INTRODUCTION
Horses have been agriculturally and culturally important to humans since ancient times. In the past, horses were primarily used for transportation, consumption, herding, breeding, and racing. In modern times, their use has diversified into areas such as leisure and tourism. Mongolian horses are one of the oldest breeds in the world [1], and are under a strong natural selection from the environment as they are typically not stabled or given supplemental feed [2].
A representative example of the use value of the Mongolian horse is the provision of milk, traditional Mongolian drinks, and meat. These horses have also been used as a major form of transportation for herding livestock and nomadic living [3]. A total of 4,093,861 horses are currently bred throughout Mongolia, accounting for approximately 6% of Mongolia’s total livestock breeding head of 67,068,486 [4].
Currently, in Mongolia, seven breeds or lineages (Mongol, Tes, Gal shar, Myangad, Undurshil, Gobi shank, and Darkhad) are recognized as morphologically or genetically distinct. The phylogenetic relationship between these Mongolian populations is yet to be identified.
The equine microsatellite (MS) marker was first characterized by Marklund et al. [5] and Ellegren et al. [6], who isolated (CA) n repeat sets and demonstrated that they are highly polymorphic in horses. DNA analysis offers several potential advantages over conventional paternity testing systems because of its accuracy and specificity [7,8]. Meanwhile, research, fertilization, regulation, and conservation of pure Mongolian breeds has been strongly promoted with the support of jurisdictions and federal boundaries under the new “Genetics of Livestock Resources” Act introduced in December 2017. Accordingly, various studies on genetic characteristics, such as securing traditional breeds, conservation of genetic diversity, genetic relationships with other breeds, and origins, are required that would help establish reasonable strategies for conservation, breeding, exploitation, and use. However, the application of MS markers in the evaluation of the genetic structure of the Mongolian horse population has not yet been conducted; this is the first study on genetic characterization based on 14 MS loci recommended by the International Society for Animal Genetics (ISAG) for Mongolian horse breeds. The purpose of this study was to investigate the genetic relationships and genetic diversity among five different horse breeds in Mongolia.
MATERIALS AND METHODS
A total of 269 whole blood samples were collected from Mongolian horses (Gobi shankh [GCH] = 93, Tes [TS] = 43, Gal shar [GSH] = 53, Darkhad [DKH] = 40, and Undurshil [SHL] = 40) (Table 1). DNA was extracted from blood samples using QuickGene 810, according to the protocol. The concentration and purity of the extracted genomic DNA were measured using an ND-1000 UV-Vis spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA). All experimental procedures involving animals were approved by the Institutional Animal Care and Use Committee of Hankyong National University using the approval code 2021-1.
| Horse types | Sample size |
|---|---|
| Gobi shankh horse (GCH) | 93 |
| Tes horses (TSH) | 43 |
| Gal shar horses (GSH) | 53 |
| Darkhad horses (DKH) | 40 |
| Undurshil horses (SHL) | 40 |
| Total | 269 |
The genetic diversity of Mongolian horses was identified using 14 MS markers (AHT4, ASB17, ASB2, ASB23, CA425, HMS1, HMS2, HMS3, HMS6, HMS7, HTG4, HTG6, HTG7, and VHL20) recommended by ISAG.
Multiplex PCR was conducted using Equine Genotypes Panel 1.1 Kit (Thermo Scientific, Waltham, MA, USA) for genotyping of the 14 MS markers. The reaction mixture (20 μL) was prepared by adding 2 μL genomic DNA (1.0 ng/μL), 9 μL master mix, and 9 μL primer mix. The PCR was then conducted using the GeneAmp PCR System 9700 (Applied Biosystems, Waltham, MA, USA). PCR amplification was conducted using the following conditions: pre-denaturation for 3 min at 98°C, followed by 30 cycles at 98°C for 15 s, 60°C for 75 s, and 72°C for 30 s. The final extension step was conducted at 72°C for 5 min after the final cycle.
