NEED FOR AN ALTERNATIVE SOURCE OF MEAT
Historians and evolutionary biologists now comprehend infectious diseases as the forces that selectively decide our future and genome. Dietary patterns of domesticated animals along with the environment drives the emergence of such infectious diseases. Proximity and interactions between humans and the animal populations are the major underlying causes of this phenomenon [1]. The recently emerged and ongoing COVID-19 pandemic is one such example, which is assumed to have originated in Wuhan, China [2,3]. Origin of this virus is possibly via a zoonotic transfer from forest-based reservoir animals to humans [4,5]. Zoonotic diseases are prone to emerge and re-emerge, whereby their incidence and frequency are intricately linked and based on the agricultural-environmental nexus. Further, a similar study has provided molecular evidence of zooanthroponosis, the transmission of disease from humans to animals [6,7].
On a global scale, emerging infectious diseases (EIDs) and growing hotspots are based on demographic, environmental, and biological factors (Fig. 1). Interestingly, these two aspects have been reported to be correlated with the rise of zoonotic EIDs, whereby forest encroachments enhance/elevate this risk.[8]. There has been a significant increase in the occurrence of EIDs. Reportedly, around 73% of them (roughly accounts to 335 EID events occurred between 1940 and 2004) are zoonotic origin [6,9]. Livestock not only contributes majorly to global warming but it also plays adverse roles in zoonotic EIDs. Maintaining health, performance, and production of livestock has turned out to be exceedingly difficult. Although constant searches for better animal feeding system to improve healthiness and productivity of livestock animal are in progress, no significant advancements suitable for existing or future populations have been discovered yet. In general, feed supplements, such as subclinical dosage of antimicrobials, plant extracts, probiotics, prebiotics, or a combination of them control livestock infections [10–14]. Although such measures are taken, EIDs and food-borne outbreaks, global warming, huge land usage, and forest encroachments are still on the rise and inevitable.
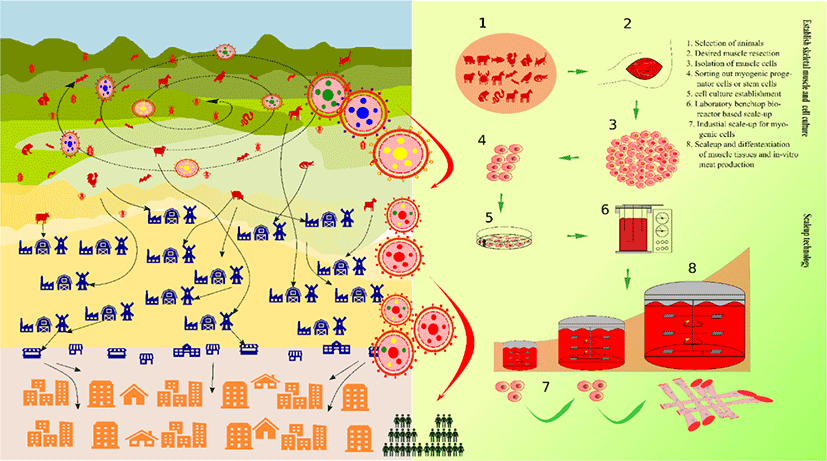
Meat is a reliable source of protein and energy. A majority of the world population constitute meat-eaters. Evolution and social interactions are the major reasons that have accounted for our carnivorous nature. Our ancestors moved from the hunter-scavenger lifestyle to animal farming by domesticating wild animals as a custom that ensured food security. However, such practices have become a threat to the planet’s biotic and abiotic resources in the contemporary times [15,16].
It is estimated that by 2100 the world population would increase by at least 9.6–12.3 billion, a number that is enormous as compared to the current scenario [17]. Furthermore, malnutrition is currently a significant problem worldwide. To overcome global hunger and undernutrition, the overall push towards food security has improved sustainable food production. Although food production has improved qualitatively and quantitatively, global availability of resources often leads to various environmental, food-related, and health issues [18–20]. It is quite evident that anthropological meat-eating affects climate change to a great extent. However, the global population cannot be forced to abandon meat consumption. Thus, novel technology-based innovations that can help overcome such issues by developing alternative forms of meat which can be multivalued based on health and environmental factors are of utmost importance.
