Background
Recently, there has been a growing interest in vitamin B3 (niacin) by the emergence of NAD+ metabolism in health and disease and the use of nicotinic acid as an important therapy for the treatment of hyperlipidemias [1]. Niacin is an important water-soluble vitamin and monocarboxylic acid derivative of pyridine which found in the nicotinic acid (C6H5NO2) form and nicotinamide (C6H6N2O) form in foods [2]. The blanket term vitamin B3 is used for both forms. Nicotinic acid (also generally known as niacin) and nicotinamide (also known as niacinamide) are similarly effective as a vitamin because they can be converted into each other within the organism [3].
Niacin, first isolated from rice bran in 1911, is readily metabolized and excreted vitamin with a recommended daily allowance of 14–16 mg/day [4]. It was later recognised to have two distinct but chemically related components, nicotinic acid and nicotinamide.
Vitamin B3, in the form of the dinucleotides, is essential for all living cells and plays a central role in energy metabolism and oxidative phosphorylation and required for protein, fat and carbohydrate metabolism in the whole organism. Niacin perform important metabolic roles in living cells as a precursors of NAD+/NADH and NADP+/NADPH [5]. With this metabolic cofactor effect, it is actively involved in preventing many pathological processes. Its deficiency causes the disease called as “pellagra” in man which is characterized by the rough or raw skin. Other problems include memory loss, vomiting and diarrhea. Niacin deficiency is also associated with an increased risk of cancer [6]. and has been shown to increase toxicity caused by reactive oxygen species [7].
B-complex vitamins that are not stored in the body, but should be taken into body daily [8]. A small amount of the niacin can be synthesized from the dietary amino acid tryptophan, but most of the daily requirement must be met by external sources of niacin [9]. The amount of niacin in the body is expressed in mg as nicotinic acid (mg) + nicotinamide (mg) + 1/60 tryptophan (mg). Additionally, riboflavin converts the amino acids into niacin [4,10,11]. In addition to being nutrients, nicotinamide and nicotinic acid are clinically applied pharmacological agents. Nicotinic acid is the oldest drug used to treat dyslipidemia. Very high intakes of niacin, many times larger than the conventionally accepted vitamin levels, are used clinically: nicotinic acid as a hypolipidaemic-hypocholesterolaemic agent [12], and nicotinamide in the treatment of schizophrenia [13] and other psychiatric disorders. Clinical studies have shown that nicotinic acid therapy reduces the number of cardiac and the fatal heart diseases [14,15] and should be given in high doses for the lipid-lowering effect. Diet is considered an important factor for the development of dyslipidemia. [16]. Blood total cholesterol (TC) and low-density lipoprotein (LDL) correlate strongly with coronary heart disease. Hypercholesterolemia is an abnormally high amount of cholesterol in the blood. One of the main effect of nicotinic acid associated with reduced lipids (low density lipoproteins, fatty acids and cholesterol) [16,17].
Nicotinamide, first isolated from horse erythrocytes in 1935, is the amide derivative of nicotinic acid [18]. While nicotinic acid is commonly effective in lowering cholesterol levels, unlike nicotinic acid, nicotinamide is ineffective on lipids.
Niacin is required to be taken with foods and found in various plant based and animal based food sources. Good sources of niacin include yeast, meat, poultry, red fish (e.g., tuna, salmon), cereals, legumes, and seeds. Milk, green leafy vegetables, coffee and tea also provide some niacin [19]. In plants, especially mature cereal grains like corn and wheat, niacin may be bound to sugar molecules in the form of glycosides, which significantly decrease its bioavailability [20]. During the cooking of cereals, particularly in products that are slightly alkaline by the use of baking soda, the availability of niacin increases [21].
In the literature, foods are associated with hypercholesterolemia in lowering cholesterol and it has been proven that foods with these ingredients to carry a health claim indicating that they reduce the risk of cardiovascular disease [22–26]. The best way to get the daily requirement of essential vitamins is to eat a balanced diet that contains a variety of foods. Thus, loss or changes of nicotinic acid and nicotinamide acid in foods also should be taken under control during food process or storage conditions. Knowing the profile of vitamin B3 is important in many ways such as in the preparation of a complete food composition table, preparation of diet therapy, nutrition education, food security, safety and regulation, food based dietary guidelines, labelling of food in the food industry, nutritional survey and also for other research purposes.
