INTRODUCTION
Poultry is the second most consumed meat by humans, providing protein and essential nutrients [1]. As the poultry industry has developed and awareness of health and nutrition has increased, the demand for high-quality meat has begun to increase. Factors affecting poultry meat quality are diverse and correlated with each other [2,3]. Among various factors determining meat quality, water holding capacity (WHC) is the most important parameter because WHC determines pH, drip loss (DL), cooking loss (CL), storage, and other qualities [3,4]. Bowker et al. [4] have reported that WHC is positively correlated with pH, but negatively correlated with DL.
Chromium (Cr) is known as a trace element that is present in feed at very small amounts. Although there is no requirement for Cr in poultry feed, many recent studies have determined effects of Cr on physiological, immunological, and growth performances of poultry [5–7]. Cr exists in its most stable trivalent form when supplemented with feed. However, inorganic Cr is poorly absorbed in broilers [8]. To improve this absorption rate problem, Cr can be chelated with substances such as yeast, methionine, and picolinate to supplement feed in the form of organic chromium (OC) [9]. When Cr is supplemented in the feed of broilers, it not only improves meat quality, but also improves antioxidant action [10,11]. Mir et al. [12] have reported that Cr supplementation in broiler feed can reduce DL and lipid oxidation in broilers.
Selenium (Se) is an essential trace element required by humans and animals. It is a nutrient that can improve nutritional value and quality of meat products [13,14]. The recommendation for Se supplementation in broiler diets is 0.15 ppm [5]. The FDA [15] has approved selenium supplementation up to 0.3 ppm in broiler diets. Previous studies have reported that 0.3 ppm Se supplementation in broiler feed is appropriate considering growth performance (e.g., body weight [BW] and feed conversion ratio [FCR]) [16–18]. Cemin et al. [19] have reported that broiler diets supplemented with Se at much higher than recommended levels can improve growth performance, carcass yield, and breast yield. Inorganic Se is toxic with a low bioavailability. It is mainly supplemented with organic Se in broiler feed, which has high bioavailability. Selenomethionine (SeMet) is a Se compound present in plants and feed grains with a high absorption rate similar to amino acids [20]. When broiler feed is supplemented with SeMet, it has antioxidant, intestinal immune function control, anti-stress, intramuscular Se deposition, and meat quality improvement effects [21–24].
Studies on effects of OC and SeMet cocktail supplementation in broiler feed are limited. Therefore, the objective of this study was to investigate effects of OC and SeMet cocktails in different ratios on broiler chickens when supplemented in feed.
MATERIALS AND METHODS
The experimental protocol was approved (CBNUA-2010-22-01) by the Institutional Animal Care and Use Committee of Chungbuk National University, Cheongju, Korea.
A total of 168 one-day-old broiler chicken (Arbor Acres) were randomly allotted to 3 groups based on the initial body weight of 37.33 ± 0.24 g with 7 replicate per 8 birds (mixed sex). The experiments period was performed 28 days. Dietary treatments were folloewd Basal diets based on corn-soybean meal (CON), CON + 0.2 ppm OC and 0.2 ppm SeMet (CS4), and CON + 0.4 ppm OC and 0.4 ppm SeMet (CS8). The NRC [5] designed all diets to meet or exceed the nutritional needs for poultry. Compositions of basal diets are shown in Table 1. The birds were fed ad libitum and they had free access to the water.
Supplied per kg diet: vitamin A, 9,000 IU; vitamin D3, 3,000 IU; vitamin E, 48 mg; vitamin K, 3 mg; thiamin, 1.8 mg; riboflavin, 6 mg; pyridoxine, 3 mg; vitamin B12, 0.012 mg; niacin, 42 mg; folic acid, 1.2 mg; biotin, 0.24 mg; pantothenic acid, 12 mg.