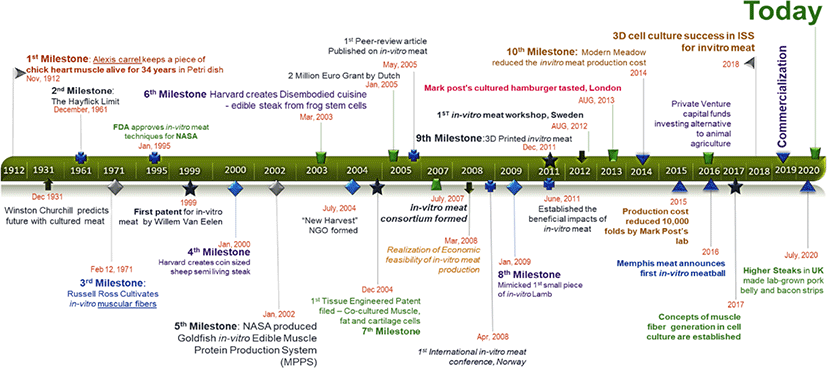
Substantial advancements in livestock can be witnessed since the commencement of the industrial revolution. The future of livestock relies on the present technological revolution, now possible with the state of the art technologies in robotics and sensing toolkits [21]. Food engineering has unlocked many such solutions till date, an alternative to animal meat being one of them. With the advent of breakthrough milestones in stem cell technology (Fig. 2), it is now possible to direct stem cells towards highly differentiated cells, for instance, satellite cells (SCs) can be directed towards skeletal muscle cells in many animals [22,23]. A single cell can be used to produce massive quantities of skeletal muscle cells by way of stem cell technology which is better known as in vitro meat (IVM), in vitro meat agriculture (IMA), or cultured meat; this innovation is a promising alternative to livestock. In a news feature in October 2009, Jeffrey L. Fox highlighted IMA’s potential to be served as a “test tube made of meat” by 2022 [24]. In August 2017, Amber Dance featured IMA approach as an option to meat and other animal-derived products, such as milk, eggs, and even leather. Therefore, IMA is a promising alternative to meat, whereby it is a multifaceted solution for food, health, and environmental issues [25]. The fact that IMA-derived products can be customized according to one’s needs is advantageous over conventional animal farming. In contrast, customization of livestock animals, such as genetic modifications, feed modifications, usage of antibiotics and synthetic hormones, etc., which are carried out for the sake of better animal performance, marbling scores, and carcass weight, often can create hurdles.
Although IMA is in its infancy stage at present, future possibilities that lie ahead are larger than expected, as witnessed in case of other technological innovations. Bovine cultured meat was commercially produced first in 2013. Since then, many startups have risen producing a variety of animal culture meat and products. At least 35 such startups worldwide have exponentially produced poultry, bovine, pork, and marine animals, including salmon, other fish, shrimps, since 2013, covering 85% of the total cultured meat market size. On the other hand, horse, kangaroo, mouse, and other animal types cover share of this market [26].
Although IVM is gaining enough attention, its production has not been streamlined. To date, startups follow the necessary measures for IMA and the cultured meat is considered safe. However, no government has assembled regulatory bodies that completely assesses the safety parameters required for producing IMA. Cultured meat production is bound to face investment crises in the absence of such regulatory bodies. Thereby, the industries expected governments to set up regulatory guidelines for large-scale production. Between July and October 2018, the Departments of Health and Human Services announced in public meetings the joint development of a regulatory framework for cell-cultured meat production, including hazards and labeling. Services of the U.S. Food and Drug Administration (FDA) and U.S. Department of Agriculture (USDA) Food Safety and Inspection Service (FSIS) hazard analysis and critical control point (HACCP) are well documented. In case of cell-cultured meat, both the agencies will work together; FDA’s risk base approach coupled with FSIS’s regular inspection oversight approach are considered to mitigate contamination problems during cell culture or cell-cultured meat production [27].
Both the FDA and USDA have assured that this regulatory framework can be successfully implemented and guarantees safety. These agencies have undertaken the responsibility to monitor initial stages of cell culture and their multi-stage governance has agreed to regulate the production and labeling of cell-cultured meat [28,29]. On March 7, 2019, USDA-FSIS and health and human services (HHS)-FDA jointly announced the formal agreement that addresses the joint regulatory frame for cell-culture meat production. The agreement includes inspecting and overseeing human food produced by cell culture, derived from cell lines of USDA-amenable species [30].
