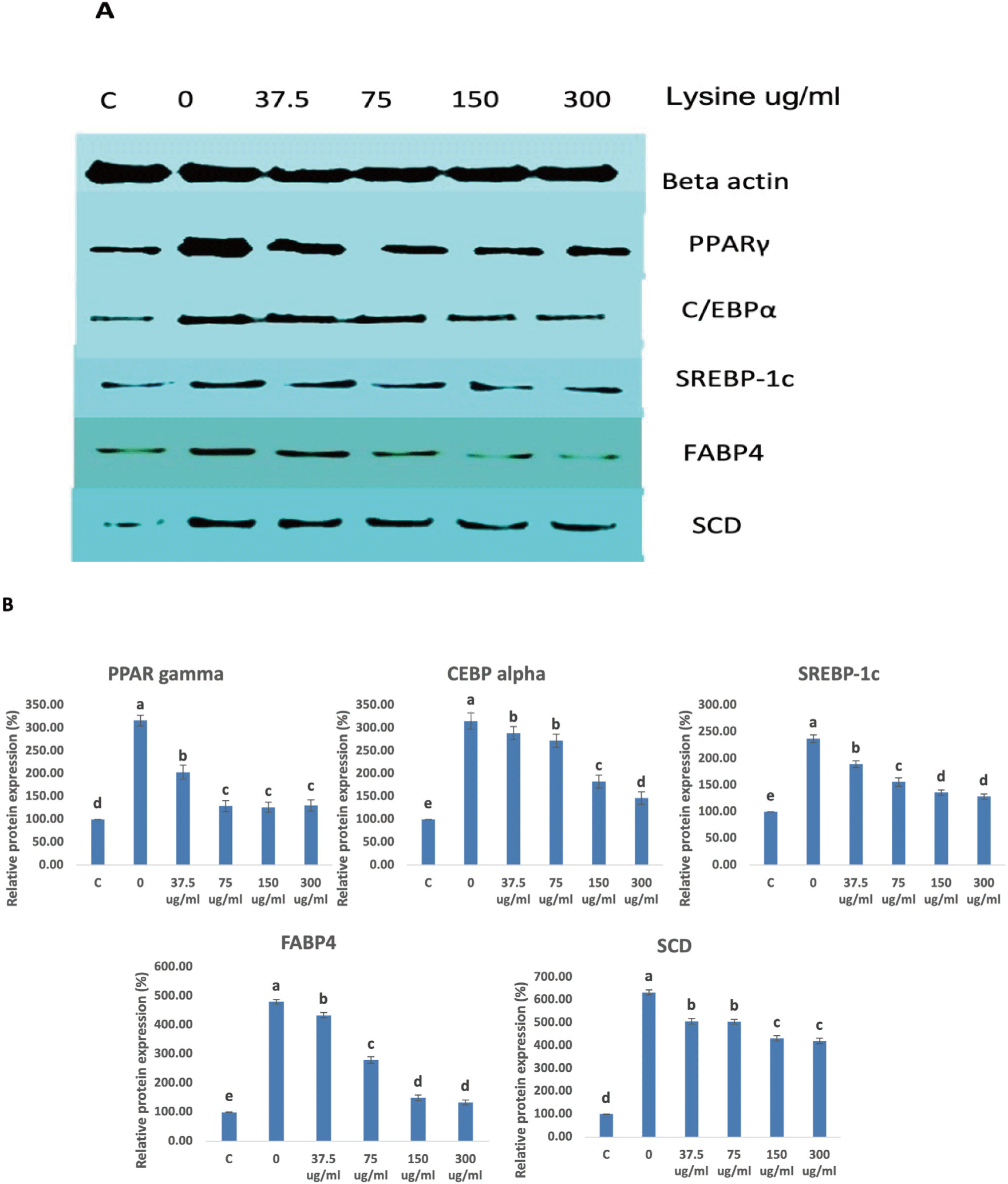
The SCD1 gene has been identified as the major the limiting enzyme in the synthesis of monounsaturated fatty acids (MUFAs) and is transcriptionally regulated by several factors [28]. SCD1 reportedly contributes to the improvement of beef quality by improving the fatty acid composition [29]. Through the action of the SREBP-1c, a lipogenic transcription factor, insulin and glucose mediated stimulation of SCD1 occurs [28,30]. Our data clearly shows that lysine levels significantly affected the expression of SREBP-1c and in return also altered SCD1 expression. This upregulation of SCD1 expression may prove useful to improve the flavor of meat with a healthier fatty acid content.
In summary, our data establish the significant role of Zfp423 in the regulation of adipogenesis in Hanwoo intramuscular SVC. Zfp423 increases the commitment of SVC to the adipogenic lineage while enhancing PPARγ and C/EBPα expression. This cascade of events results in the upregulation of important adipogenic genes, FABP4, SREBP-1c and SCD1. Oil Red-O staining clearly showed enhanced adipogenic differentiation in lysine deficient SVC. These datasuggests that low lysine levels upregulated Zfp423 expression and resulted in an increased preadipocyte determination and enhanced intramuscular adipogenesis in Hanwoo SVC.
