INTRODUCTION
Embryo transfer in cattle can accelerate the rate of genetic improvement compared to artificial insemination, particularly through the breeding of high-value female donors [1,2]. In Hanwoo cattle (Bos taurus coreanae), embryo transfer is performed for commercial purposes to enhance the quality and quantity of beef meat, with males typically raised for fattening and females for breeding [3,4]. With the accelerating development in embryo transfer technologies, understanding their impact on pregnancy rates and the male-female sex ratio remains an important area of research to improve the livestock industry.
Numerous factors can influence the success of embryo transfer, with the quality and developmental capacity of the embryos being the most critical. Achieving a high-quality embryo, technically skilled embryo transferers, and specifically managing the recipient can greatly increase the chances of successful pregnancy to around 70%–80% [5,6]. The sex ratio of the offspring can also be affected by various characteristics of the embryo, including developmental rate, freezing resistance, and the ability to maintain pregnancy after embryo transfer [7].
The commercial availability of frozen/thawed embryo technology began in the early 1980s, and a significant improvement was achieved in the 1990s using ethylene glycol instead of glycerol as a cryoprotectant [8,9]. This allowed for the transplantation of thawed embryos under field conditions instead of depending on the laboratory microscope. However, the freezing process exposes embryos to various stresses that can negatively affect their viability and, consequently, the pregnancy rate [10,11]. The developmental stage and quality of the embryo also affect pregnancy rates. It is important to use frozen/thawed embryos for efficient breeding programs and timed parturition in farms and to consider the developmental stage of the embryos when selecting embryos for transfer. Embryo survival after transfer is a critical factor in the productivity and profitability of cattle breeding. Premature embryo death and abortion can lower pregnancy rates and increase calving-to-pregnancy intervals, leading to higher production costs and financial losses [12-14]. Therefore, it is necessary to monitor pregnancy rates in relation to the conditions and developmental stages of the embryos.
In Angus beef cows, the pregnancy rates of in vivo-derived (IVD) fresh embryos were higher than those of frozen/thawed embryos (53% vs. 44%, respectively). However, there was no significant difference between grades 1 and 2 (57% and 56% respectively) [1]. In Holstein cows, there was no difference in pregnancy rates between stages 4 to 7 and grades 1 and 2; however, in another study [15], cows with grade-1 embryos had a higher pregnancy rate than cows with grade-2 embryos, with no significant difference in pregnancy rates among different stages of grade-1 embryos. Heat shock (40°C)-exposed sperm were found to decrease embryonic development and the male ratio [16,17]. Moreover, male embryos show rapid development and strong freezing resistance compared with female embryos [18]. Additionally, midsummer heat stress reduces feed intake, increases the negative energy balance, induces changes in ovarian follicular dynamics, reduces the estrus detection rate, and changes fallopian tube function, leading to fertilization failure and early embryo death [19]. However, on the other hand, the pregnancy rate with embryo transfer is higher than artificial insemination under these stressful conditions [20]. Conversely, frozen/thawed embryos are more sensitive to heat stress and develop slower when exposed to heat than fresh embryos [21]. During a heat wave in Korea in August 2018, a temperature of 41°C was recorded in Hongcheon-gun, Gangwon-do, Korea. In dairy cows, the artificial insemination rates are 55.6% in the summer and 87.8% in the cool season.
Therefore, it is necessary to study the differences in pregnancy rates and sex ratios according to developmental stage, quality grade, and fresh and frozen/thawed IVD embryos in Hanwoo cows to improve the efficiency and success of breeding programs. Additionally, the effect of ambient temperature on embryo transfer is an important consideration for maximizing pregnancy rates and minimizing early embryo death.
MATERIALS AND METHODS
Hanwoo heifers raised in cattle breeding farms in Hongcheon-gun, Gangwon-do, Korea with a carcass weight of 500 kg or more, backfat thickness of 120 cm2 or more, marbling score of 1 + + or more, and 60 days since calving underwent rectal examinations. Heifers with healthy reproductive organs and negative test results for the four major diseases (Leukosis, Brucellosis, John’s D., and Tuberculosis) were selected (68 cows).
