INTRODUCTION
Meat consumption is continuously increasing worldwide. As interest in health increases, people are gradually prioritizing quality over quantity. Nevertheless, cooking meat and fish at >150°C may produce carcinogens, such as heterocyclic amines (HCAs) and polycyclic aromatic hydrocarbons [1]. First discovered on the surface of cooked fish and beef in 1977, HCAs are produced via the Maillard reaction at high temperatures; cooking temperature, time, method, type, or ingredient of meat considerably influence HCA production [2,3]. HCAs can produce DNA adducts via CYP1A2 enzymes in the body [4], and these adducts can be transformed into DNA sequences that are genetically consistent with mutations in carcinogenic inhibitory proteins, leading to cancer development [5,6]. Furthermore, HCAs pose human and animal health risks, such as developing tumors in the liver, breast, skin, gastrointestinal tract, colon, and prostate, when they consume meat containing HCAs [7–9].
For this reason, natural materials such as various antioxidants are used to reduce the production of various carcinogens and HCAs; studies have described the inhibition of Maillard reactions by antioxidants and sulfur compounds and the protection of intestinal cells and barriers by probiotics [10,11]. However, studies on the occurrence and reduction of HCAs in pork belly, which is highly favored and consumed by Koreans [12], remain insufficient. Therefore, further research should be performed to reduce carcinogens by seasoning pork belly with natural ingredients such as blackcurrants, gochujang, and natural spices. Blackcurrants are reported to have about 50% of the activity of CYP1A2, the first substance of HCA metabolism, because of the abundance of vitamins and anthocyanins in berries [13,14]. Previous studies found to contain 160–285 mg/100 g of vitamin C, 500–1,342 mg/ 100 g of polyphenols, and 160–411 mg/100 g of anthocyanin [15–17]. In addition, as a traditional fermented food in Korea, gochujang has been a favorite food and excellent nutritional food seasoning used in various foods; it shows anticancer effects against gastric cancer cells [18]. Gochujang contained 0.71–6.40 mg GAE/g of phenol content, and 0.67–0.94 mg QE/g of flavonoids [19–21]. Moreover, natural spices such as garlic, ginger, cinnamon, cloves, octagonal, and licorice contain phenol compounds or flavonoids are used as examples and treatments against oxidative stress, tumor, inflammation, cognitive ability, and memory improvement, respectively [22–27]. Antioxidants isolated from natural spices were quercetin, ferulic acid, eugenol, gallates, darylheptanoids [28,29]. Therefore, this study was conducted to reduce HCA intake by comparing HCA contents in pork belly cooked with various methods. Pork belly seasoned with natural materials was examined to confirm the reducing effect of natural materials on HCA production.
MATERIAL AND METHODS
Pork belly sample was purchased from local market (Anseong, Korea). Since the ratio of fat and protein of pork belly is different, a large amount of pork belly was taken from the same entity and part and homogenized after seasoning to avoid an individual difference. Unseasoned pork belly and pork belly seasoned with natural ingredients were cut to a thickness of 1 cm, vacuum-packed, and frozen (−20°C) until they were used. The frozen samples were thawed at 4°C for 12 h and then heat treated. Pork belly samples were prepared using natural ingredients (garlic, ginger, cinnamon, licorice, star anise, clove, blackcurrant, and gochujang; Table 1). Gochujang was prepared by using sun-dried red pepper, and blackcurrant was salted using 100% concentrated solution. Garlic, ginger, cinnamon, licorice, star anise, and cloves were mixed with each powder, dissolved in purified water to prepare natural spices, and cured. The criteria for mixing natural spices were adjusted using least cost formulation (LCF) program that consider the legal standard, quality terms and least cost [30]. Afterward, 1 kg of pork belly was seasoned with 100 g of each seasoning, mixed well, aged under refrigerated conditions (0°C–4°C) for 24 h, and used in the succeeding experiments.