Using Hi-Di™ formamide, the amplified PCR products were diluted from 1:50 to 1:100 depending on the concentration, and the diluted PCR products were further diluted using Hi-Di™ formamide and GeneScan™- 500LIZ™ size standard. After conducting capillary electrophoresis using a Genetic Analyzer 3730xl (Applied Biosystems), the size of each MS marker was determined using GeneMapper version 5 (Applied Biosystems). The determined alleles were collated using Microsoft Excel (Microsoft, Redmond, WA, USA) and used for statistical analysis. The mixture for genotyping contained 1 μL of PCR product, 8.9 μL of Hi-Di formamide (Applied Biosystems), and 0.1 μL a GeneScan™ 500LIZ size standard (Applied Biosystems).
Using the MS Toolkit software [9] program, the number of alleles, expected and observed heterozygosity (HExp and HObs, respectively), and polymorphism information content (PIC) values were calculated. Principal coordinate analysis (PCoA) and factorial correspondence analysis (FCA) were conducted using GenAlEx 6.4 [10] and Genetix [11] using each marker-specific allele frequency to identify genetic correlations between groups. Nei’s DA genetic distance [12] was calculated, and phylogenetic trees were estimated using the DISPAN program [13]. Population structure [14,15] was used to estimate the uniformity of the population, and the K value was set to estimate the number of distinct populations (∆K). To calculate the average estimate and standard deviation of each K value, the length of the burn-in period and the number of Markov chain Monte Carlo (MCMC) Reps after burn-in frequency was set, and the optimal K value and genetic uniformity for each cluster were calculated. The results were applied to the Structure Harvester [16] using the Evanno method [17].
RESULTS
The mean number of alleles (MNA), HExp, HObs, and PIC values for the five breeds used in the study are summarized in Table 2. Among the breeds, the values for HExp, HObs, and PIC values were highest for GSH (0.787, 0.789, and 0.751, respectively) and the lowest for DKH (0.751, 0.738, and 0.706, respectively).
The number of alleles, HExp, HObs, and PIC values for the markers used in this study are summarized in Table 3. The MNA was 11 and alleles ranging from 6 (HTG7) to 22 (ASB2) of the 14 selected MSs were identified. HExp and HObs ranged from 0.559 (HTG4) to 0.883 (ASB17) and 0.543 (HTG4) to 0.865 (ASB17), with mean values of 0.767 and 0.752, respectively. PIC values ranged from 0.519 (HTG4) to 0.862 (ASB17), with a mean value of 0.729.
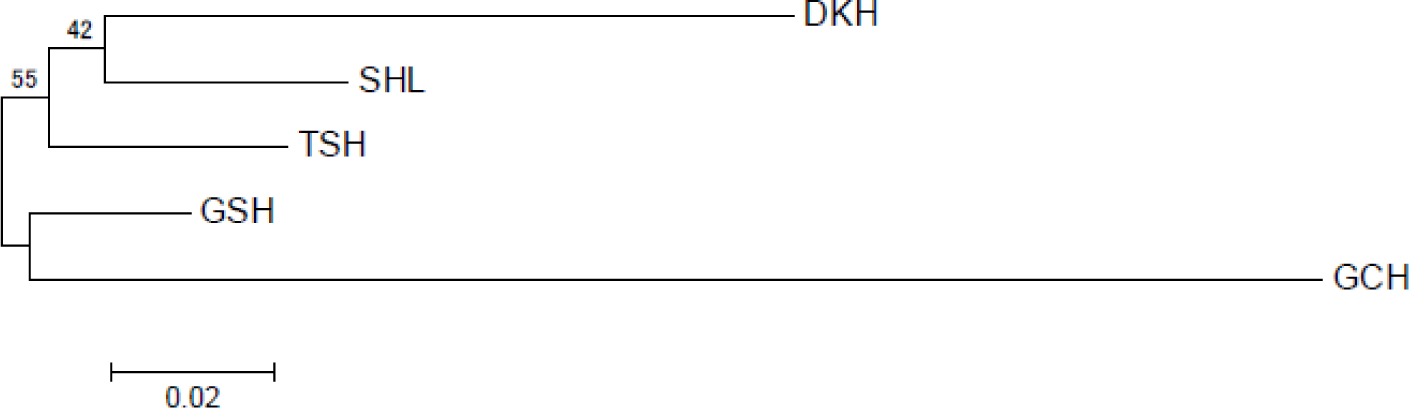
The genetic divergences among the populations based on allele frequencies were calculated according to the DA genetic distance [18]. Table 4 shows the DA genetic distance values for the five populations. Among the populations, the GSH and Tes hourse (TSH) were the closest (DA = 0.0535), and the largest difference was calculated for GCH and DKH (DA = 0.2703). Also, the genetic distance analyzed through statistical tests is indicated. The p-value was the lowest between TSH and GSH (p-value = 0.01269), and the largest between DKH and GCH (p-value = 0.08277). The phylogenetic relationship among the five horse populations using DA genetic distance is shown in Fig. 1. Grouping values were determined using 10,000 repetitive “bootstrap” tests to assess the reliability of the neighbor-joining tree (NJT) and were specified at the branching points of the tree. The Mongolian horse population was mainly divided into two groups. The five populations were divided into two clusters. DKH and SHL were in the first group, and GSH and GCH in the second group. The TSH was located between the two clusters.
