INTRODUCTION
Obesity is a physiological state defined by an excessive accumulation of adipose tissue in the body. Although controversial, canine obesity is generally defined as over 20% of the ideal weight of adult dogs depending on the variety, body size, and age [1]. Additionally, the obesity of dogs can be estimated by body condition score based on observation and palpation [2]. Globally, the obesity rate in companion dogs is estimated to be approximately 59%, and it is increasing yearly [3]. This trend directly causes an increase in the health care cost incurred by the pet owner [4]. Diseases associated with obesity in humans such as musculoskeletal disorders, cardiovascular diseases, diabetes, and osteoarthritis are also observed in dogs, as they experience severe health complications and deterioration when obese [5]. These diseases also cause several metabolic disorders [6,7] which reduce the life span [8] and quality of life [9] of companion dogs. Hence, obesity prevention and weight control in companion dogs is an important social issue and a prerequisite for animal welfare. Obesity, in animals and people, is attributed to an imbalance between energy intake and energy expenditure caused by excessive food intake and insufficient physical activity [10]. Although restriction of food to reduce energy intake may result in a temporary reduction in body weight, it is not a successful strategy for the long-term goal of weight control [11]. In addition, there are practical difficulties associated with weight control of companion dogs, because it requires active intervention and a strong will of dogs and pet owners. To achieve successful long-term weight control in companion dogs, it is essential to provide a nutritionally balanced diet composed of ingredients that keep obesity in check.
Resistant starch (RS) has physiological functions similar to dietary fibers; it is degraded by the gut microbiota but not by the digestive enzymes in monogastric animals [12]. RS has been classified into five types by their features: RS type 1 is the starch of physically inaccessible to enzymes, RS type 2 is high-amylose starch, RS type 3 is retrograded starch that is formed mainly due to heating-cooling cycle, RS type 4 is chemically modified starch, and RS type 5 is the formation of starch that the amylose-lipid complex [12,13]. Hence, RS reduces blood glucose and body fat mass, and enhances insulin sensitivity [14-16]. In rats, the intake of RS not only reduces body fat mass [17] but also the size of the mesenteric fat [16,18]. In a clinical study, the intake of RS triggered the expression of genes encoding hormone sensitive lipase, adipose triglyceride lipase, and perilipin, which are attributed to lipid metabolism; RS also decreased the level of homeostatic model assessment for insulin resistance [19]. Several studies have reported the positive anti-obesity effect of RS; however, only a few studies have analyzed RS feed in dogs while monitoring the digestibility and postprandial blood glucose. Moreover, to date, no study has associated RS supplements with anti-obesity in canines. Therefore, this study was conducted to investigate the anti-obesity potential effects of corn starch with an increased RS content in healthy adult beagle dogs. We evaluated the anti-obesity effects of corn RS feeding on body weight, nutrient digestibility, and obesity-related hormones in beagles.
MATERIALS AND METHODS
All animal experiments were approved by the Institutional Animal Care and Use Committee of the National Institute of Animal Science, South Korea (Approval Number: NIAS-2019-370). Dogs used in this study were observed according to the ethical guidelines for animal protection.
Four spayed beagle dogs and six castrated beagle dogs (2.9 ± 0.05 years) were enrolled and divided into a control group (CON, n = 5, two females and three males) and fed a diet consisting of rice and chicken breast meal, and a treatment group (TRT, n = 5, two females and three males) and fed a diet of corn-resistant starch and chicken breast meal. Each dog was housed in an independent room (170 cm × 210 cm) maintained at consistent room temperature (22 ± 1°C) and 60 ± 10% relative humidity during the study period. Each dog was fed twice a day at 10:00 and 16:00 h with a 1.2-fold higher intake (132 kcal × kg BW0.75) than the daily recommended metabolic energy requirement (MER) suggested by the European Pet Food Industry Federation (FEDIAF). The dogs were provided ad libitum drinking water [20]. For the last week of the experiment, each experimental diet containing 0.5% chromium oxide was provided to the CON and TRT groups. The amount of feed intake was recorded each day and the body weight was measured every two weeks. The rate of body weight change was calculated by dividing the body weight for each week measured at 2-week intervals by the initial body weight (0 weeks), and multiplying by 100. Data for each individual were tested as replicates.