There lacks an appropriate direction that allows choice of cells for cell-cultured meat production, as of now. The underlying technology and strategy rely on stem cells or precursor cells, which are of many types. In addition, right from proliferation to differentiation of the skeletal muscle cells, various culture conditions and materials, including the use of animal-based serum, encounter many ethical and technical issues. Thus, the scientific, ethical, and legislative issues that span collection of tissue samples up to mass cultivation must be crucially considered and successfully resolved.
Moreover, use of genetically modified organisms (GMOs) or engineered products, such as growth promoters and serum alternatives, in cell culturing or cell-cultured meat production is a great challenge. The harvested cells should be free of any microbial contamination, including bacteria, fungi, and viruses. Contamination by other genetic materials, such as drug-resistant plasmids, should be monitored as well. Further, presence/use of toxic substances, allergens, and any adulterants should be avoided. Moreover, biomass produced from microorganisms, such as microalgae, is the alternative potential source of cell culture media [31]. Recent amendments of the regulatory system by the EU (European Union) and US-based aforementioned bodies are expected to accelerate this technology in the forward direction in both research and industry. However, the current regulations are primitive; thus, only minimal extent to mitigate the hazards and future risks are possible as of now. Additionally, regulatory bodies must periodically update these guidelines to prevent any unexpected hazards effectively.
REQUIREMENTS FOR PRODUCTION OF CELL-CULTURED MEAT
In 1961, Alexander Mauro for the first time reported SCs, wherein he assumed them to be related with muscle fiber regeneration [32]. Today, this is an established concept and is a key component for the future IMA revolution. Molecular regulations involved in culturing of embryonic stem cells and skeletal muscle cells has been well established and reviewed previously [33]. Briefly, during the course of muscle development an animal requires coordinated events, namely regulated myogenic signaling cascade followed by proliferation, differentiation, and maturation of the progenitor cells, to form skeletal muscle cells. Anatomically, these stem cells reside beneath the basal lamina surrounding the myofibers; they are self-renewal and function as the source of regeneration [34]. SCs express paired box transcription factors paried box (Pax) 3 and Pax7, along with basic helix-loop-helix factors myoblast determination protein (MyoD), myogenic factor (Myf) 5, Myf6, and myogenin (often termed as the myogenic regulatory factors, MRFs). These transcription factors can be observed in cluster of differentiation (CD) 56+CD29+CD31−CD45− cell population; they are highly conserved and have been recently characterized in porcine cells [35,36]. Apart from SCs, another suitable progenitor candidate is PW1+ interstitial cell (PIC). Although they express Pax3 and Pax7 similar to SCs, PICs are more plastic with the expression of octamer-binding transcription factor (Oct) 3/4, sex determining region Y (SRY)-box transcription factor (Sox) 2, and Nanog. Moreover, SCs are only confined to skeletal muscle cell differentiation, whereas PICs can differentiate to skeletal as well as smooth muscle cells and adipocytes. In addition, differential gene expression analysis revealed that SCs express only the myogenic commitment gene sets, while PICs are also related to mesenchymal stem cell markers, indicating their multipotent nature. Certainly, stem cells antigen (sca)-1 marker can be utilized to identify PICs’ isolation at certain time point during development, since this marker is present only in the first three weeks of postnatal age, but it disappears later [37–40]. Other molecular regulators of proliferation and differentiation include microRNA(miR)-1, miR-206, and miR-133.
Many model approaches have been used for the mass cultivation of animal meat. With the passage of time and corresponding technological advancements, various methods have progressed in this regard. Although cell-cultured meat has garnered much attention, its production continues to face economic and technical challenges. The cultivation methods include 2 dimension (2D) and 3D culturing models, which have been discussed previously [41]. The following section briefly summarizes these models.
2D models where cells can be cultured in Petri dishes or cell culture factories. Although the 2D model has limitations with respect to mass cultivation, it serves as an efficient and beneficial model at laboratory research for small-scale optimization. On the other hand, 3D models provide an array of options; they are comparatively less limited as compared to the 2D models for cultivation. However, it is exceedingly difficult to optimize the cultivation condition, which is still at the preliminary research stage and would require more efforts and time before it reaches large-scale production [35,42,43].