To induce corpus luteum regression, 2 mL of prostaglandin F2α (PGF2α; cloprostenol 250 μg/mL, Synchromate, Pfizer, New York, NY, USA) was injected intramuscularly. On day 0, to induce the emergence of new follicles, an intravaginal device containing 1.9 g of progesterone (EAZIBREED™ CIDR®, InterAg, Hamilton, New Zealand) was inserted into the vagina, and 1 mL (2mg/mL, Esrone, Samyang Anipharm, Seoul, Korea) was injected intramuscularly. From day 5 after controlled internal drug release (CIDR) device insertion, 300 mg of follicle-stimulating hormone (FSH; Folltropin V, Bioniche Animal Health Canada, Belleville, ON, Canada) was intramuscularly injected eight times at 12-hour intervals as a reduction method, and 2 mL of Synchromate was administered twice at 12-hour intervals intramuscularly on days 7 of FSH administration. The CIDR was removed on day 8. Estrus was checked approximately 12 hours after the final administration of FSH, and artificial insemination was performed twice, 60–72 hours after the administration of PGF2α (Fig. 1A).
On day 7.5 after estrus detection, 5 mL of 2% lidocaine (Lidocaine HCl Hydrate, Daihan, Seoul, Korea) was administered to induce epidural anesthesia. Both uterine horns were flushed with flushing media (BIOLIFE Advantage Complete Flush Media, Agtech, Manhattan, KS, USA) warmed to body temperature. The embryos were recovered by perfusion through a 3-way catheter (IMV, L’Aigle, France). The collected perfusate was filtered using an Em-con filter (Agtech) and transferred to a 100-mL Petri dish. Embryos were examined under a stereomicroscope at 40× magnification according to the International Embryo Technology Society standards [22]. They were visually evaluated and transferred to a holding medium (BIOLIFE Holding and Transfer Medium, Agtech) on a warm plate at 37.5°C. In the case of fresh embryo transfer, embryos were transferred within 5 h after quality grade 1–2, stage 4–7, and freezing was started within 1 h after testing quality grade 1 and stage 4–7 freezing.
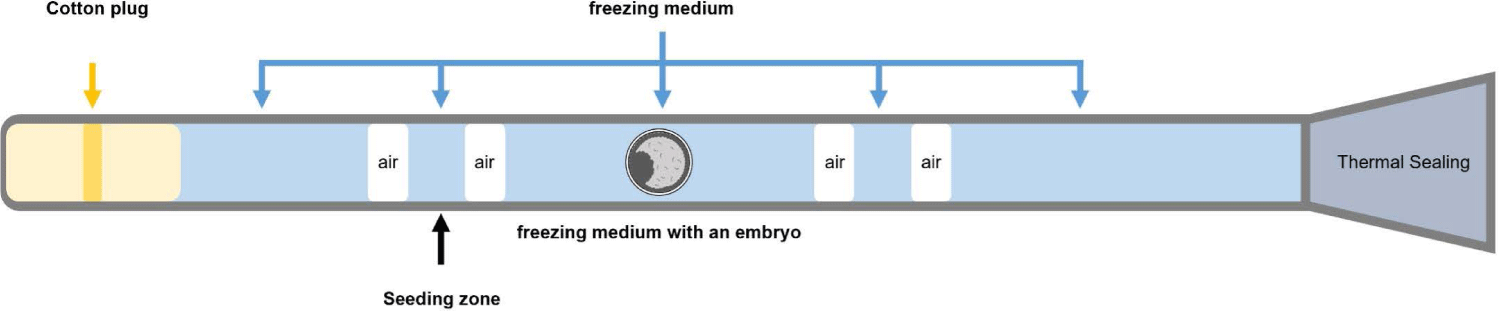
Embryos were washed thrice with holding medium (BIOLIFE Holding and Transfer Medium, Agtech) and immersed in freezing medium (BIOLIFE Freeze medium ethylene glycol with sucrose, Agtech) for 5 min. After equilibration, the embryo was loaded into a 0.25-mL plastic straw, and the straw was placed in a portable embryo freezer (CL8800i, CryoLogic, Blackburn, Australia) containing liquid nitrogen. After a 2-min exposure to −6°C, the seeding zone was inoculated using a cotton swab dipped in liquid nitrogen. When the temperature dropped to −32°C at a rate of −0.3°C/min, the straw was immediately immersed in liquid nitrogen and stored (Fig. 2).