The heating-cooling cycle to increase the RS (type 3) content of corn starch was as follows; steam heating at over 100°C for 30 min, and cooled at 4°C for 24 hours. The RS content in rice, corn starch, and experimental diets was measured using Megazyme RS assay kit (K-RSTAR, Megazyme, CT, USA), according to the manufacturer’s manual. The RS content of rice and corn starch was 2.33% and 6.69%, respectively, and the degree of starch degradation by amylase enzyme in vitro was 97.0% and 92.5%, respectively. The rice and corn starch with increased RS content were used as raw ingredients for each experimental diet.
Diets were prepared according to a previously described method with minor modifications [21]. The raw materials of the diets were commercially available as food products that were mixed, heated, molded, cut, dried, and cooled. The experimental diets used to evaluate nutrient digestibility were prepared separately using the same method; each experimental diet was supplemented with 0.5% chromium oxide. Table 1 lists the formulation ratios and chemical compositions of the diets used in this study.
Vitamin and mineral premix supplied per kg of diets: 3,500 IU vitamin A; 250 IU vitamin D3; 25 mg vitamin E; 0.052 mg vitamin K; 2.8 mg vitamin B1(thiamine); 2.6 mg vitamin B2 (riboflavin); 2 mg vitamin B6 (pyridoxine); 0.014 mg vitamin B12; 6 mg Cal-d-pantothenate; 30 mg niacin; 0.4 mg folic acid; 0.036 mg biotin; 1,000 mg taurine; 44 mg FeSO4; 3.8 mg MnSO4; 50 mg ZnSO4; 7.5 mg CuSO4; 0.18 mg Na2SeO3; 0.9 mg Ca(IO3)2.
Blood samples were collected from the anterior cephalic vein after the dogs were subjected to fasting for at least 16 h at the beginning (0 weeks) and the end (16 weeks) of the experiment. The collected blood was immediately divided between EDTA vacutainer tubes (ref 367861, BD Vacutainer, NJ, USA) and serum vacutainer tubes (ref 367812, BD Vacutainer). The blood collected in the EDTA tubes were used in the complete blood cell count (CBC) analysis using an automated blood analyzer ProCyte Dx (IDEXX Laboratories, Westbrook, ME, USA) immediately after collection. The blood collected in the serum vacutainer tubes were centrifuged at 1,650×g for 15 min to separate the serum; the serum supernatant was stored at –80°C until further use. Serum biochemical composition analysis was performed using an automated biochemistry analyzer, Hitachi 7180 (Hitachi High-Technologies, Tokyo, Japan). Adiponectin (MBS2607889, MyBioSource, San Diego, CA, USA), immunoglobulin G (IgG, ab157701, Abcam, Cambridge, MA, USA), interleukin-6 (IL-6, ab193686, Abcam), insulin (MBS031096, MyBioSource), leptin (MBS037935, MyBioSource), and tumor necrosis factor-α (TNF-α, ab193687, Abcam) were measured by enzyme-linked immunosorbent assay using a microplate spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) in duplicates, according to the manufacturer’s instructions.
The feces of the dogs were collected for five days after the dogs fed a diet containing 0.5% chromium oxide. The fecal samples were stored at –20°C until analyses. The diets and fecal samples were dried in a hot-air oven at 75°C and homogenized for further analyses. The chemical composition of the diets and fecal samples were analyzed according to standard Association of Official Analytical Chemists methods [22]. Nutrient digestibility was calculated using the following equation:
All statistical analyses were performed using SPSS version 17.0 (SPSS Statistics, Chicago, IL, USA). The data were presented as mean ± SEM. All data were analyzed using the student’s t-test. Time-dependent changes between 0 and 16 weeks were analyzed using a paired t-test. Differences were considered statistically significant when p < 0.05.
RESULTS
To promote weight gain, the dogs were fed a diet with a 1.2-fold increase in the MER. The metabolizable energy (ME) intake was 941.9 ± 40.09 kcal/day in CON and 944.8 ± 51.99 kcal/day in TRT groups, indicating no significant variation (p = 0.966; Table 2). The body weight of dogs in each group after 16 weeks was 15.1 ± 0.96 kg in CON and 14.1 ± 1.42 kg in TRT, which indicated a lower body weight increase in TRT than in CON (p < 0.05). Compared to the start of the experiment, the body weight gain (BWG) increased by 1.4 ± 0.24 kg in CON, whereas a marginal increase in BWG of 0.22 ± 0.42 kg was observed in the TRT groups (p < 0.05). Consequently, the feed conversion ratio (FCR) was 4.0 ± 0.55 in CON and 0.4 ± 1.19 in TRT after 16 weeks, indicating that the FCR was lower in TRT than in CON (p < 0.05). Considering the individual variations in body weight, the final body weight was normalized using the initial body weight (Fig. 1).