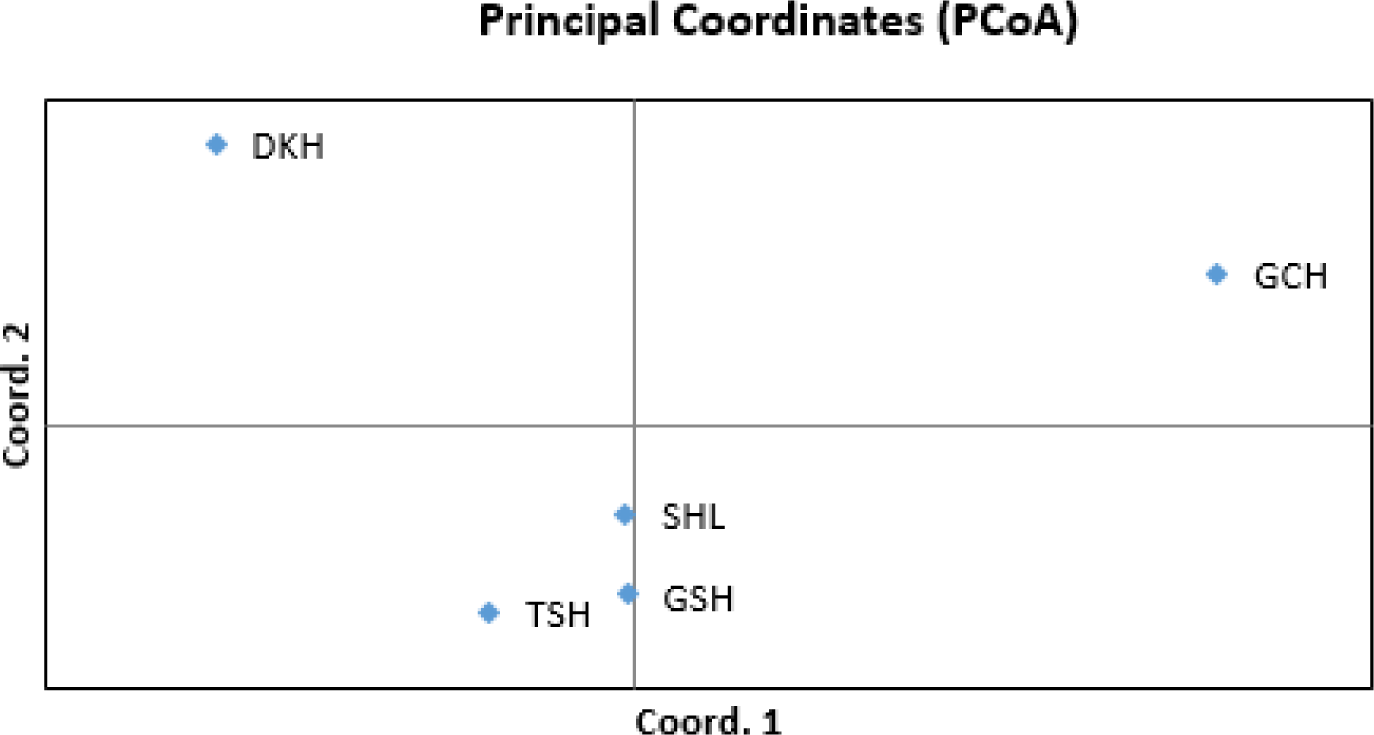
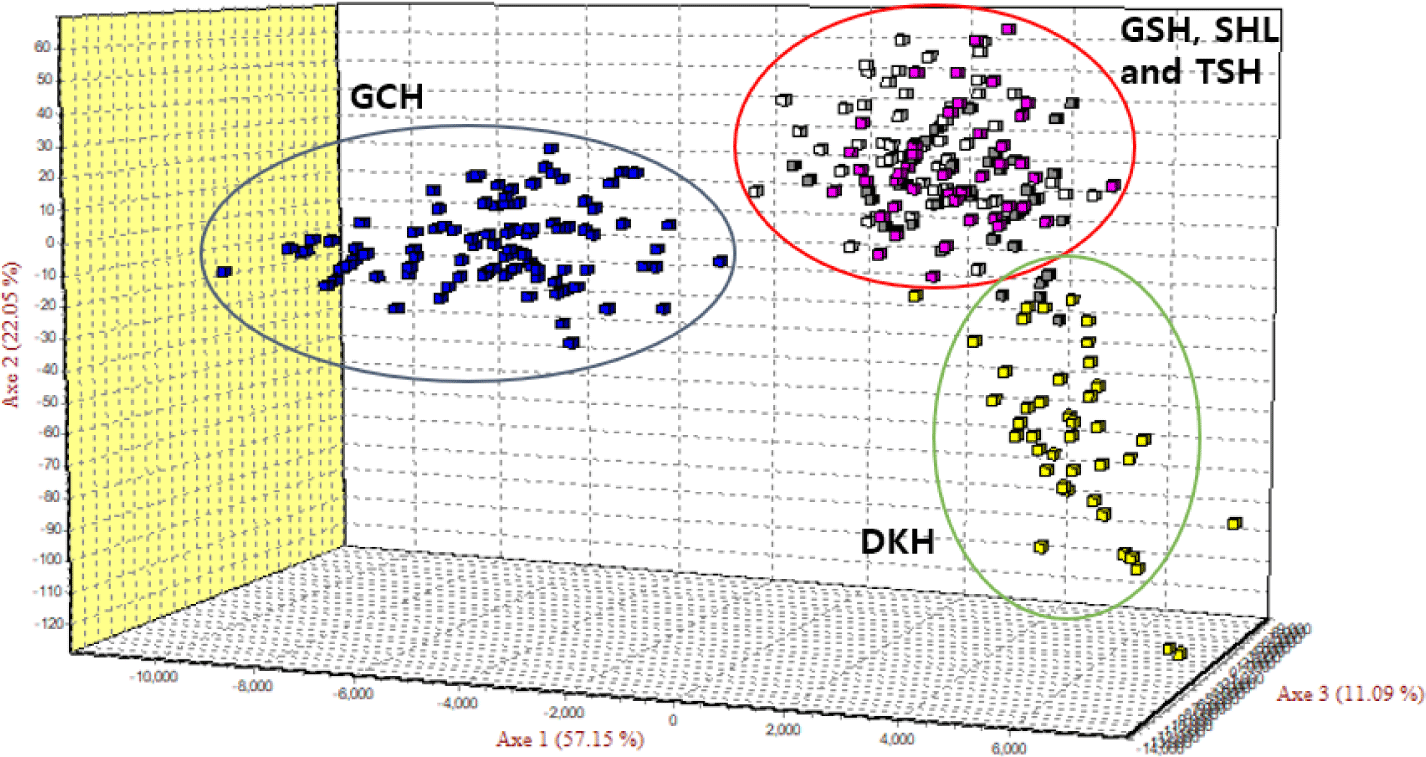
As the phylogenetic tree may not take into account the effects of admixture between the five populations, we conducted PCoA and FCA, using allele frequencies of the 14 MS markers, as an alternative approach to understand the genetic relationships among populations. The principal coordinates contributed to 93% of the variation, including the third ingredient. The first three principal coordinates corresponded to 56.77%, 23.46%, and 13.20% of the total variation. Fig. 2 shows that DKH and GCH were distinct from the other populations. In contrast, TSH, GSH, and SHL were closer in terms of the genetic distance. The FCA analysis revealed that the three dimensions contributed to a total of 90.29 with Axis 1 at 57.15, Axis 2 at 22.05, and Axis 3 at 11.09. Fig. 3 shows that the results of the PCoA analysis were consistent with those of FCA.