Growth performance was measured by weekly BW and feed intake (FI). The body weight gain (BWG) was calculated by subtracting the BW of the previous week from the BW of this week, and the BWG of 1 to 7, 8 to 14, 15 to 21, 21 to 28, and 0 to 28 were measured. The FI was derived by subtracting the residual amount from the feed amount. FCR was calculated by dividing the measured FI by BWG.
All treatment groups were supplied with 0.2% Cr2O3 in the feed 3 days before the end of the experiment. Two days before the end of the experiment, fresh poop was collected through rectal massage, sealed, and stored at −20°C. The feed and feces of all treatment groups were dried in an oven at 50°C for 72 hours and then ground to a fine powder. The sample was multiplied by 6.25 and the crude protein was determined by titrating N in accordance with the Kjeldahl Method. The equation for calculating apparent total tract digestibility (ATTD) follow as: 100 − [(concentration of nutrient in fecal Cr2O3 feed) / (concentration of nutrient in diet Cr2O3 fecal) × 100].
All broilers had 2 mL of blood drawn from the wing veins prior to slaughter, which was collected in vacuum tubes containing K3EDTA and a tube free of heparin for serum analysis. The collected blood samples were centrifuged for 20 minutes at 12,500×g at 4°C. An automatic hematology analyzer (XE2100D, Sysmex, Kobe, Japan) was used to examine red blood cell (RBC), white blood cell (WBC), heterophil, and lymphocyte samples. For total antioxidant status (TAS) analysis, the serum samples obtained by centrifugation of the blood were measured using a total antioxidant capacity assay kit, such as the ELISA kit (EK780137, AFG Scientific, Northbrook, IL, USA). The uric acid levels were measured using the Qualigent UA (Qualigent UA, SEKISUI Medical, Tokyo, Japan) reagent through an enzymatic assay method (Labospect008AS, Hitachi, Tokyo, Japan).
At the end of the experiment, all broilers had a 2 cm resection of the duodenum, jejunum, and ileum. The duodenum was resected midway, the jejunum was resected from the entrance of the bile duct, and the ileum was resected toward the junction of the Meckel’s diverticulum and the cecum. Samples taken for intestinal morphology analysis were fixed in 10% neutral buffered formalin ([NBF] Sigma-Aldrich, St Louis, MO, USA). The sample was put on the slide, then paraffined and stained with hematoxylin and eosin. Using an Olympus IX51 inverted phase-contrast microscope, field morphology was examined. When analyzing intestinal morphology, it is important to consider the villus height (VH), crypt depth (CD), and villus height to crypt depth ratio (VH : CD).
At the end of the experiment, broiler breasts and drum stick meat were collected and stored in vacuum packs. Meat content analysis of breast meat and durm stick meat water, protein, fat, and ash contents were analyzed according to the AOAC method. The Laakkonoen et al. [25] method was used to analyze WHC. DL was calculated as the weight ratio (%) of the initial sample by measuring the DL produced during the circular shaping of a 2 cm thick breast and drum stick, placing it in a polypropylene bag, vacuum-packing it, and storing it in a refrigerator at 4°C for 24 hours. The CL was calculated using the weight of a 3 cm thick chicken breast and drumstick meat that had been formed into a circle, heated in a hot water heater to 70°C, and allowed to cool for 30 minutes. A rheometer (Compac-100, Sun Scientific, Tokyo, Japan) was used to measure shear force (SF) during a shear force cutting test. After homogenization with a homogenizer (Bihon seiki, Ace, Tokyo, Japan), the pH was determined using a pH meter (Mteeler Delta 340, Mettler-tolede, Leicester, UK). Using a spectro colormeter (Model JX-777, Color Techno. System, Tokyo, Japan) calibrated on a white plate, the meat’s color was assessed (L*, 94.04; a*, 0.13; b*, −0.51). Se analysis is performed using inductively coupled plasma mass spectrometry (ICP-MS, ELAN DRC II, Perkin Elmer, Waltham, MA, USA) on a solution obtained by adding 0.5 g of breast meat and drumstick meat samples to a microwave digestion system and treating them with nitric acid.