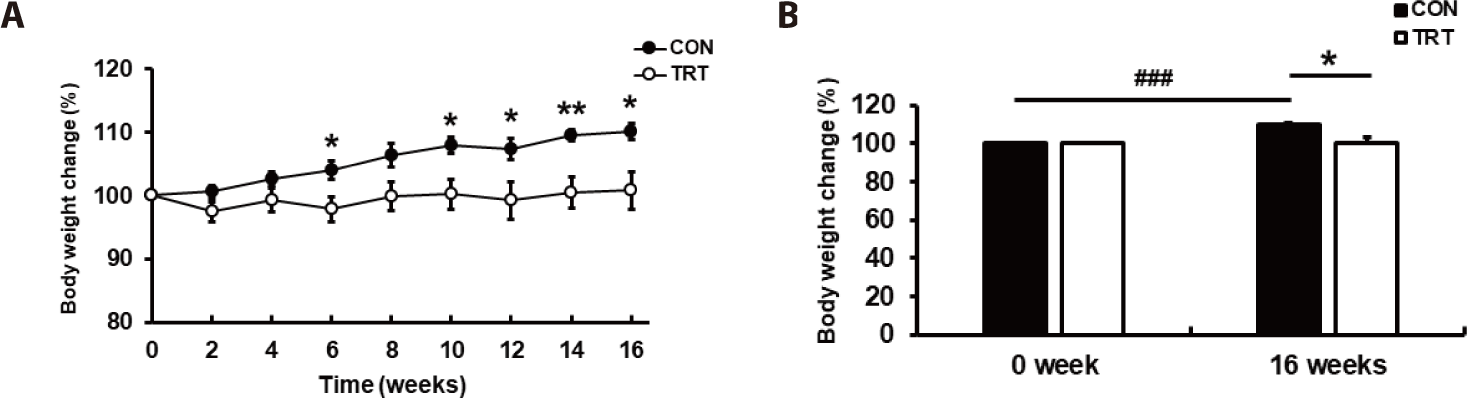
The rate of change in body weight was lower in TRT than in CON after ten weeks (p < 0.05; Fig. 1A). In addition, the rate of change in body weight in CON was higher at 16 weeks compared to at 0 weeks of the experiment (p < 0.01), while no significant difference in body weight was observed in TRT (Fig. 1B). This suggests that the corn-RS diet could help prevent obesity because it does not increase body weight even with excessive energy intake. This also suggests that the corn-RS diet did not affect weight gain in beagle dogs as much as the rice-based feed.
Table 3 presents the results of apparent total tract nutrient digestibility (ATTD). The ATTD of dry matter (DM), nitrogen-free extract (NFE), and organic matter (OM) were reduced in the TRT group compared with CON. The ATTD of DM was lower in TRT than in CON by 2.4% (p < 0.01); the digestibility of DM in CON was 92.9 ± 0.53%, whereas the DM was 90.5 ± 0.29% in TRT. The ATTD of NFE was lower in TRT than in CON by 7.2% (p < 0.05); 93.9 ± 1.06% was digested in CON, while 86.7 ± 2.14 % was digested in TRT. Similarly, ATTD for OM was 3.1% lower in TRT than in CON (p < 0.05). However, the ATTDs of crude protein (CP) and acid hydrolyzed fat were not affected by the dietary treatments. In addition, although there was no significant difference, the ATTD of ME was 89.2 ± 1.10% in TRT, which was lower than that of the CON, 91.7% (p = 0.061).
To evaluate the suitability and safety of corn RS as an ingredient in pet food, we analyzed the CBC and serum biochemical parameters at 0 weeks and at the end of 16 weeks. The CBC parameters, including leukocytes and erythrocytes, were within the normal reference range in both CON and TRT (Table 4). No significant difference was found in the leukocyte counts (white blood cell; lymphocyte; monocyte; neutrophil; eosinophil; basophil) between CON and TRT. Moreover, the erythrocyte counts (red blood cell; hemoglobin; mean corpuscular hemoglobin; mean corpuscular hemoglobin; red cell distribution width) were not affected in the dogs fed corn RS.