Pork belly samples were divided into four treatments: unheated (Raw, R), boiling (BO), pan frying (PF), and barbecue (BBQ) method). The cross-section of the pork belly after cooking is shown in Table 1. Cooking temperature was adjusted using an infrared thermometer (TM-969, Lutron, Taiwan). BO was cooked in a stainless steel pot (30 cm in diameter and 31 cm in height) with enough water to soak the sample and at 100°C for 30 min. PF was heated using an electric grill (55 cm width, 31 cm length, and 14 cm height; kitchen art) and cooked at 190°C for 6 min on the front and 6 min on the back. BBQ was cooked in a charcoal brazier (55 cm width and 34 cm length) at ≥ 600°C for 3 min on the front and 3 min on the back.
The HCA standards (2-amino-3-methyle-3H-imidazo[4,5-f]quinoxaline [IQx], 2-amino-3-methyl-3H-imidazo[4,5-f]quinolone [IQ], 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline [MeIQx], 2-amino-3,7,8-trimethyle-3H-imidazo[4,5-f]quinoxaline [7,8-DiMeIQx], 2-amino-3,4,8-trimethyl-3H-imidazo[4,5-f]quinoxaline [4,8-DiMeIQx], and 2-amino-1-methyl-6-phenylimidazo[4,5-b] pyridine [PhIP]) are the six most commonly found and potentially carcinogenic substances in meat (Toronto Research Chemicals, Toronto, Canada). The standard material was dissolved in high-performance liquid chromatograph (HPLC)-grade methanol to prepare a concentration of 500 mg/L and diluted before analysis. Extrelut® NT 20 column and Extrelut® NT refill material were purchased from Merck (Darmstadt, Germany). Bond-elut propyl sulfonic acid (PRS) cartridge (200 mg, 3 mL) and Bond-elut Jr-C18 cartridge (500 mg) were bought from Agilent (Cornia; Santa Clara, CA, USA). All reagents and solutions were purchased and used with HPLC or extra pure grade.
Solid-phase extraction (SPE) was conducted to extract HCAs from pork belly in accordance with the method of Gross and Grüter [31] and modified according to laboratory conditions. The pork belly sample was thawed at 4°C for 24 h and used for the experiment. Then, 3 g of the sample was placed in a 50 mL tube, added with 12 mL of 1 M sodium hydroxide, vortexed for 1 min, reacted at 250 rpm in a water bath at 30°C for 30 min, and decomposed via ultrasonic treatment for 15 min. The mixture was mixed with 13 g of Extrelut® NT refill materials and filled with empty Extrelut® NT 20 columns. Afterward, 75 mL of dichloromethane, 6 mL of 0.1 M hydrogen chloride, 15 mL of methanol/0.1 M hydrogen chloride (45/55 v/v), and 2 mL of distilled water were sequentially flowed and eluted into a pretreated PRS cartridge. The PRS cartridge was then washed with 15 mL of distilled water and connected to a pretreated C18 cartridge in the order of 5 mL of methanol and 5 mL of distilled water. Subsequently, 20 mL of 0.5 M ammonium acetate (pH 8.5) adjust with sodium hydroxide was eluted in two connected cartridges, and the PRS cartridge was removed. A mixed cellulose ester (MCE) membrane filter (0.45 μm, 13 mm) was connected to the C18 cartridge and extracted with 1 mL of 10% ammonia solution in methanol. The extract was concentrated with a vacuum concentrator, dissolved with 200 μL of methanol, and injected into HPLC.
HPLC (AA) used Fortis H2O (250 × 4.6 mm, 5 μm) columns by modifying the methods of Zhao et al. [32]. As the mobile phase, 50 mM ammonium acetate pH 3.6-adjusted acetic acid (A) and acetonitrile (B) were used. The composition of solvent B was set to 10%–60% at 0–15 min, 60%–10% at 15–20 min, and 10% at 20–30 min. The sample was measured for 30 min. Then, 10 μL was injected, flowed at a flow rate of 1 mL/min, and analyzed at 263 nm absorbance.