The 16S amplicon sequencing was commercially commissiond to Sanigen (Sanigen, Anyang, Korea). In brief, the sequencing data were produced by MicroSeq with 300-bp paired-end. Raw reads were trimmed, quality filtered, pair-merged and denoised using divisive amplicon denoising algorithm 2 (DADA2) plugin [26] in Qiime2 software 2021.11 distrubution [27]. With end product of DADA2, amplicon sequence variants (ASV) were defined and used in downstream analyses. Taxonomic classification analysis was performed for the ASVs using Qiime2 Naïve bayes classifier.
The experimental unit in all studies was the cage, and the collected data were analyzed using the General Linear Model (GLM) procedure of SAS software (SAS Institute, Cary, NC, USA). Differences in statistical analysis were assessed using Tukey’s multiple range test, with statistical significance set at p < 0.05.
RESULTS
OC and SeMet cocktail supplementation were not significant in weekly measured BW (Table 2). BWG, FI, and FCR did not show significant differences by supplementation with different levels of OC and SeMet cocktails.
The ATTD of DM, CP, and GE did not show significant differences by supplementation with different levels of OC and SeMet cocktails (Table 3).
| Items (%) | CON1) | CS4 | CS8 | SE | p-value |
|---|---|---|---|---|---|
| DM | 77.30 | 78.51 | 79.98 | 1.094 | 0.273 |
| GE | 79.30 | 80.38 | 82.08 | 0.997 | 0.195 |
| CP | 75.10 | 74.92 | 75.58 | 1.300 | 0.935 |
WBC, RBC, heterophil and lymphocyte were not significantly affected by OC and SeMet cocktail supplementation (Table 4). In uric acid, CS8 group was significantly lower than CON group. CS8 group was significantly (p < 0.05) higher TAS compared to CON group.