To thaw frozen embryos, the straw was taken out from the liquid nitrogen and was left at room temperature for about 10 s, slowly immersed in a 35°C water bath for 10 s, and then kept for an additional 10 s. The straw was removed from the water bath, dried on the outer surface, and transplanted.
In total, 297 recipient cows were selected from 20 Hanwoo cow farms in Hongcheon-gun, Gangwon-do, Korea. Of these recipient cows, 116 received fresh embryos and 181 received frozen IVD embryos. On day 0, a CIDR insertion and Estradiol benzoate (1 mL) were injected intramuscularly. On day 8, 2 mL of PGF2α was intramuscularly injected while the CIDR was removed. Estradiol benzoate (0.5 mL) was intramuscularly injected approximately 24 h after PGF2α administration and heat was confirmed approximately 12 h thereafter (Fig. 1B). At 7.5 days after the discovery of estrus, the ovaries were palpated, and 5 mL of 2% lidocaine was applied for epidural anesthesia to the donor cow with the corpus luteum, and then an embryo transfer syringe (IMV) was used on the uterine horn in the direction of the corpus luteum. Fresh and frozen embryos were transferred.
For the diagnosis of pregnancy, individuals who did not undergo re-estrus after embryo transfer were confirmed by rectal palpation between 60 and 70 days. Data were collected by searching delivery records based on the records of transplantation one year after transplantation to investigate the delivery and sex of live births. The pregnancy rate was defined as the number of pregnancies divided by the number of recipients, and the delivery rate was defined as the number of calves divided by the number of recipients.
RESULTS
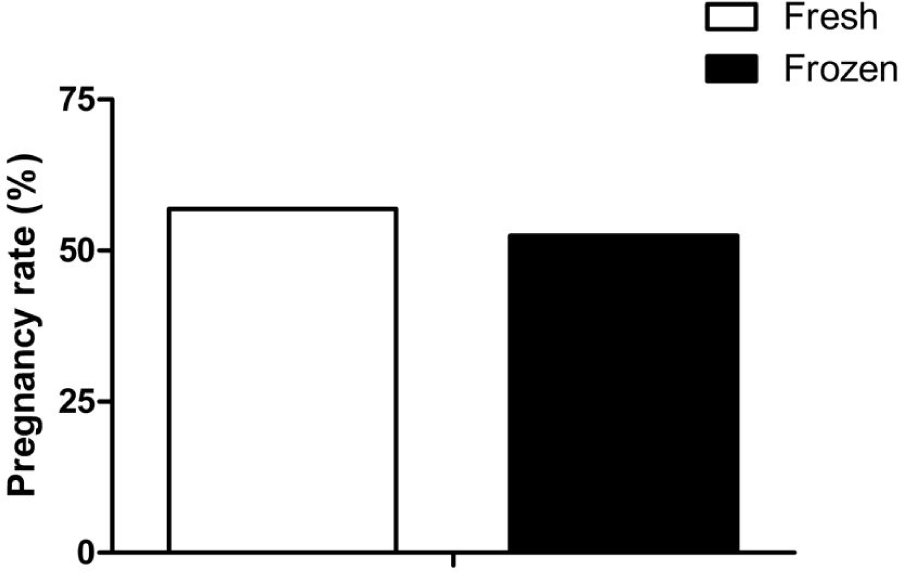
We transferred 116 fresh and 181 frozen embryos to 297 recipient cows. Pregnancy rates were compared between fresh and frozen embryos. The fresh embryos had pregnancy rates of 56.90% (66/116), and the frozen embryos had a lower pregnancy rate of 52.49% (95/181) (Fig. 3). However, there was no significant difference in pregnancy rates between fresh and frozen embryos (p = 0.4).