Presence rates of nicotinic acid and nicotinamide, which are the available forms of vitamin B3, are different for each food. However, the studies in the literature are generally based on the analysis of total amount of vitamin B3 in foods [27–30] and the studies determining the profile of vitamin B3 in foods are limited [2,21,31–36]. Therefore, the aim of the study was to investigate the presence and levels of vitamin B3 in some animal based and plant based foods for determining their vitamin B3 profiles in these foods which have been proven to have positive effects on health particularly on blood lipid level.
Materials and Methods
This study has focused on the vitamin B3 profiles of some animal based foods and plant based foods. Post-column UV derivatization system by HPLC (high-performance liquid chromatography) was used to determine the presence and concentrations of nicotinic acid and nicotinamide in the 20 food samples consisting of 10 meat samples and 10 cereal and legume samples. In this study, 10 kinds of animal based food samples consisting of veal (veal steak fillet), chicken (breast), turkey meat (thigh), goat meat (leg, belly), lamb (leg, back, arm), mutton (belly), bovine meat (loin) and 10 different plant based food samples namely; barley, rye, wheat (bread), wheat (durum), oat, rice, dried pea, green lentil, red lentil and chickpea were studied.
The vitamin standards (nicotinic acid and nicotinamide) and teflon tube “tubing” (length: 20 m diameter: 0.5 mm), used in this study, were obtained from Sigma-Aldrich (St. Louis, MO, USA). UVA lamp (20 W, 60 cm) was used for post-column derivatization. Sodium hydroxide (NaOH), hydrochloric acid (HCl), hydrogen peroxide (H2O2), copper sulfate (CuSO4), trichloroacetic acid (C2HCl3O2) and potassium dihydrogen phosphate (KH2PO4) were obtained from Sigma (St. Louis, MO, USA). In this study, all other chemicals were used in high purity.
Samples of veal (veal steak fillet), chicken (breast), turkey meat (thigh), goat meat (leg, belly), lamb (leg, back, arm), mutton (belly), bovine meat (loin), barley, rye, wheat (bread), wheat (durum), oat, rice, dried pea, green lentil, red lentil and chickpea were purchased from local markets in Turkey and grounded in the laboratory.
The profile of vitamin B3 was determined by HPLC, consisting of Shimadzu Nexera-İ LC-2040C 3D pump with a Shimadzu RF-20A fluorescence detector (Shimadzu Corporation, Kyoto, Japan) according to the procedure described by Lahely et al. with some modifications [37]. The Zorbax eclipse X08-C18 column (5 μm, 4.6 × 150 mm) (Agilent, USA) was used and the flow rate was 1 mL/ min. The column oven temperature was maintained at 25 °C , the analysis time was 40 min and the injection volume was 20 μL.
Standard stock solution of nicotinic acid and nicotinamide (100 μg/mL) was prepared in 0.1 N HCI solution. Each standard is freshly prepared daily. Working standards in five concentration levels were prepared from stock solution.
Homogenized 1–10 g of the samples (based on the estimated amount of niacin it may contain) were placed in 100 mL erlene, then 60 mL of 0.1 N HCI was added and autoclaved at 121°C for 30 minutes. Then, the samples were taken from the autoclave and cooled down to room temperature, 2 mL of 20% tricycloacetic acid (C2HCl3O2) solution was added to the solution, proteins were precipitated, the volume was completed with 0.1 N HCI, filtered through filter paper. The final solution filtered through 0.45 μm filter into HPLC vials.