In the jejunum and ileum, supplementation with OC and SeMet cocktail had no affected (Table 5). In the duodenum, the CS4 and CS8 groups had significant (p < 0.05) increased in VH compared to the CON group.
In breast meat content, Se deposition was significantly (p < 0.05) higher in CS8 group compared to CON group (Table 6). Also, in breast meat characteristics, WHC was significantly (p < 0.05) higher in the CS8 group than in the CON group. CL was significantly (p < 0.05) lower in CS4 group compared to CON group.
In drum stick meat content, ash was significantly (p < 0.05) higher in CS8 group than in CON group (Table 7). In the groups supplemented with CS, the WHC was significantly (p < 0.05) higher than CON group. In CL, the CS-supplemented groups were significantly (p < 0.05) lower than CON group.
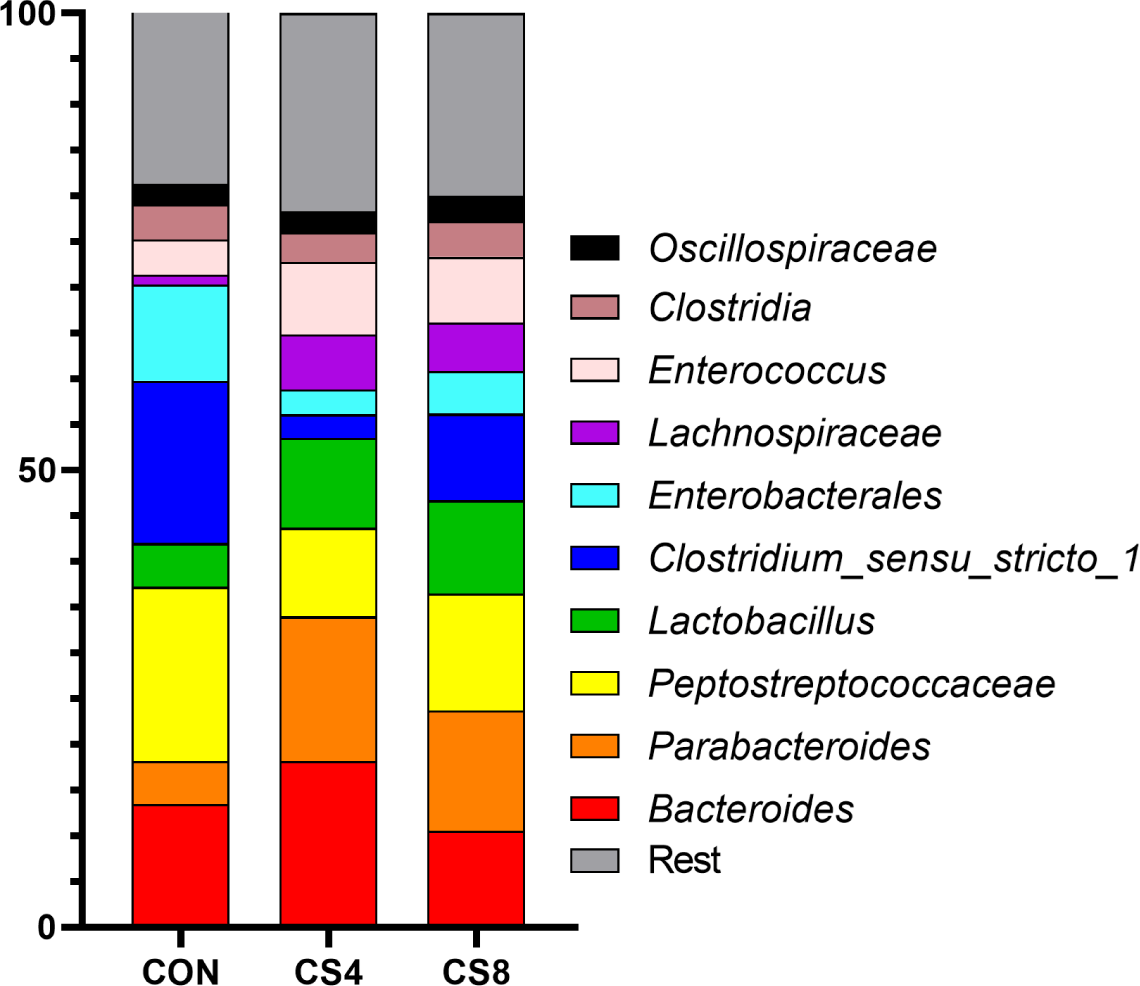
In genus level, the CS8 group showed significantly (p < 0.05) higher levels of Lactobacillus than the other groups (Table 8) (Fig. 1). In addition, Enterococcus was significantly (p < 0.05) lower in CON group compared to CS supplemented groups.
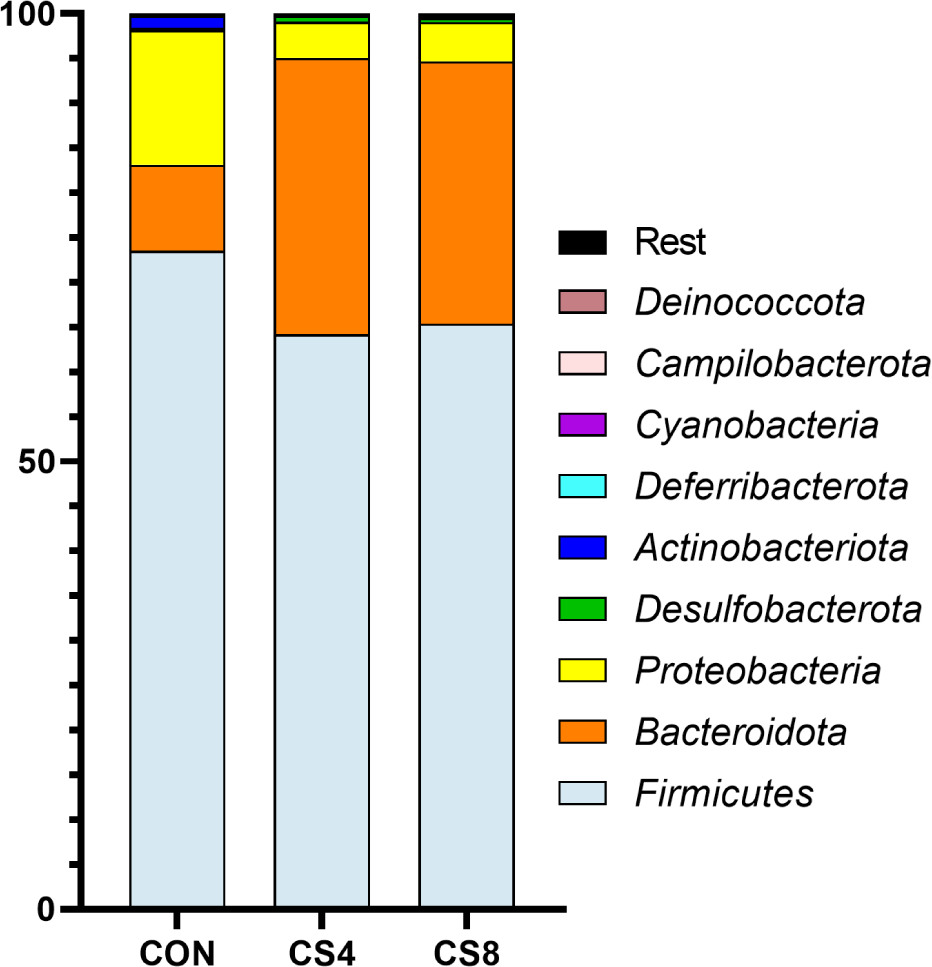
In phylum level, Bacteroidota showed a significantly (p < 0.05) higher CS8 compared to CON group (Table 9) (Fig. 2). Actinobacterota was significantly (p < 0.05) higher in CON group than in CS-supplemented groups.
DISCUSSION