Animal experiments were conducted at the Animal Laboratory of Chung-Ang University’s Anseong Campus in accordance with the animal ethics guidelines with approval from the Institutional Animal Care and Use Committee of Chung-Ang University (Approval No. 2019-00039). For the animal experiment, Institute of Cancer Research (ICR) mice were used. According to experimental purposes, 4-to-8-week-old mice were purchased from Orient Bio (Seongnam, Korea) and DooYeol Bio (Seoul, Korea). Pico 5030 (Orient Bio) was fed as the basal experimental diet. The inside of the animal room was maintained at 22 ± 3°C and 60 ± 10% humidity, and lighting was adjusted at a 12 h interval. The experiment was conducted for 21 days, and an adaptation period of 7 days was set before the experiment. Then, 25% of the total feed was mixed with pork belly samples, the remaining 75% was mixed with general feed, and 100% of the general feed was provided as a control group. Body weight, feed intake, and water intake were measured once every 3 days during the experiment. Symptoms such as changes in general conditions, hair mass, voluntary activities, reaction rate, and death that might occur during visual appearance and toxicity tests were observed and recorded.
All experiments were repeated thrice, and results were presented as average ± standard deviation. Data were statistically analyzed using SPSS Statistics 26 (IBM, Armonk, USA). Significant differences were determined using a t-test, one-way ANOVA was conducted using a generalized linear model, and post-validation was performed with Tukey’s multiple range test at p < 0.05.
RESULTS AND DISCUSSION
The content of HCAs produced in pork belly cooked with different methods was analyzed. The results revealed four HCAs (IQx, IQ, MeIQx, and 7,8-DiMeIQx) among six types of HCAs in the pork belly treatment group (Table 2). However, HCA was detected in pork belly cooked via the BBQ but not in pork belly cooked with the other methods. The total HCAs were found in pork belly heated via the BBQ method, and 7,8-DiMeIQx accounted for the highest proportion of 28.32 ng/g. Since pork belly BBQ was cooked at ≥ 600°C, a large amount of HCAs was produced by promoting the Maillard reaction. This reaction, which isa non-enzymatic browning reaction, occurs between amino acids and reducing sugars at high temperatures. It begins with an initial condensation reaction in the presence of reducing sugars and amino groups, which produce N-substituted glycosylamine from aldose or N-fructosylamine from Heyns and H2O [33]. Primary amines with nucleophilic amino groups (−NH2) react with carbonyl groups of reducing sugars (−C=O), yielding a Schiff base via beta-elimination, which generates an Amadori or Heyns product via isomerization [34,35]. The Amadori product, which is a ketoamine, degrades via oxidative composition, resulting in the formation of a wide range of reactive carbonyl and dicarbonyl compounds, such as fission products and reductones under alkaline conditions or hydroxymethylfurfural under acidic conditions via dehydration, sugar fragmentation, and Strecker reaction [36]. Some reductones undergo aldol condensation that produces hydroxyacetone, dihydroxyacetone, hydroxyacetyl, pyruvaldehyde, or glycolaldehyde via alcohol and carbonyl condensation. The Strecker degradation is another possible mechanism of HCA production during meat cooking. In this mechanism, acetaldehydes and aminoketones initially form, resulting in the production of volatiles such as pyridines, oxazoles, imidazoles, pyrroles, and thiazoles [37]. The final steps involve an aldol type condensation, aldehyde–amine condensation, and heterocyclic nitrogen compound formation [10,38]. For example, heterocyclic pyridine and pyrazine are produced by hexose and free amino acids via the Maillard reaction; imidazoquinoxaline and imidazoquinoline are formed through the transformation of creatine and aldehyde and the Strecker degradation of free amino acids [7].
Means with different superscript letters within the same row are significantly different from the control at p < 0.05.
HCA, heterocyclic amine; IQx, 2-amino-3-methylimidazo[4,5-f]quinoxaline; IQ, 2-amino-3-methyl-3H-imidazo[4,5-f]quinolone; MeIQx, 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline; 7,8-DiMeIQx, 2-amino-3,7,8-trimethyle-3H-imidazo[4,5-f]quinoxaline; 4,8-DiMeIQx, 2-amino-3,4,8-trimethyl-3H-imidazo[4,5-f]quinoxaline; PhIP, 2-amino-1-methyl-6-phenylimidazo[4,5-b] pyridine; nd, not detected; nq, not quantifiable.