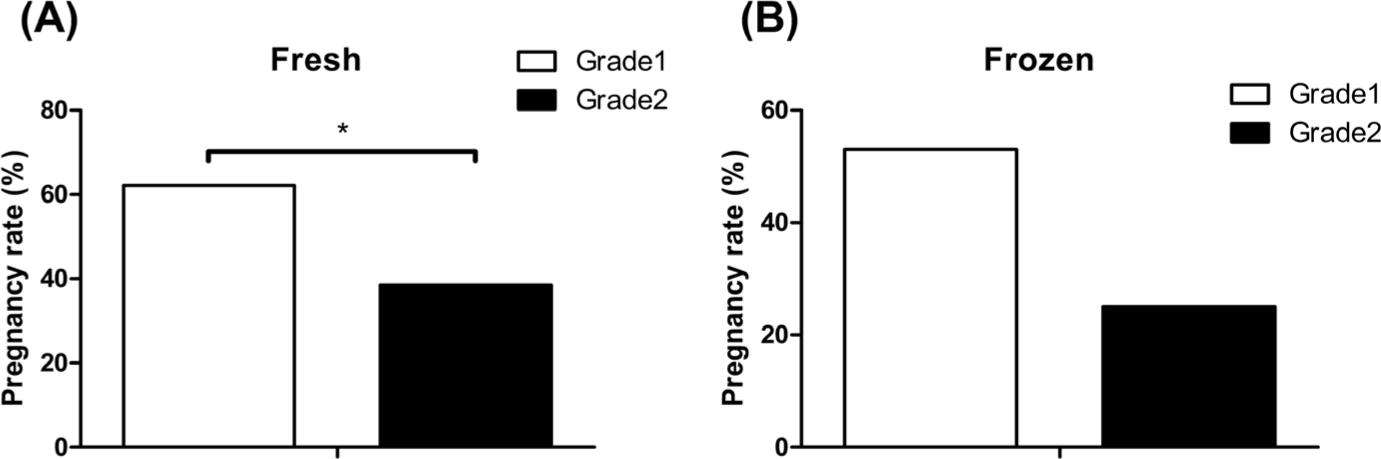
Pregnancy rates were examined according to the quality grades of both fresh and frozen embryos. Among all transferred embryos, the pregnancy rate was higher in grade-1 than in grade-2 embryos. Furthermore, there was a significant increase in the pregnancy rate of fresh embryos for both quality grades, with a 9% increase in grade 1 and a 13% increase in grade 2 (p < 0.05) (Fig. 4).
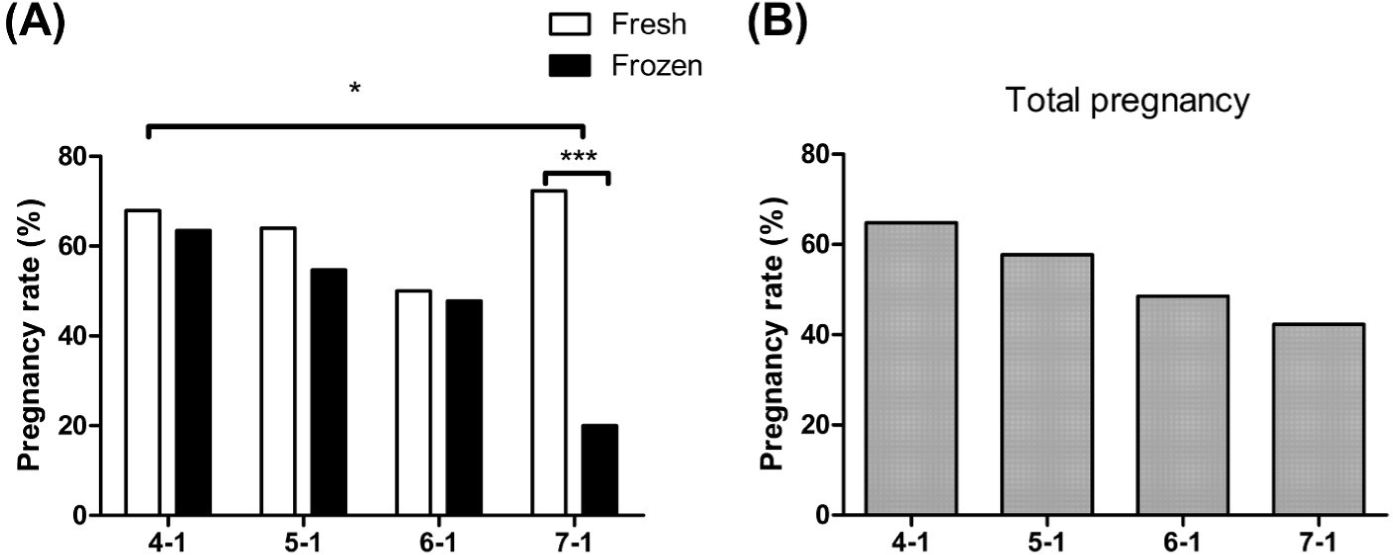
We then investigated how the embryo stage affects pregnancy rates under both fresh and frozen conditions. Initially, we found that fresh stage-7 embryos exhibited the highest pregnancy rates. Surprisingly, there were no significant differences in pregnancy rates across the different stages, and the overall rates remained stable and comparable to those of quality grade-1 embryos. However, our analysis of frozen embryos revealed a notable decline in fertility as the stage progressed from 4 to 7. Specifically, when examining pregnancy rates by stage in frozen embryos, the percentages were 63.5% for 4-1, 54.7% for 5-1, 47.8% for 6-1, and 20.0% for 7-1 (p < 0.05) (Fig. 5). Remarkably, frozen embryos at stage 7-1 displayed the lowest rates of fertilization compared to the other groups, and this decrease was significant compared to fresh embryos at stage 7-1 (p < 0.05) (Fig. 5).