Post-column derivatization is required to determine the profile of vitamin B3. For post-column derivatization, a photo-chemical derivatization system was established in the laboratory. Detection methods described by Lahely et al. was used with some modifications [37]. The derivatization system was made by wrapping a teflon tubing with a length of 20 m and a diameter of 0.5 mm on a UV-A lamp 60 cm long. The system was connected between the analytical column and the fluorescence detector. The mobile phase prepared daily and protected from light. The mobile phase was prepared by mixing 500 mL of deionized water and 9.5 g of potassium dihydrogen phosphate (KH2PO4). Then, 7.5 mL of hydrogen peroxide (H2O2) and 2 mL of copper (II) sulfate solution (CuSO4) (0.12 copper (II) dissolved in 100 mL of deionized water) was added and the volume was completed with 1 L of deionized water. Finally, the mobile phase was filtered through a 0.22 μm filter. The fluorescence detector set to excitation wavelength of 322 nm and emission wavelength of 380 nm, respectively. An Agilent eclipse X08-C18 column (5 μm, 4.6 × 150 mm, Agilant Technologies) was used and flow rate of 1 mL/min. The injection volume was set at 20 μL, and separation was completed in 40 min.
Results and Discussion
Linearity was evaluated by analysing five different concentrations of standard solutions in triplicate.
The calibration curve was obtained in a concentration range from 1 μg/mL to 10 μg/mL and found to be linear for nicotinic acid and nicotinamide with coefficient of determination greater than 0.996 as shown in Table 1.
Limit of detection (LOD) and limit of quantification (LOQ) for nicotinic acid and nicotinamide were calculated from the calibration line at the lowest concentration using the following equations: LOD = 3 × SD, LOQ = 10 × SD, (SD: standard deviation). LOD and LOQ values have been shown in the Table 1.
Dried pea sample was used for precision and accuracy assessment in the study. The precision study of the analytical method included repeatability and reproducibility tests. The repeatability was tested by analysing the same sample in ten replicate within the same day. The reproducibility was determined by analysing the same sample in 16 replicates in three independent days. The relative standard deviation (RSD) values of repeatability are 2.0% and 2.0% respectively, RSD values of reproducibility are 2.08% and 2.04% respectively, for nicotinic acid and nicotinamide as shown in Table 1.
The accuracy of the method was evaluated by analysing the Standard Reference Material 1849a, Infant/Adult Nutritional Formula (108±10 mg/kg), demonstrating the validity of the assays. The recoveries for the dried pea sample was determined by spiking standard solution at concentration of 0.5 μg/mL. Recoveries were found to be in the range of 96.4% and 104.2% for nicotinamide and nicotinic acid respectively, as shown in Table 1.
Blank samples of ultrapure water and reagents were also prepared using the same procedures as for the food samples. In the determination of nicotinic acid and nicotinamide no obvious interferences was observed from the blank chromatograms.
The quality procedures of analytical method were based on ISO/IEC 17025 requirements. A Standard Reference Material (SRM 1849a) Infant/Adult Nutritional Formula was provided from National Institute of Standards & Technology (Gaithersburg, MD, USA) and was proceed in a similar way to the unknown samples.
For the quality of the research, we participated in the FAPAS® test (2018), the proficiency test for analysing breakfast cereal, which organized by FAPAS® (Food Analysis Performance Assessment Scheme in UK). All analyses performed in triplicate, and the average value used. Our result was found in the acceptable range (−2 ≤ Z score ≤ +2).
HPLC is one of the most convenient and accurate analytical techniques to analyze the vitamin B3 from foodstuffs. The results indicating nicotinic acid and nicotinamide acid concentrations given in Table 2 for some animal based foods such as meat and HPLC chromatograms of the standard and the goat meat (belly) sample presented in Fig. 1 and 2. Table 3 shows the vitamin B3 profiles of some plant based foods such as cereals and legumes.