HCAs are formed while cooking meat, poultry, and fish at high temperatures (> 150°C) for long periods. Epidemiological studies have also confirmed that these reaction products show high mutagenic and carcinogenic activities and pose a cancer risk in meat products during high-temperature cooking [39–41]. The results of this study are similar to those of Oz and Kaya [42] and Warzecha et al. [43] who reported that the production of HCAs increases as cooking temperature increases. As shown in this study, the comparison of the concentration of HCAs in pork belly raises the concerns about the high health risk of consumers who cook BBQ meat at > 600°C at home. In addition, this study demonstrated that the Maillard reaction was strongly influenced by proteins in meat at high temperatures (> 600°C). However, consuming BBQ meat every day under the conditions used in this study is unreasonable. A previous study also reported that a 70 kg adult male can develop cancer if he ingests 1.82 g of HCA (e.g., IG), which is equal to about 3,000 tons of bacon [44]. In fried beef patties, 0.35 ng/g HCA is found in commercial hamburgers, and 142 ng/g HCA is detected in beef patties cooked over a barbecue [45]. Furthermore, the amount of HCAs produced when meat products are cooked is extremely low [46]. Therefore, HCA is detected in meat cooked at very high temperatures, not under general cooking conditions considered to be a normal range that does not pose a risk on human health. In this study, high-temperature BBQ method was selected to analyze the effect of reducing HCAs by natural materials based on the results; other cooking methods were excluded because HCAs were not detected.
Animal experiments showed a low feed efficiency in PF and BBQ; visual observation, weight, and length measurement of organs after the end of the experiment revealed no toxic abnormalities or significant changes due to the ingestion of pork belly cooked with BBQ method. Conversely, hematological analysis indicated that PF was significantly lower than other samples in the hematocrit (HCT) parameter (Table 3). HCT indicates the degree of distribution of red blood cells in the blood and decreases below normal (approximately 36%–55%) when red blood cell production is reduced or destroyed; a low HCT mostly indicates anemia, which can be an indication of suspected bleeding, cirrhosis, or cancer [47,48]. In addition, there was no significant difference in blood urea nitrogen (BUN) and crea levels between samples. BUN represents the concentration of urea nitrogen in the blood, and the normal value is 8–33 mg/dL; increased test results may lead to kidney failure, kidney disease, or gastrointestinal bleeding [49]. Crea is a waste of creatine produced in muscles and has a normal value of 0.2–0.9 mg/dL; increased test results may lead to suspected kidney bleeding, kidney stones, or bacterial infection of the kidney [50]. PF shows the results of blood and serum analysis within the normal range, but it significantly differs in HCT compared with CTL. As mentioned above, a low HCT level can be estimated to develop anemia because iron deficiency can occur by a decrease HCT level. In a previous study, heme iron was measured using four cooking methods in raw and cooked lamb, and the result showed that the total iron and heme iron are lower in pan frying and grilling method than in other methods (raw and boiling) [51]. Turhan et al. found that the total and heme iron contents of anchovy (Engraulis encrasicholus) are the highest in grilled samples but the lowest in boiled samples. These differences may be due to sample types (meat and fish) and different time and cooking methods [52]. Although significant differences were found in the hematological examination (i.e., red blood cell, HCT, mean platelet volume, and procalcitonin), these differences remained within normal ranges [53−58]. Therefore, this study suggested that pork belly cooked by different cooking methods did not induce toxicity and pose human health risk because all values were within the normal range of hematological parameters.
Other analysis items of CBC and serum are not shown. Data presented as mean ± standard deviation (n=3).
CTL, regular diet; G1, regular diet+raw pork belly; G2, regular diet+boiled over cooking pork belly; G3, regular diet+pan fried over cooking pork belly; G4, regular diet+barbecue over cooking pork belly.