We then examined the outcomes of fresh and frozen embryo transfers in a cohort of pregnant cows. Among 66 pregnancies resulting from fresh embryo transfer, 12 (18.18%) resulted in miscarriages. Within this group, there were 27 female (50.00%) and 27 male (50.00%) calves with an equal male-to-female ratio of 1:1. In contrast, among the 95 pregnancies resulting from frozen embryo transfer, 20 (20.05%) resulted in miscarriage. The resulting offspring consisted of 29 female (38.67 %) and 46 male (61.33%) calves with a male-to-female ratio of 6:4. The miscarriage rate was approximately 3% higher in pregnancies involving frozen embryos than in those involving fresh embryos. Additionally, frozen embryos exhibited a 2% higher miscarriage rate and a higher percentage of female births than fresh embryos. However, it is important to note that these observed differences were not statistically significant (Table 1).
| No. of recipient | Sex ratio of calves (%) | |||
|---|---|---|---|---|
| Pregnant | Aborted (%) | Male | Female | |
| Fresh | 66 | 12 (18.1) | 27/54 (50.0) | 27/54 (50.0) |
| Frozen | 95 | 20 (21.1) | 46/75 (61.3) | 29/75 (38.7) |
We investigated the sex ratio and miscarriage rate based on the stage of grade-1 embryos. The male-to-female ratio varied across the different stages, with ratios of 4.5:5.5 in stage 4, 3.2:6.8 in stage 5, 5.2:4.7 in stage 6, and 2.9:7.1 in stage 7. The miscarriage rates for stages 4–7 were 28.8%, 24.4%, 3.9%, and 36.4%, respectively. Notably, stage 6 exhibited a relatively low miscarriage rate; however, no significant difference was observed between stages (Table 2).
We conducted a comprehensive study to examine the impact of season on the pregnancy rate, miscarriage rate, and sex ratio of both fresh and frozen embryos. From February to April, nine embryos were transferred, resulting in three pregnancies with a pregnancy rate of 33.3%. Between May and August, out of 194 embryos transferred, 108 embryos were successfully conceived, yielding a pregnancy rate of 55.7%. Similarly, from September to November, 47 of the 89 transfers resulted in pregnancy, yielding a pregnancy rate of 52.8%. Finally, from December to January, three out of five transfers led to embryo pregnancy, resulting in a pregnancy rate of 60.0%. When comparing the pregnancy rates of fresh and frozen embryos across different seasons, the following percentages were observed: 42.9% for fresh embryos vs. 0.0% for frozen embryos in spring, 59.8% for fresh embryos vs. 52.7% for frozen embryos in summer, 51.9% for fresh embryos vs. 53.2% for frozen embryos in autumn, and 60.0% for frozen embryos in winter. Notably, frozen embryos displayed higher fertility rates in autumn and winter than fresh embryos. The following male-to-female ratios were observed in different seasons: 6.7:3.3 in spring, 4.0:6.0 in summer, 5.5:4.5 in autumn, and 3.3:6.7 in winter. These ratios suggested a slight predominance of female births in spring and autumn, whereas summer and winter exhibited relatively balanced sex ratios (Table 3).