RS, resistant starch; CON, rice-based diet; TRT, corn-RS-based diet; WBC, white blood cell; LYM, lymphocyte; MONO, monocyte; NEU, neutrophil; EOS, eosinophil; BASO, basophil; RBC, red blood cell; HGB, hemoglobin; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; RDW, red cell distribution width.
Table 5 shows the serum biochemical parameters of dogs fed corn RS. All parameters were within the normal reference range; no significant differences were observed between CON and TRT. The concentration of serum glucose (GLU) was 108.40 ± 2.29 mg/dL and 99.40 ± 4.20 mg/dL in CON and TRT, respectively, indicating a reduction in serum glucose in dogs fed corn RS despite no significant difference (p = 0.097). Total cholesterol (T-CHO) was 316.6 ± 22.6 mg/dL and 267.4 ± 8.4 mg/dL in CON and TRT groups, respectively. Here again, the results were not significant (p = 0.076), but there was a reduction in T-CHO in the dogs fed the corn-RS diet.
RS, resistant starch; CON, rice-based diet; TRT, corn-RS-based diet; TP, total protein; AST, aspartate transaminase; ALT, alanine transaminase; GGT, gamma-glutamyl transferase; CREA, creatinine; GLU, glucose; LD, lactate dehydrogenase; CHO, cholesterol; TG, triglycerides; UN, urea nitrogen; TBIL, total bilirubin; CK, creatine kinase.
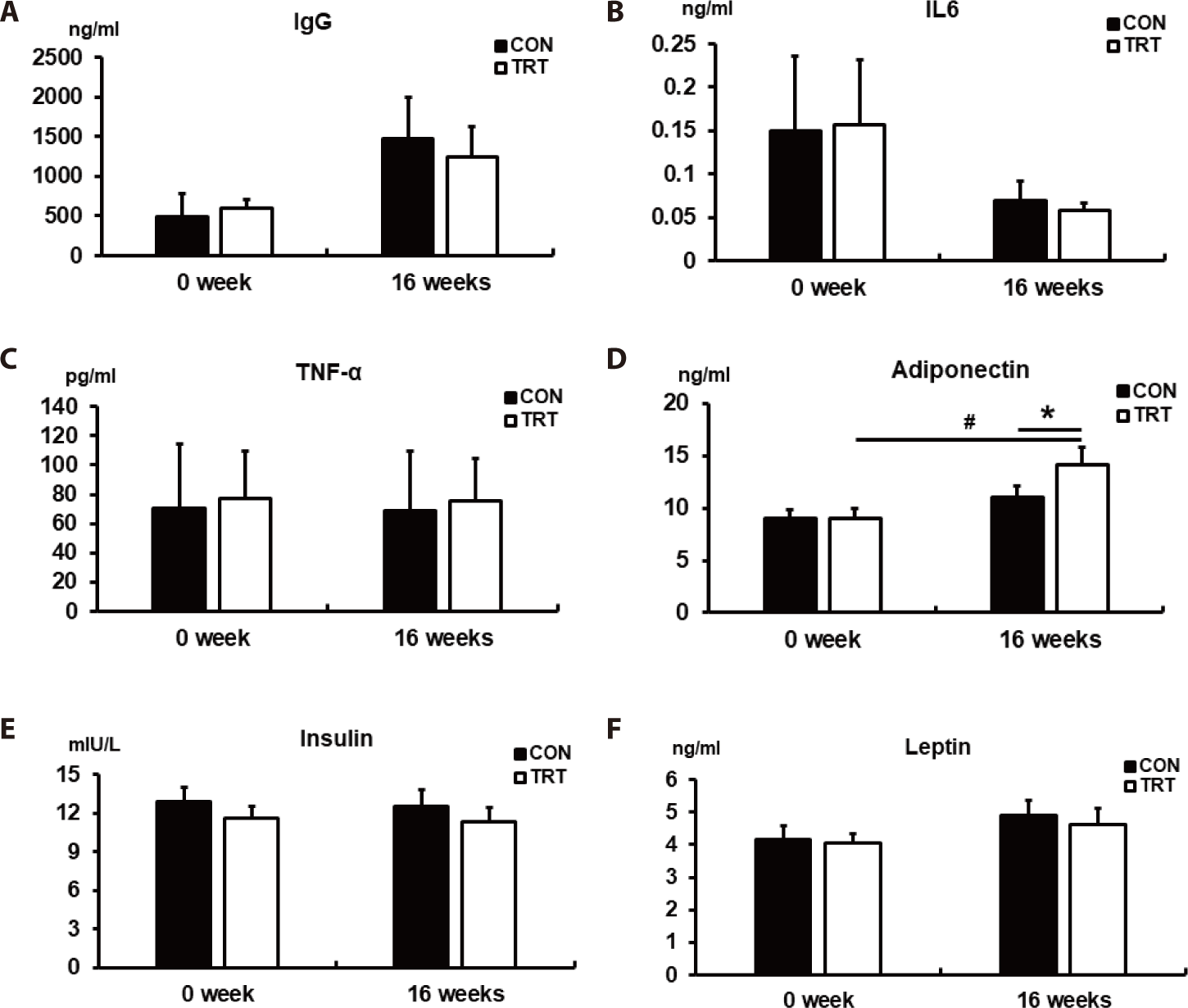
To evaluate the effects of the corn-RS diet on immune function and anti-obesity in dogs, immune-related hormones and cytokines (IgG, IL6, and TNF-α), as well as obesity-related hormones (adiponectin, insulin, and leptin), were analyzed in the serum (Fig. 2). We found that the immune-related hormones were not affected by the corn-RS diet (Figs. 2A, 2B, and 2C). Furthermore, we observed an increase in adiponectin, an obesity-related hormone secreted from adipose tissues with beneficial effects on lipid metabolism, in TRT compared with CON (p < 0.05; Fig. 2D); serum adiponectin was higher at 16 weeks than at 0 weeks in TRT (p < 0.05). In contrast, no significant change in insulin and leptin was observed within the groups between the beginning and the end of the experiment or between the CON and TRT groups (Figs. 2E and 2F).
DISCUSSION
This study was performed to evaluate the anti-obesity effects of corn RS in dogs. The MER of dogs is recommended based on their body weight, age, variety, and activity. The MER for healthy adult dogs is approximately 110 kcal/kg0.75 according to FEDIAF [20]. In this study, healthy adult beagles were given 132 kcal/kg0.75 ME at a 1.2-fold higher level than the recommended MER for 16 weeks to induce obesity. This level was approximately between 110 kcal/kg0.75 for dogs with moderate activity (1–3 h/day) and 150–175 kcal/kg0.75 for dogs with high activity (3–6 h/day), according to the MER recommendations of FEDIAF [20]. In our previous study, the body weight of beagle dogs was shown to increase when they consumed 132 kcal/kg0.75 ME per day [23]. Additionally, cereal grains such as rice, corn, and wheat are commonly used energy supply ingredients in pet food as well as livestock feed [24,25]. With the increased prevalence of obesity, RS, which is insensitive to the digestive enzymes in the body, has recently been in the spotlight as a novel food source for weight control in companion animals [26]. RS has physiological properties such as fiber that is not degraded by digestive enzymes in monogastric animals and is fermented by microorganisms in the large intestine. We focused on RS as a substance with an anti-obesity effect. Corn RS was selected because of its high RS content, and rice was selected as a control.