Cr plays a role in regulating insulin action and improving glucose and lipid metabolism through regulation of fatty acid synthase and lipase [28–31]. Se is known to be an important trace element for the growth of broilers. It is involved in antioxidant reactions and hormone secretion pathways. However, in this study, it showed no effect on growth performance or nutrient digestibility. Cr and Se are supplements widely used to protect broilers from environmental stress. They are considered to have no effect under normal stress-free conditions like in this study.
Uric acid in blood can be used as an indicator of amino acids utilization. Changes in serum urea nitrogen and concentration are consistent [32]. Although uric acid has an antioxidant function, its high levels in blood can cause health problems in broilers [33]. Sun et al. [34] have reported that Se deficiency can increase blood uric acid. However, Cr supplementation is not known to reduce blood uric acid in broilers [35–37]. In this study, CS8 group decreased blood uric acid compared to CON group. This suggests that CS8 group can improve amino acid utilization in broilers and Se deficiency in CON group.
Se is incorporated into selenoproteins in the form of selenocysteine, an amino acid, to function as glutathione peroxidase and melanocytes to protect against oxidative stress [38]. Tang et al. [24] have reported an increase in total antioxidant capacity when feed is supplemented with OH-SeMet. Zduńczyk et al. [39] have reported that 0.3 ppm Se supplementation can increased TAS of laying hens compared to 0.15 ppm Se supplementation. Cr can act as an indirect antioxidant by reducing elevated insulin and preventing glucose oxidation [40,41]. In the existing environment, OC can enhance the antioxidant capacity compared to inorganic chromium [42]. In this study, the CS8 group showed higher TAS compared to the CON group.