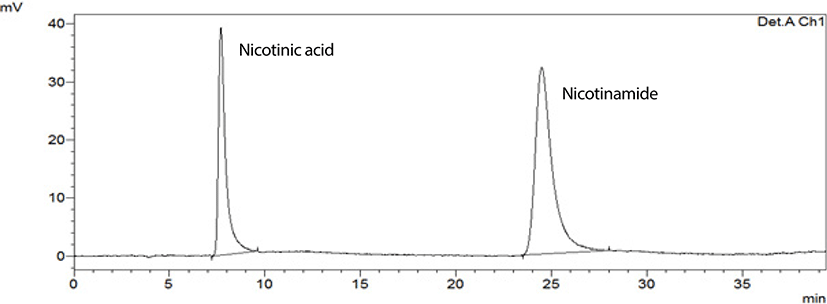
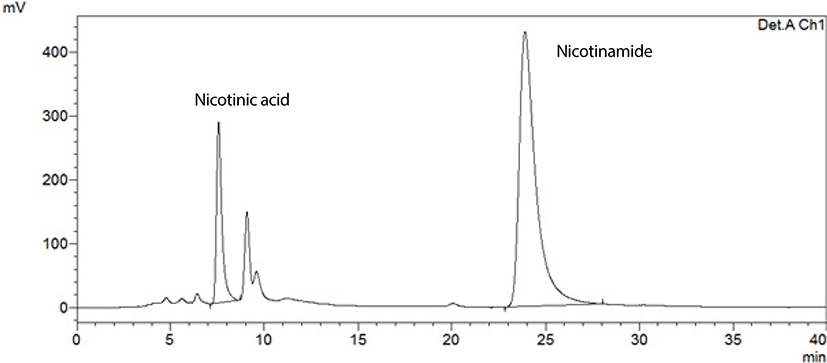
The analytical method was successfully applied to determine the vitamin B3 profile of 10 meat samples; veal (veal steak fillet), chicken (breast), turkey meat (thigh), goat meat (leg, belly), lamb (leg, back, arm), mutton (belly), bovine meat (loin), and the results are shown in Table 2.
Fig. 1 and 2 show typical chromatograms for standard solution and the goat meat (belly) sample, respectively.
The concentrations of nicotinic acid were found in the range of values between 8.5% and 49.6% in the meat samples while the concentrations of nicotinamide were found between 59% and 91.5%. Nicotinic acid form and nicotinamide form were found together in all meat samples.
Among the samples, the highest amount of nicotinic acid was found in chicken breast with 3.739 mg/100 g. However, since the chicken breast sample also contained 5.442 mg/100 g nicotinamide, the concentration of nicotinic acid was listed in the third as a percentage. In terms of the nicotinic acid profile, the highest amount of nicotinic acid was found in the chicken breast sample in a quantitative amount among the meat samples, but the highest percentage was the lamb arm sample. The lamb arm sample contains 2.112 mg/100 g nicotinic acid, but also contains 2.143 mg/100 g nicotinamide so the presence of nicotinic acid and nicotinamide in the lamb arm was found to be 49.6% and 50.4% respectively.
The lowest amount of nicotinic acid in the samples was the lamb back with 0.424 mg/100 g and 8.5%. If we list the nicotinic acid levels in the meat samples from high to low; chicken (breast), turkey meat (thigh), veal (veal steak fillet), lamb (leg), lamb (arm), mutton (belly), bovine meat (loin), goat meat (belly), goat meat (leg) and lamb (back), respectively.
Another remarkable point is that the nicotinic acid levels in the meat samples differed in the meats belonging to the different regions of the animal. However, when comparing the amounts of nicotinic acid in the meat samples belonging to a region of the animal, it is also wrong to make a generalization. For example, while in 3 different lamb meat samples, the highest amount of nicotinic acid (mg/100 g) was found in lamb leg, in goat belly meat the amount of nicotinic acid was higher than goat leg meat.
The analytical method was successfully applied to determine the vitamin B3 profile of 10 cereal and legume samples; barley, rye, wheat (bread), wheat (durum), oat, rice, dried pea, green lentil, red lentil and chickpea, and the results are shown in Table 3.
In the cereals and legumes samples, the amounts of nicotinic acid were in the range of 30.4% to 100% and the nicotinamide concentrations were between 0% and 69.6%. In 10 plant based food samples, the highest amount of nicotinic acid containing sample was the durum wheat with 6.668 mg/100 g, while the lowest nicotinic acid containing sample was the oat with 1.025 mg/100 g.
The presence and amounts of nicotinic acid of the samples in mg per 100 g from high to low, wheat (durum), wheat (bread), barley, rye, green lentil, red lentil, rice, chickpea, dried pea and oat, respectively. When we assess in terms of the profile, 7 of 10 samples (barley, rye, wheat (bread), wheat (durum), oat, rice, green lentil) did not contain nicotinamide and as a consequence the concentration of nicotinic acid in these samples was 100%. In 70% of the cereal and legume samples was observed that only nicotinic acid form was present (there were only nicotinic acid form). While 70% of the cereal and legume samples were not in nicotinamide form, in 3 samples (dried pea, red lentil, chickpea) nicotinic acid form and nicotinamide form were seen together.