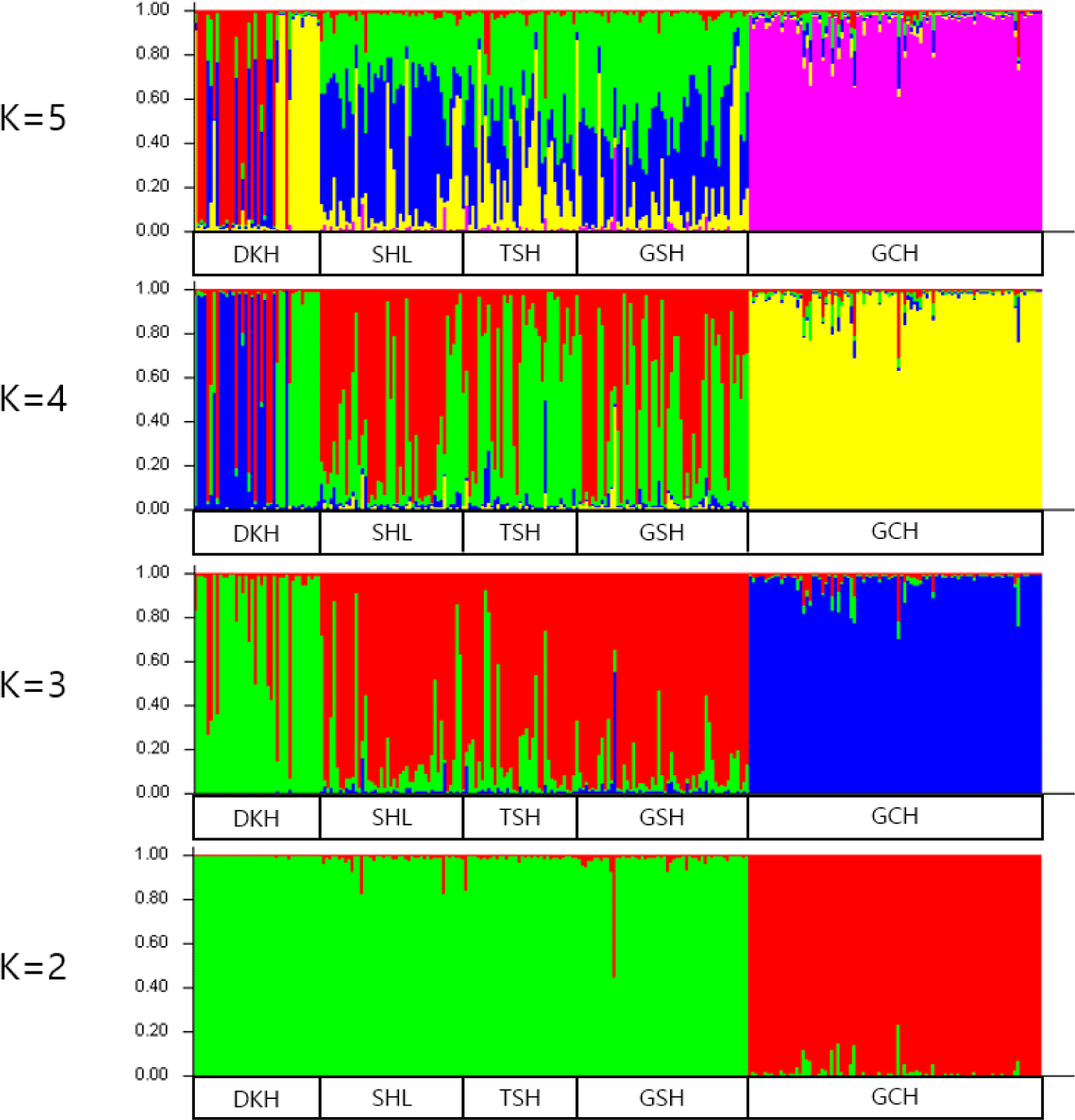
A Bayesian clustering method and population structure were used for clustering algorithms of multilocus genotypes to identify the population structure and pattern of admixture within the populations. Population structure was used to estimate the number of groups. The Bayesian analysis set K values from 2 to 5 and examined the formation of colonies by group (Fig. 4). The bar plot of Fig. 4 shows that DKH and GCH were distinct from the other three populations when the K values were between 3 to 5. The remaining three populations were found to be similar. DKH and GCH were separated into different clusters at K = 3, and the SHL, TSH, and GSH breed were similar. When the K value reached 4 or 5, DKH and GCH were still separated into different clusters, but the SHL, TSH, and GSH formed a single. Burn-in and MCMC repetitions (200,000 times and 1,000,000 times each, respectively) were conducted to estimate the optimal number of groups (∆K values) by setting the K values from 2 to 5, and the ∆K value was estimated using structure harvester. In Table 5, the highest ∆K value (43.763%) was obtained with a K value of 4.
DISCUSSION
In this study, 269 Mongolian horses from five breeds raised in Mongolia were used to analyze the genetic diversity and genetic relationships between each breed. The Tes breed has a large body and feet, which are well-adapted to regions where there is substantial snowfall and temperatures as low as −50°C in the winter, and hot sandy soft soils in summer. Dominant colors are red and reddish-brown. The Gal shar breed is well-adapted to the steppe and moves at a high speed. These horses are small in stature and have sharp eyes, small ears, thick tendons, and strong hooves. Dominant colors are red and yellow. The Darkhad breed is well-adapted to the cold taiga and high mountain ranges at 2,000 m above sea level, mainly living in pastures. Bone development is good, and hair growth begins early, depending on the ecological environment. It has strong vigilance, and its dominant colors are white and brown. The Undurshil breed is well-adapted to the harsh environment of the gobi and has a tall body height, strong hooves, and thin skin. Dominant color is brown. The Gobi shankh is a group of horses native to a specific area; the morphologic characteristics of this horse require further study.