DISCUSSION
This study investigated the impact of seasonal transfer on the pregnancy rate, miscarriage rate, and sex ratio in recipient cows using fresh and frozen embryos. The comparison was based on the stage and quality grade of the IVD embryos. The recipient cows were synchronized using conventional synchronization methods [23]. While no significant difference was observed, the pregnancy rate of fresh embryos exceeded that of frozen embryos by over 4%, and the miscarriage rate was 2% higher for frozen embryos. These results are consistent with those of Ferraz et al. [24] who demonstrated a higher pregnancy rate with fresh embryos than with frozen embryos. This suggests that freezing affects the pregnancy rate of IVD embryos [25]. However, the present study revealed that frozen embryos transferred using the current freezing protocol exhibited pregnancy rates similar to fresh embryos.
Evaluation of embryo quality is based on visual assessment, which, although subjective, serves as a predictor of embryo viability [26]. Grades 1–4 are assigned based on the characteristics of the zona pellucida, cytoplasmic regularity, and viability, with grades 1 and 2 considered suitable for embryo transfer [27]. This is consistent with previous findings reported by Phillips and Jahnke [27] and Erdem et al. [15], who observed a higher pregnancy rate with grade-1 than with grade-2 embryos. However, another study [1] reported no significant differences between grade-1 and grade-2 embryos. In grade 1, the pregnancy rate of fresh embryos is 9% higher than that of frozen embryos. In grade 2, there is a 13% higher pregnancy rate for fresh embryos compared to frozen embryos. Notably, grade-2 embryos exhibited a pregnancy rate of less than 40%, representing a difference of approximately 25% compared with grade-1 embryos. For grade-2 embryos, prioritizing the transfer of fresh embryos over freezing is recommended. However, for grade-1 embryos, the results indicated that the pregnancy rate was not significantly affected by the convenience of using fresh or frozen embryos. Therefore, the findings of this study in Hanwoo cows coincide with those of Holstein embryos, in which grade-1 and -2 transfers achieve the highest rates of pregnancy [28].
To investigate the impact of fresh and frozen embryos on the pregnancy rate based on embryo stage, we observed that the pregnancy rate may vary according to embryo stage. The positioning of the embryo within the female genital tract corresponds to its developmental stage, ranging from the morula to the late blastocyst stage on days 6–8 of fertilization [29,30]. Given that the synchronization protocol utilized in this experiment involved transferring embryos 7.5 days after estrus detection, the pregnancy rate was expected to be highest when embryos were transferred at stages 6 to 7. Surprisingly, the highest pregnancy rate was observed in patients with stage-4 embryos (64.8%). As the stages progressed from 4 to 7, the pregnancy rate gradually decreased by 7%, 9%, and 6%, respectively, with stage 7 demonstrating the lowest pregnancy rate of 42.3%. In the case of fresh embryo transfer, stage 7 exhibited the highest pregnancy rate at 72.3%, whereas stage 6 exhibited the lowest rate at 50%. Nevertheless, the overall pregnancy rates exceeded 50% across all stages, indicating stable pregnancy rates, regardless of whether stages 4–7 were used during fresh embryo transfer [31-33].
Pregnancy rate is also influenced by the freshness of the embryo and the stage of development [34]. In our study, there was a significant drop of approximately 10% in the pregnancy rate from stages 4 to 7. This suggests that the developmental capacity of embryos is more susceptible to damage caused by freezing. Slow freezing during cryopreservation leads to a decrease in viability due to the formation of ice crystals and the physical destruction of organelles caused by the presence of free water inside the cells [35,36]. As the embryo develops and progresses to the blastocyst stage, the size of the blastocyst cavity and the amount of water within it increases, thereby increasing the likelihood of damage during freezing. While no significant difference in pregnancy rates between fresh and frozen embryos was observed at stage 4, the difference in pregnancy rates became more pronounced as the embryos progressed to stage 7. Therefore, to achieve a stable pregnancy rate after embryo cryopreservation, it is advisable to select and freeze embryos at stages 4–5.
Pregnancy rates based on developmental stages of fresh and frozen embryos have not been extensively investigated in previous studies. Erdem et al. [15] reported that stages 5 and 6 exhibited the highest pregnancy rates, which aligns with the findings of Hasler [37] who observed the highest pregnancy rates at stage 5. However, contrasting results were reported by Putney et al. [38] and Ferraz et al. [24], who reported higher pregnancy rates in stage 7 and lower rates in stage 5. Vieira et al. [39] reported that the developmental stage of the embryo did not significantly affect pregnancy rates. The results of the present study differ from those of previous studies, suggesting that pregnancy rates during embryo transfer vary according to the developmental stage of embryos in different countries and breeds. These variations highlight the importance of considering specific factors such as geographical location and breed when interpreting and comparing pregnancy rate outcomes across studies [40,41].