Table 4 shows the content of HCAs produced when pork belly was cooked via the BBQ method. Overall, HCA contents were detected in BBQ pork belly samples; however, considering the toxicology test results, their levels unlikely affected the human health risk. Among the pork belly marinated in natural materials (such as natural spice, blackcurrant, and gochujang), pork belly containing blackcurrant did not have HCAs except for 7,8-DiMeIQx. The comparison of the total amounts of HCA production in terms of the different seasonings used showed that the least amount was observed in pork belly seasoned with blackcurrant. Specifically, the amount was decreased by about 54% compared with that of general pork belly. Blackcurrants (Ribes nigrum L.) have an antioxidant activity and high contents of polyphenol (ferulic acid) and anthocyanin; they also exhibit higher antibacterial activities against pathogenic and spoilage bacteria (Bacillus subtilis, Listeria monocytogens, Pseudomonas aeruhinosa, and Escherihia coli) than other fruits [59,60]. Previous studies reported that blackcurrant as a natural antioxidant is responsible for the inhibition of HCAs or biogenic amines, which are produced in meat and meat products under extreme cooking method with high temperatures, by decreasing oxidative stress and genetic toxicity [34,59,61]. A high intake of HCAs is associated with oxidative stress [62]. At the beginning of HCA metabolism, reactive species are produced by cytochrome P450 (CYP), which can cause oxidation of lipids, nucleic acids, and proteins, resulting in cell damage, oxidative stress, and biological dysfunction that leads to the potential development of cancer and cardiovascular disease [63,64]. CYP1A2 is induced to accelerate N-acetyltransferase activity, producing N-hydroxylarylamine; N-hydroxylarylamine is further converted by N-acetyltransferases (NATs) such as NAT1 and NAT2, which promote DNA adducts related to cancer development [6,34]. Polyphenols attenuate the mutagenicity of HCAs through the competitive inhibition of NADPH CYP reductase, thereby indirectly interfering with the CYP450 activity [65]. In addition, the inhibitory activity of polyphenols on HCA production is mainly correlated with the free radical scavenging activity and trapping of reactive carbonyl species, such as phenylacetaldehyde, which is a thermal degradation product of phenylalanine relate to form PhIP [39]. For these reasons, blackcurrants with antioxidant activities to minimize oxidation or DNA adduct production in meat constituent when it is applied to high temperature or cooking can prevent the production of carcinogenic substances by interfering with HCA metabolism via their antioxidant activity. Moreover, other indirect mechanisms can regulate the Maillard reaction in meat products, and previous studies demonstrated the effect of natural products on the reduction of HCAs [66,67]. In the present study, gochujang ingredients increased by about 8% compared with that of regular pork belly, indicating that gochujang is the seasoning that produces the highest amount of HCAs. In pork belly seasoned with gochujang, the content of IQx was significantly higher than that in regular pork belly. Although the exact mechanism is unclear, the production of HCAs may have increased because the Maillard reaction is promoted at ≥ 600°C by a large amount of sugar, starch, and amino acid contained in gochujang materials. Sugar is an important factor in providing the sweetness of gochujang that contains a high amount of free sugar, which can be fructose, glucose, and maltose ranging from 300 mg/g to 400 mg/g [68]. In addition, free amino acids detected in commercial gochujang are glutamine, asparagine, proline, arginine, and leucine. Soybean-based gochujang increases the reducing sugar content at 4.87%–9.06% to 11.40%–17.71% up to 60 days of fermentation [21]. Kim and Lee demonstrated that the total sugar content and reducing sugar contents of traditional gochujang are 18.58%–30.45% and 13.96%–19.03% during fermentation, respectively [3]. The free sugar compositions of gochujang are glucose (4.43%), fructose (1.44%), maltose (3.98%), and maltotriose (1.92%) [69]. Increased temperature plays a role in HCA production; Lee and Shibamoto reported that browning is promoted by sugar or amino acid during heating [70]. Hasnol et al. showed that grilled chicken marinated with different types of sugars (table sugar, brown sugar, and honey) can produce HCAs. Among the sugars, the level of HCAs except IQx in the samples marinated with table sugar is higher than than that in samples marinated with brown sugar [71].