While the nicotinamide form was either absent or low level from nicotinic acid form throughout the samples, only in the pea sample the amount of nicotinamide was more than 2 times than the amount of nicotinic acid in quantity.
In consistent with the results of this study for the lamb meat total niacin content in the range of 4.3–5.8 mg/100 g, Williams reported in his study that 5.2 mg/100 g total niacin for the lamb meat in nutrient composition (per 100 g) of lean red meat list in the ‘Nutritional composition of red meat’ study [30].
According to the Table 3, we can say that the cereals and legumes are the best sources of nicotinic acid. Although nicotinic acid form and nicotinamide form were present together in the all meat samples, in 70% of cereal and legume samples, only nicotinic acid form was present.
In the cereal and legume samples, nicotinic acid concentrations were found to be significantly higher than the meat samples. Nicotinic acid concentrations were found in low amounts in the meat samples. Nicotinamide concentrations were much higher than nicotinic acid concentrations in all meat samples. The presence rates of nicotinic acid and nicotinamide were determined in the meat samples as 30% and 70% and as 87% and 13% in the cereal and legume samples, respectively.
As can be clearly seen from Table 2 and 3, in terms of vitamin B3 profile, the profile in the meat samples appears as a combination of nicotinic acid and nicotinamide while the profile in the cereal and legume samples show the predominant characteristic of nicotinic acid with 100% nicotinic acid form in 70% of the samples.
Nicotinic acid has been proven to have positive effects on blood lipid level. Clinically, high-dose nicotinic acid leads to reduced lipidemias, reduced progression of coronary heart disease, and reduced mortality [16]. Dyslipidemia is the abnormal lipid metabolism which is regarded as a strong predictor for the development of cardiovascular diseases (CVDs) and one of the most important risk factors for cardiovascular disease and diet is considered an important factor for the development of dyslipidemia [18].
Ahmad has been reported that incorporation of lentils in human diets may help in protection and management of CVDs and related disorders according to the results of the study which investigates the effects of lentil on serum lipidemia and cardiovascular indexes in cholesterol-fed rats [38].
Niacin is a type of B vitamin and is also important for converting food to energy. It is water-soluble, which means it is not stored in the body and leftover amounts of the vitamin leave the body through the urine. That means human need a regular supply of vitamin B3 in their diet.
In the literature, there are studies based on total niacin content of foods. The results of this study were compared with the US Department of Agriculture Nutrient Database and Turkish Food Composition Database. For example, for the wheat (durum), total niacin content was found 6.668 mg/100 g in this study. In consistent with the findings of this study, according to the USDA, total niacin content of wheat (durum) is 6.738 mg/100 g in National Nutrient Database [39]. In addition, according to Turkish Food Composition Database (TURCOMP), total niacin content of wheat (durum) 6.650 mg/100 g [40].
In the literature, niacin sources are generally reported as meat, fish and poultry etc., but in particular their profiles should also be taken into account. The results of the analysis showed that the total niacin content of meat samples was high but, their profiles say many other things, the profiles showed a very valuable information as the levels of nicotinic acid were low.
Jenkins et al., stated that the National Cholesterol Education Program Adult Treatment Panel III (ATP III) and the American Heart Association have been recommended the use of functional foods or foods high in components that reduce cholesterol as additional options to enhance the effectiveness of cholesterol-lowering diets to increase the relevance of dietary advice for the primary prevention of cardiovascular disease. These functional foods and components include viscous fibers, soy protein, plant sterols, and nuts. Jenkins et al., have been assessed the efficacy of these cholesterol-lowering components when combined in the same diet to determine whether predicted reductions in LDL cholesterol of 30% could be achieved by diet, in a series of metabolically controlled studies. They found that, more than 30% of motivated participants who ate the dietary portfolio of cholesterol-lowering foods under real-world conditions have been able to lower LDL-cholesterol concentrations > 20%, which has not been significantly different from their response to a first-generation statin taken under metabolically controlled conditions [25].