In the ingredients used in this study, the RS content of corn starch (6.69%) was approximately 3-fold higher than that of rice (2.33%). The RS content of each experimental diet containing rice (CON) or corn RS (TRT) was 1.09% and 3.12%, respectively. In this study, the feeding of a rice-based diet to the CON group for 16 weeks resulted in an increase in body weight, while the corn-RS-based diet with a high RS content showed a lower weight gain. While the effect of the corn-RS diet on weight loss could not be verified in the TRT group, it is notable that the body weight of the dogs in the TRT group did not increase compared with the weight gain observed in the CON group. The change in body weight also showed a significant decrease in TRT compared with CON. Therefore, to identify the cause of reduced rate of change in body weight with a corn-RS diet, the nutrient digestibility in dogs fed rice- or corn-based diets was analyzed. The results showed that the digestibility for DM, NFE, and OM were decreased in TRT, as well as the digestibility of ME decreased compared to that of the CON group. The nutrient digestibility of commercial pet food for dogs varies greatly depending on the price and brand of pet food. Previous studies have reported that the digestibility of each nutrient is within the following ranges [27,28]: 66.9%–84.4% for DM, 70.4%–82.5% for CP, 76.1%–95.8% for b), 65.0%–87.6% for OM, and 72.6%–87.7% for energy. In this study, the digestibility for all measured nutrients in both CON and TRT groups was similar to or higher than that reported in previous studies [27,28]. According to Corsato Alvarenga et al. [29], the digestibility of DM in corn-based diets for companion dogs was 80.23% on average at a level similar to or lower than that of the rice-based diet. The digestibility of DM and OM, and gross energy was also lower in corn-based diets compared to Brewer’s rice-based diet [25]. The nutrient digestibility of pet foods can be influenced by various factors such as chemical composition, dietary fiber content, trace minerals, and moisture [30,31]. In particular, the digestibility of starch can vary depending on the characteristics of the starch in the raw ingredients used, interactions between starch and protein, physical properties, and starch forms [32]. The reduced digestibility of DM, OM, and NFE in this study may be attributed to their resistance to digestion and absorption, because RS is insensitive to the digestive enzymes in the body. Thus, it can be concluded that the suppressed weight gain in the TRT group is attributable to the decrease in digestibility of DM, OM, NFE, and ME in dogs fed a high RS diet. The RS also functions as a prebiotic for microorganisms similar to the fiber in the large intestine of animals. In the intestines of rodents, RS increased the gut microbiota population [33]; fermented RS was also shown to play a critical role in weight loss [34]. Hence, further studies should be conducted to provide an in-depth analysis of potential changes in the gut microbiota in dogs feeds with high RS.