Mongolia is making continuous efforts to protect and utilize its livestock genetic resources, but molecular genetic studies on livestock genetic resources are scarce [19]. Thus, there is a need to conduct modern molecular genetics research. Another important aspect of this study is the assessment of the breed status of the horse population in Mongolia based on genetic differences. As insemination and paternity management in Mongolia has not been controlled for more than 30 years, this study aimed to investigate whether these horse populations have crossed with each other and lost their specific allele frequency.
The purpose of this study was to determine the genetic diversity and relationships of Mongolian horses using 14 MS markers. The results of genetic variability (Tables 2 and 3), Nei’s DA genetic distance (Table 4, Fig. 1), PCoA (Fig. 2), FCA (Fig. 3), and population structure (Table 5, Fig. 3) provided genetic evidence for the differentiation of the five breeds.
MS markers have been previously used to assess the genotypic diversity of heterozygosity and PIC in animal breed selection [20]. The genotyping among the five Mongolian horse populations using the 14 MS markers showed that the population with the highest HExp, HObs, and PIC values was GSH (0.787, 0.789, and 0.751, respectively) and the population with the lowest value was DKH (0.751, 0.738, and 0.706, respectively). Among the 14 MS markers, the marker with the highest values of HExp, HObs, and PIC was ASB17 (0.883, 0.865, and 0.862, respectively) and the marker with the lowest values was HTG4 (0.559, 0.543, and 0.519, respectively). According to Botstein et al. [21], polymorphisms of MS markers are determined using the following criteria: if the sum of HExp is ≥ 0.6, and PIC is ≥ 0.5, then the marker is determined to be highly polymorphic. Therefore, except for the HTG4 marker (HExp: 0.559; PIC: 0.519) used in this study, the other MS markers were considered to be highly useful in analyzing polymorphism in Mongolian horse populations.