The 3D models (Table 1) include gel-based and scaffold-based approaches. In case of an in-gel system, a variety of biomolecules, such as collagen, fibrin, etc., can be embedded. Moreover, these gel systems can contain uniformly distributed cells, rendering efficient mimicking of the natural tissue mechanical responses production [35,42,43]. In the scaffold-based approach, biopolymer utilization is the alternative. Various scaffolds are available that can be used for requirement-based customization. Scaffolds are highly customizable with respect to mechanical stiffness, degradation upon vasculature, flexible architecture, and in vivo mimicking [41]. Apart from the animal origin biomaterials alternatively plant, microbial origin or synthetic edible food grade polymers should be considered for the scaffolds and microbead synthesis. One of the major issues in producing scaffolds are the synthesis methodology involves various harsh chemicals which makes the end product as non-edible scaffolds though the initial raw materials are edible grade. Few plants based promising biopolymers are polysaccharides like amylose and its derivatives, polyesters, alginates, chitin, hyaluronic acids etc. In case of scaffold based 3D culture perfusion of nutrition is also one of the major issues, apart from that accessibility for the cells to migrate inside the matrix is also difficult if the porous space is too small. Considering both the factors together in scaffold-based technology both nutrition perfusion and increased porous with high degree of matrix will enhance the cell growth which will avoid the cell growth only on the surface. Notably, these methods are called tissue engineering techniques. Further, availability of recent advancements including 3D printing has elevated these approaches. Recently, 3D bioprinting of the human skeletal muscle cells and tissues restored muscle function [44]. The final product from the scaffold should be able to hold the water upon cooking and integrity of the structure also plays a key role, hence the material’s stability also influence the texture and palatability, so the market value. On the other hand, microbeads-based cultivation has so far progressively up-scaled cell production, owing to their relatively larger surface area [45]. However, this technique can only increase the cell number, which is its major limitation [46]. Further, cell-based whole meat production is not yet possible, which requires further processing that can reproduce proper texture, appearance, taste, and flavor like meat from animal. In summary, the aforementioned culturing methods have various advantages and are highly popular in the present times owing to their feasibility and success rates. Even though such advance technologies exist, the question of scaling up cell-cultured meat production to an industrial level still exists.
In 1917, Warren H. Lewis and Margaret R. Lewis first described muscle formation [47] and the terms myotubes and myofiber were coined by Jorge Francisco Tello in the same year [48]. However, no sustained control over the cell types and their final fates has been reported to date. This gap can be filled by a combination of biophysical and biochemical elements that render controlled methods in IMA [49].
Skeletal muscle cells are the basic units of myotubes and myofibers. Differentiating progenitor cells exhibit limited mitotic proliferation prior to myotube formation, but subsequently, exhibit no nuclear proliferation. Skeletal muscle cell cultures involve two phases, namely proliferation and differentiation. Proliferation of progenitor cells determines the quantity of cultured meat production, whereby higher rate of expansion of cells is achieved by increasing the efficiency of cell doublings. Differentiation is an important phase to achieve the required characteristics for IVM. Moreover, as mentioned before, controlling SC population in its progenitor state is crucial, wherein many factors are involved to maintain the stem cell or progenitor state [50].
In case of IMA culture methods, extrinsic regulators should be chosen to avoid GMO issue. Extrinsic regulators involved in myogenesis that have been hardly trialed and optimized till date, should be considered for mass culture methods such that further progress in the field of IMA is met. A decade ago, there was no proper evidence to be considered for controlling IVM culture in a controlled manner. One such paradigm is fibronectin, an essential and adequate factor for wingless-type protein (Wnt) 7a signaling through frizzled class receptor (FZD) 7/stearoyl-CoA desaturase (Scd) 4 that can regulate the number of SCs [33]. In addition, SC status is regulated by metabolic activity, for instance reduced nicotinamide adenine dinucleotide (NAD)(+)-silent mating type information regulation 2 homolog (SIRT) 1 activity retains its progenitor property, by way of metabolic control of H4K16 acetylation. Strategic metabolic control over NAD(+)-SIRT1 activity can help in sustained retention of the SC status [51]. Other extrinsic factors that regulate SC status via facilitating proliferation but antagonizing differentiation are collagen IV, transforming growth factor (TGF), insulin-like growth factor (IGF), Hepatocyte growth factor (HGF), basic fibroblast growth factor (bFGF), brain-derived neurotrophic factor (BDNF), epidermal growth factor (EGF), Tumor necrosis factor-like weak inducer of apoptosis (TWEAK), and Delta-1 [42,43,52]. In differentiation and myofiber formation, soluble extracellular domain of collagen XXV and alpha 6 integrin are cleaved, which is sufficient to promote formation of multinucleated myofibers [53,54].