The CBC, serum biochemistry, immune- and obesity-related hormones in the serum were analyzed to verify the safety and anti-obesity effects of the corn-RS diets in dogs. Although the dogs were fed a diet with an energy level higher than their daily MER, the CBC and serum biochemical parameters were within the normal reference range [35,36], and the concentrations of immune-related hormones were similar between the CON and TRT groups. These results verified the safety of corn RS for its use as an ingredient for pet food, as it does not cause any negative effects on the health of the dogs. Although no significant difference was observed between CON and TRT groups; serum GLU and T-CHO showed a decrease in TRT compared to their levels in the CON group. These results are consistent with a study reported by Kimura [37] in which, based on oral glucose tolerance tests, the serum GLU level was reduced in beagles fed an RS diet. In a study, analyzing the changes in postprandial glucose in dogs fed a diet containing lentils, peas, Brewer’s rice, and corn as the raw materials, the corn-based diet showed the lowest area under the curve [25]. In another study, the levels of T-CHO and triglycerides (TG) decreased in mice fed a high RS diet when compared with those fed a low RS diet [38]. In a mouse-diabetes model, RS improved insulin resistance and increased the mass of the pancreas [39]. Although our study did not show a significant difference on the levels of GLU and T-CHO in the TRT group compared to those in the CON group, a decreasing tendency of these parameters was observed using the corn-RS-based diet for 16 weeks in dogs. This suggests the potential use of RS as a dietary material to improve diabetes and obesity in companion dogs, as observed by Kimura [37].
Additionally, the adiponectin concentration in the TRT group increased in dogs fed the corn-RS diet for 16 weeks. Adiponectin is of scientific interest due its positive role in lipid metabolism and its anti-obesity effects [40]. Adiponectin is mainly secreted by white adipose tissues, with functions that include the suppression of various metabolic disorders like oxidative stress, inflammation, obesity, and insulin resistance [41]. Clinically, plasma adiponectin concentration is negatively correlated with body weight, fat mass, and insulin resistance [42]; for example, adiponectin knockout mice displayed insulin resistance and glucose intolerance [43,44]. Adiponectin exhibits three different structures (trimer, hexamer, and multimer) and its activation involves binding with AdipoR1 and AdipoR2 [45]. The main signaling pathway for adiponectin facilitates the phosphorylation of AMP-activated protein kinase (AMPK), which plays a key role in the maintenance of cellular energy homeostasis as a positive regulator of insulin sensitivity. Also, the trimeric and hexameric forms of adiponectin can penetrate the blood-brain barrier and the blood-cerebrospinal fluid barrier to bind with AdipoR1 in the arcuate hypothalamus (ARH) to increase the phosphorylation of AMPK, which promotes food intake [46]. However, adiponectin concentrations increase to control the energy homeostasis during fasting; expression of AdipoR1 in ARH decreased after refeeding [46]. Although we only measured the concentration of total adiponectin in this study, our results suggest that an increase in serum adiponectin in the TRT group was not due to starvation because sufficient energy was provided (above their MER) to dogs. Comprehensively, the diet based on corn RS with an increased RS content might be suitable for dog food and could be helpful in reducing BWG in dogs fed a high energy intake diet.