Means with different superscript letters within the same row are significantly different from the control at p < 0.05.
IQx, 2-amino-3-methylimidazo[4,5-f]quinoxaline; nq, not quantifiable; IQ, 2-amino-3-methyl-3H-imidazo[4,5-f]quinolone; nd, not detected; MeIQx, 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline; 7,8-DiMeIQx, 2-amino-3,7,8-trimethyle-3H-imidazo[4,5-f]quinoxaline; 4,8-DiMeIQx, 2-amino-3,4,8-trimethyl-3H-imidazo[4,5-f]quinoxaline; PhIP, 2-amino-1-methyl-6-phenylimidazo[4,5-b] pyridine; HCA, heterocyclic amine.
Antioxidants such as alicin, eugenol, gingerol, annetol, and glyciridine in natural spices likely cause free radical scavenging activity, resulting in reduced HCAs [13,61,72]. Phenolic compounds, vitamins, and anthocyanins in blackcurrants reduce HCAs in meat heated at high temperatures [13,61]. Therefore, blackcurrant can reduce HCA production by more than 50% because of high-temperature heating. Our preliminary study on the HCA inhibitory activity of antioxidants suggested that the HCA content in the sample marinated with vitamin C and gochujang was lower than that in the sample marinated with gochujang (data not shown). Numerous strategies for regulating Maillard reaction in foods by adding functional ingredients such as vitamins and peptide derivatives have been found to inhibit Maillard reaction by targeting reactive sites, intermediates, or products. For example, synthetic antioxidants remove reactants (reducing sugars or amino groups) or compounds with sulfur groups, and some compounds inhibit Maillard reactions by scavenging Maillard-derived radicals and trapping Strecker aldehydes and acrylamide [66]. Therefore, using natural materials with antioxidant activities that interfere with HCA metabolism and inhibit the Maillard reaction or avoiding sugar-rich ingredients in meat products may effectively minimize potential carcinogenic substances that can only occur under extremely high temperatures or not general cooking conditions.
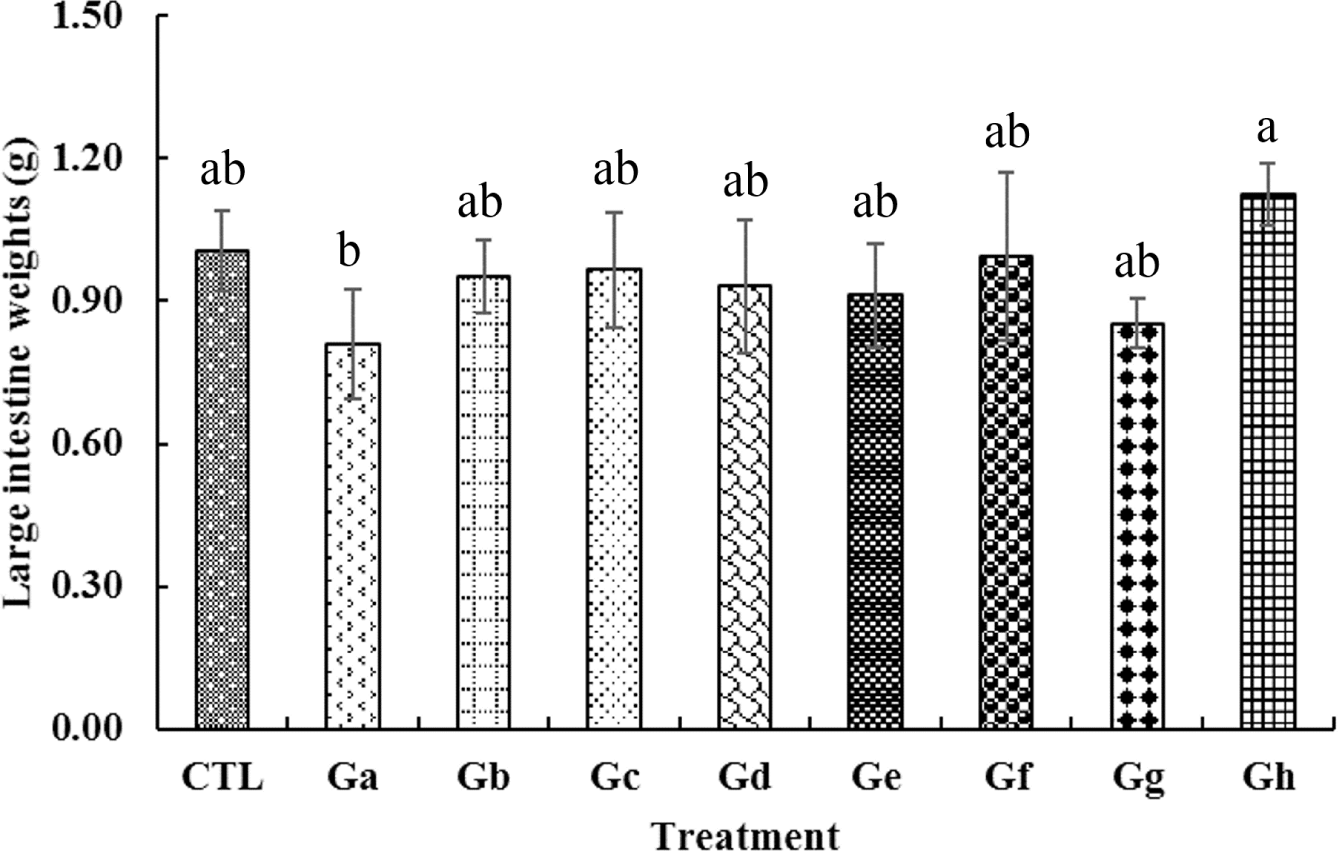
In this study, the levels of hematological, serological, and length of the large intestine for indirect colitis expression were measured using the blood and organs in mice as predictors of toxicity in a mouse model that ingested pork belly marinated with natural materials. The animal experiment demonstrated that the feed efficiency of the experimental animals that consumed seasoned pork belly was higher than that of