Pre-selection of calf sex in dairy and beef cattle breeding offers significant economic advantages and has a profound impact on management strategies. To investigate the potential influence of embryo developmental stage on the sex ratio of live Hanwoo cows, we conducted a follow-up observation of the sexes based on embryo stage. In fresh embryos, a 1:1 male:female ratio was observed, whereas in frozen embryos, the male:female ratio was 3:2, indicating a relatively higher proportion of males (approximately 20%). However, no significant differences were observed between the groups.
Determining offspring sex from embryos has been a subject of great interest, with several studies attempting to establish associations between embryonic sexes and morphological and temporal assessments, leading to conflicting results. Early bovine studies suggested that early embryos were more likely to be male, implying a potentially faster developmental rate of male embryos compared to female embryos [42,43]. However, other studies have found no significant effect of embryo sex on the timing of bovine embryo division [44-46]. Consequently, investigators have used morphological and temporal evaluations to identify potential developmental differences between male and female bovine embryos. However, the effect of embryo sex on the timing of division remains controversial. Previous studies by Sugimura et al., Holm et al., and Magata did not find a relationship between developmental dynamics and bovine embryo sex [47-49].
Monthly embryo transfer records were reanalyzed to investigate the impact of temperature and other environmental factors on the pregnancy rates of frozen and fresh embryos. Lee et al. [28] highlighted the negative effects of cumulative heat stress during the hot summer season in Korea, particularly from September to early October, resulting in poor embryo grades. In the present study, embryo transfers were rarely performed between December and February due to local business practices.
In Korea, spring is considered as February to April, summer as May to August, autumn as September to November, and winter as December to January [17]. In this study, over 65% of embryo transfers took place between March and August, as farmers preferred to deliver calves during the warm season following hot summers. Fresh embryos had a higher pregnancy rate during summer, whereas frozen embryos exhibited a higher pregnancy rate during autumn.
Ferraz et al. [24] reported a negative correlation among temperature, humidity, and pregnancy rates. Heat shock-induced aging of sperm at 40°C results in decreased embryo development and male sex ratios [16]. Heat stress during midsummer reduces feed intake, increases negative energy balance, alters ovarian follicular dynamics, decreases the estrus detection rate, and affects fallopian tube function, leading to fertilization failure and early embryo mortality [19]. Consequently, embryo transfer shows higher pregnancy rates than pregnancies by artificial insemination [20]. However, frozen embryos display increased sensitivity to heat stress and slower development under heat exposure than fresh embryos [21]. Although the temperature used for embryo exposure was 41°C, it should be noted that an actual heatwave in Hongcheon-gun, Gangwon-do, Korea recorded a temperature of 41°C in early August 2018. In dairy cows, the artificial insemination rate was 55.6% in summer and 87.8% in the cool season [50]. Although embryos can be influenced by seasonal ambient temperatures [51,52], no significant differences were observed in this study because of the use of an embryo warmer to maintain optimal conditions until immediately before transfer. Furthermore, 93% of the actual embryo transfers occurred after mid-October during the fall. This schedule allowed sufficient recovery from heat stress, thereby contributing to a stable pregnancy rate. Male-to-female ratios for embryo transfer were 6.7:3.3 in spring, 4.0:6.0 in the summer, 5.5:4.5 in the autumn, and 3.3:6.7 in the winter. It can be concluded that there was no significant effect on the male-to-female ratio during the summer and autumn.
In conclusion, the comprehensive findings of this study provide valuable insights into the optimization of embryos in Hanwoo cows. By exploring the factors that influence the viability and success of IVD embryo transfer in Hanwoo cows, this study contributes to enhancing the desired fertility rate, pregnancy rate, and sex ratio of the resulting calves. The determinants identified to achieve optimal outcomes in Hanwoo cattle reproductive management have significant implications for animal breeding. Moreover, the results of this study offer potential solutions to improve the efficiency and effectiveness of embryo transfer techniques in Hanwoo cattle, thereby benefiting the overall productivity and sustainability of the Hanwoo cattle industry.