Intestinal morphology characteristics of VH and CD are important for nutrient absorption and intestinal health [43]. When villi are shortened, the surface area is reduced and the rate of nutrient absorption is reduced [44]. Antioxidant supplementation can protect enterocytes from apoptotic oxidative stress and improve intestinal morphology [45]. Huang et al. [7] have reported that supplementation with chromium propionate in broiler diets can increase VH under heat stress conditions. However, the exact mechanism by which CS8 could improve growth performance of poultry is not clear. Cr and Se are mostly supplemented under heat stress [46,47]. Research in the existing environment is insufficient. Further studies are needed to determine effects of Cr and Se supplementation on intestinal morphology in the conventional environment.
Recently, research on the development of high-quality meat is increasing, in line with consumers who are increasingly aware of functional foods. In this study, CS was added to feed to improve meat quality. As a result, Se deposition in breast meat was improved. Characteristics of breast and drum stick meat were improved. Among meat quality factors, WHC is one of the most important characteristics of chicken related to DL, CL, pH, and protein with the ability to retain water [3,48,49]. Silva et al. [50] have reported that WHC could be measured by DL and CL. Supplementation of Nano-Se [51], SeMet [50], and OH-Met [24] to broilers could improve WHC. Mir et al. [12] have reported that WHCs of refrigerated breast and thigh meat are improved when broilers are supplemented with 1.5% chromium in flaxseed compared to flaxseed supplemented broilers. WHC and pH are known to be correlated. However, in this study, there was no significant difference in pH between breast and drumstick meat [4]. Cai et al. [51] has reported that WHC could be improved by reducing DL by improving antioxidant level. Considering that the CS8 group in this study had higher TAS than the CON group, WHC was improved by improving antioxidant ability regardless of pH. In this study, ash was significantly higher in the CS8 group in drum stick meat because the mineral intake due to supplementation of SeMet and OC was higher than that of the CON group. However, various studies reported no significant difference in the ash content of broiler meat due to chromoium supplementation. Based on this, the evidence of ash content in the drum stick meat of the CS8 group is considered to be Se or other minerals [52–54]. As a functional food, meat that can supply Se to humans is an important nutrient source [24]. Se is an essential component of the antioxidant enzyme system. It is an important nutrient for broilers as it interacts with vitamin E within the cell membrane [55]. Many researchers have conducted studies on Se supplementation in feed for broiler nutrition and Se deposition in meat [47,50,56]. In this study, the CS 8 group showed higher deposition of Se than CON group grow up. It coincided with a previous study reporting that organic selenium is more efficiently deposited in the body than inorganic selenium [57]. Various studies have reported that supplementation with 0.2 and 0.4 ppm Se can increase Se deposition and that improved antioxidant levels might contribute to selenoprotein expression [58,59]. Silva et al. [50] have reported that supplementing broilers with 0.6 ppm SeMet can promote deposition of 0.267 mg/kg breast meat, which is 40.05 μg, 70% of adult human requirement. In this study, 0.23 mg/kg Se was deposited in breast meat, which was 57% of adult human requirement. Taken together, these results suggest that CS8 supplementation in broiler feed is effective in improving meat quality and depositing selenium in meat.
The gut microbiome plays a crucial role in the health and gut environment of broiler chickens. The composition of this diverse microbiome is influenced by various factors, such as diet and stress [60,61]. Supplementing antioxidants in the feed can also impact the gut microbiome [62, 63]. Gangadoo et al. [64] reported that including nanoselenium (0.9 mg/kg) in the diet led to an increase in the abundance of Lactobacillus. In this study, similar results were observed at the genus level for the treatment groups supplemented with CS. However, the mechanism of OC and SeMet supplementation on the broiler gut microbiome has not yet been fully understood, and further research is needed. Therefore, further studies are required to investigate the changes in Firmicutes, Bacteroidota, Proteobacteria, and Actinobacteriota at the phylum level.
CONCLUSION
When supplemented with 0.4 ppm of OC and SeMet in the broiler feed, Se deposition was the highest in breast meat, and uric acid, TAS, jejunum morphology, and meat quality were improved. In conclusion, OC and SeMet cocktail supplementation may have beneficial effects on high-quality meat production and physiology in broilers.
