Culture medium is a key factor of any cell culture-based technology, including IVM production. The media preference for both proliferation and differentiation is variable and largely dependent on media composition as well as its cost. Therefore, culture media constitutes a major portion of the economic and technical issues associated with IMA currently. Till now there is no specific media based on the cell status or condition. Currently the media utilized is only a generalized medium which supports the growth for broad range of cells. In IMA, the isolated cells would be stem cells which will be further proliferated to specific progenitor cells and upon which is induced to differentiate into skeletal muscle cells. Hence, though everything are same cells but the cell’s state is different, so the requirements of the cells also changes. Thus, there is need to formulate new medium for every particular metabolic state. This formulation may help in faster completion of the cell cycle hence a faster production can be achieved. Although most of the currently using media components required for proliferation and differentiation are similar, portions such as growth factors and molecules that stimulate differentiation are crucial for the latter phase. One such supplement is fetal bovine serum (FBS) that is widely used in cell culture owing to its non-activated immune system and absence of potential cytotoxicity. FBS is a rich source of growth factors and thus promotes cell growth; it also exhibits potential buffering capacity. Although FBS is beneficial in cell culturing techniques, there are limitations associated as well. These include batch to batch variability, ethical issues, and high cost due to extensive demand. Alternatively, FBS can be replaced by cloned growth factors, single cell proteins, or serum equivalent extracts of microbial-origin to cut down the production cost substantially. However, technological barriers that exist in realizing these options must be solved so as to overcome costs of large-scale production efficiently. Interestingly, this field opens a new forum for research.
Culture conditioning is extremely crucial in IMA as the conditioning source can modulate the cells. Conditioning can be categorized into two types: physical and chemical. These stimuli include electrical, mechanical, topographic, flow, co-culturing with other types of cells, and growth factors. A tissue in its 3D space is dependent on all aforementioned factors at any given time. Combination of soluble or tethered signaling molecules that are spatially and temporally controlled renders successful physiological establishment [41,44,55]. This provides evidence that the extracellular matrix (ECM) controls cell fate. The ECM environment has its own mechanical properties; these properties are sensed by cells and direct SCs to fulfill proliferation, differentiation, and maturation [46]. These mechanical properties transfer via ECM-integrin attachment [56]. Reportedly, electro-mechanical stimulation of cells aligned on a polymer enhanced myotube maturation as compared to non-stimulated differentiated cells [57]. Another such signal is the elasticity of cell-adhering substrates, wherein adhering substrates that can mimic in vivo environment (approximately in the range of 8–12 kilopascal; KPa) exhibit higher regeneration potential than the common Petri dishes with stiffness strength equivalent to ~106 kPa [49,58,59]. The required elasticity should be equivalent to the tissue material measured in terms of the transcellular contractile force exerted by adhesion complexes and actin-myosin cytoskeletons [60,61]. Thus, assessing the required stiffness for stemness and differentiating SC will pave innovative approaches. Further, if other similar factors are considered and IMA requirements are optimized successfully, it is possible that media conditioning in the absence of serum but presence of only the basic supplement may be sufficient for successful IMA.
Apart from the above-mentioned factors, any given tissue growing above the size of 1 to 1.5 mm is difficult to be grown due to the limitation of nutrition and oxygen diffusion, leading to nutrition scarcity and hypoxic conditions, and ultimately, necrosis. The tissue requires angiogenesis plexus or intercalated vasculature to gain access to nutrition, oxygen, or any mitogens. One such model was engineered with a sponge-like biopolymer scaffold and co-cultured with myoblasts, fibroblasts, and endothelial cells. Accordingly, this “prevascularization” technique helped in angiogenesis, and as a result, proper perfusion of the nutrients was observed [62].
CONCLUSION
Cell-cultured meat can be an essential product that has the potential to meet our future food demands. IMA aids in animal welfare, reduction in EIDs, sustainable utilization of land and other available resources, and exhibits environmental benefits. Present focus of research should be the development of alternatives for media composition (for example, serum-free media), complete in vitro maturation of the cells, controlled SC cultivation, development of 3D matrix and microcarriers, appropriate selection of physical and chemical signals for specific cell types and regulation of cell proliferation, differentiation, and maturation, which is not only compatible with physiochemical properties but also edible [41,55,63]. Past and ongoing pandemics along with the increasing global demands for meat compels us to realize the emergent need for developing alternatives for livestock. These technology-based alternatives will ameliorate economic and food crises, human loss and suffering, and emergence of new infectious agents, thereby promising a better and safer future.