the experimental animals that consumed general feed. Conversely, no significant difference was observed in the visual observation, weight, length of the organ hematology and serological analysis after the end of the experiment (data not shown). Although the length of the large intestine did not show any difference in all the treatment groups, the weight of the large intestine slightly increased in the gochujang pork belly treatment group heated by the BBQ method (Fig. 1). Park and Kim suggested that the increase in the ratio of the weight to the length of the large intestine is one of the indicators that exacerbate colorectal inflammatory responses [73]. The colon weight/length ratio in the colitis model increases compared with that in the non-colitis model, which can predict the development of colon edema [74]. Although pork belly marinated with gochujang by the BBQ method may suggest the possibility of colorectal inflammation through colon weight, its toxicity level in the animal study was not dangerous; blackcurrant and natural spices did not also induce toxicity in the mouse model. Therefore, no toxicity occurred when pork belly was cooked using different cooking methods or seasoned with natural materials. Furthermore, the intake of pork belly seasoned with any of the natural materials did not elicit toxic effects except when general cooking was not performed.
CONCLUSION
In this study, the amount of HCA production that can occur when pork belly is cooked at a very high temperature was investigated. Various natural materials that can reduce of HCA production were selected to confirm the reduction effect, and the material with the highest reduction effect was identified. The results confirmed that HCAs was produced only when pork belly was cooked via BBQ at very high temperature for a long time, and the highest HCAs was 7,8-DiMeIQx. Furthermore, seasoning pork belly with natural materials containing antioxidants could reduce the production of HCAs, even if pork belly was heated to high temperatures. Although the toxicity levels were within normal range regarding to human health risk, the method yielding the relatively high toxicity among various cooking methods was barbecue, and the natural material with reduction effect for toxicity was blackcurrant. Thus, this study confirmed not only a manufacturing method capable of reducing HCAs but also the reduction of HCA production through the addition of an antioxidant.