Chen et al., also summarized in their review that the findings of recent studies on the production, application, efficacy, and mechanisms of popular cholesterol-lowering nutraceuticals and functional foods. According to them; a nutritionally balanced diet that reduces saturated fat and cholesterol intake has traditionally been the first goal of dietary therapy in lowering plasma TC and recently nutraceuticals and functional foods have attracted much interest as possible alternative therapies for lowering plasma TC, especially for hypercholesterolemia patients, whose blood cholesterol level is marginally high (200–240 mg/dL) but not high enough to warrant the prescription of cholesterol-lowering medications [27].
As it is well known, the soluble fiber in foods reduces the concentration of elements required for cholesterol synthesis in the liver by inhibiting the absorption of bile acids in the intestine. In addition, gamma tocopherol, which is found in fiber sources such as oats, barley, rice husks, reduces serum cholesterol by preventing cholesterol synthesis in the liver. Shamliyan et al. reported that the presence of 5–10 g soluble fiber in daily diet reduces the risk of cardiovascular disease and death and consuming foods rich in viscous soluble fiber reduces LDL cholesterol blood levels 10% to 15% with expected reduction in CVD events by 10% to 15% [26].
Mclntosh et al., studied the influence of two sources of dietary fiber (nonstarch polysaccharides, NSP) on blood lipids and glucose concentrations. 21 mildly hypercholesterolemic men aged 30–59 y have been provided with comparable barley and wheat foods for each of 4 week in a crossover-designed experiment. Barley contains β-glucan as a source of soluble dietary fiber whereas wheat contains the largely insoluble cellulose and hemicellulose fiber. Consumption of barley relative to wheat foods has been associated with a significant fall in both plasma TC (6%,p < 0.05) and in low-density-lipoprotein cholesterol (7%,p < 0.02) whereas triglyceride and glucose concentrations has not been changed significantly. They have been concluded that barley dietary fiber is more effective than wheat dietary fiber at lowering blood cholesterol in hypercholesterolemic men [24].
Kirby et al. suggested that palatable and inexpensive high-fiber foods such as oat bran may have a role in the treatment of certain patients with hypercholesterolemia. They have been fed control and oat-bran diets in an alternating sequence to eight men with previously documented hypercholesterolemia to evaluate selected metabolic effects of plant fibers. They found that serum TC concentrations were stable on control diets whereas a progressive reduction was observed in seven men on oat-bran diets. On oat-bran diets, average reductions in serum TC concentrations found 13% (p < 0.01, n = 8); plasma LDL cholesterol concentrations found 14% lower (p < 0.05) while high-density lipoprotein cholesterol concentrations have not been changed [23].
Health is vital for sustaining life and a healthy, adequate and balanced diet is essential for the preservation and maintenance of health. In order to have a healthy diet, everyone needs to know the nutritional composition of the foods they eat, as well as the amount of important vitamins and their profiles in the nutrients. In literature, no recent data is available about the profiles of vitamin B3 of foods such as meat, cereal and legume that are eaten everyday by human. Moreover, numerous people frequently suffer from lack of nicotinic acid in their daily diets like people with high blood cholesterol levels. Nicotinic acid deficiency is one of the major dietary problems of people with hypercholesterolemia. People usually think they meet the needs of nicotinic acid by consuming meat, fish and poultry etc.in their daily diet, because of vitamin B3 is often referred to as nicotinic acid in the literature, which are known as vitamin B3 rich foods.
Conclusion
Niacin has many important biological functions in humans and we need to have more detailed and accurate information about it therefore, the determination of niacin profiles in biological samples is important for evaluating nutritional conditions. This study was conducted in order to determine the vitamin B3 profiles of 10 kinds of animal based food and 10 different plant based food samples by HPLC using post-column derivatization system. There is no available data and comprehensive study published in the literature regarding the vitamin B3 profile in meats, cereals and legumes. This study will provide the vitamins B3 profile of some animal based foods and some plant based foods. However, this study reported of vitamin B3 profiles in a limited number of samples and hopefully, these new values will serve as a useful means to calculate dietary intake of vitamin B3 in the general population. These data will also be useful in the preparation of a complete food composition table, preparation of diet therapy, food based dietary guidelines, nutrition education, food security, safety and regulation, labelling of food in food industry, nutritional survey and also for other research aims.
