Previously, population relationship research on domestic horses has tended to compare their datasets with Mongolian horse samples. These results are almost consistent with the results of this study. Our estimate of genetic diversity (HExp = 0.767, HObs = 0.752, and PIC = 0.729) in Mongolian horse population was found to be in a similar range as reported for Korean horse breeds, HExp = 0.770, HObs = 0.771, and PIC = 0.699 [22], HExp = 0.760, HObs = 0.749, and PIC = 0.728 [23], HExp = 0.809, HObs = 0.833, and PIC = 0.761 [24], HExp = 0.801, HObs = 0.771, and PIC = 0.764 [19] and other horse breeds, HExp = 0.780, HObs = 0.790, and PIC = 0.770 [25], HExp = 0.768 and HObs = 0.728 [26], HExp = 0.797 and HObs = 0.649 [27], and HExp = 0.740, HObs = 0.628, and PIC = 0.706 [28].
The number of detected alleles was similar to findings in the report by Cho et al. [24], in which the MNA of the Mongolian horse population using 11 MS loci was 8.30 alleles per locus. The MNA values in Choi et al. [22] and Ling et al. [26] were less than the results in our study. However, the MNA in the present study was similar to that reported by Jung et al. [27]. When Nei’s genetic distance was analyzed based on alleles to measure the genetic distances between the populations used in the study, the genetic distance between DKH and GCH was 0.2703, which was the furthest. The genetic distances between DKH and TSH, SHL, and GSH were 0.1154, 0.1156, and 0.1258, respectively, and those between GCH and TSH, SHL, and GSH were 0.2042, 0.1925, and 0.1799, respectively. Contrastingly, the genetic distance between TSH and GSH was 0.0535, which was the closest, and the genetic distance between TSH and SHL was 0.0736, and that between GSH and SHL was 0.0668. Therefore, it can be seen that the genetic distances for the 3 populations, except for DKH and GCH, were similar.
The results of genetic relationship analysis based on allele frequencies obtained by genotyping were visualized using PCoA and FCA. The results of PCoA showed that DKH and GCH were genetically distinct from the other three populations. In contrast, TSH, SHL, and GSH were genetically close. The results of FCA showed that the DKH and GCH formed a cluster for each group. However, TSH, SHL, and GSH were not clearly separated. Therefore, both the PCoA and FCA analyses and the population structure analysis showed that the DKH and GCH populations were significantly distinct from the other populations. This suggests that both these populations are genetically distinct from other horse populations and have not lost specific alleles. There are also population groups that are genetically different from other populations. In Mongolia, SHL, GSH, and TSH populations originated from different countries, but were not classified into different clusters. This is possibly because they intercrossed with each other. This was similar to the results of other studies on Mongolian horses [26].
Based on the allele frequencies used in the study, K was analyzed to determine the optimal number of clusters that can separate five populations. When K was between 3 to 5, DKH and GCH showed different patterns from the other three populations, and the other three populations showed similar trends. In addition, by analyzing the most suitable value when K was between 2 to 5 using likelihood estimates, it was found that the most suitable value was ΔK = 43.763 (K = 4). The values of ΔK are used when estimating the number of populations required to divide the five populations used in the study into two to five populations. Therefore, it was most appropriate to divide the Mongolian horses used in this study into four populations.
The results of this study suggest that the MS markers, except for the HTG4 marker, can be used to aid the conservation, traceability, and future improved abilities of horse populations in Mongolia. We hope that the results will be of great help in the breeding, conservation, and protection of their genetic resources. These results are expected to bridge the information gap and contribute to strengthening the conservation and effective management of domestic traditional equine genetic resources, which is expected to provide a theoretical foundation for proper evaluation, protection, use, and studies of origins and evolution. In addition, this study provided basic data to secure the genetic resources of five Mongolian horse breeds.
