Background
The United States of America is one of the largest producers of livestock and number one producer of beef cattle in the world [1]. According to the USDA, as of July 2015, there are 98.4 million beef cattle in the United states [2] and approximately 1.5 billion kg of manure (according to ASABE Standard D384.2, manure production from a beef cattle is 20–34 kg of manure per day) is generated daily only from beef cattle. Livestock manure is a nutrient source for crops. At the same time, it is also a major source of pollutant gases (ammonia-NH3, hydrogen sulfide-H2S, etc.), greenhouse gases (GHGs), volatile organic compounds (VOCs), odor, and particulate material (PM). Emission of pollutant gases and GHGs are becoming an important issue for human and animal health, and environment [3, 4]. In a livestock production systems, the rate and amount of gaseous emissions depend on animal species, diet composition, manure management, weather, types of housing system, and topographic features [5].
In a confined livestock operation, the emission of pollutant gases can impact workers’ health, livestock welfare and productivity. The exposure of pollutant gas like H2S can cause dizziness, headache, respiratory problem, bronchitis, pulmonary paralysis, and unconsciousness and higher concentration (>100 ppm) can have lethal outcomes [6–8]. Similarly, the concentration more than 25 ppm of NH3 can cause respiratory irritation, chemical burns to the respiratory track, skin and eyes, severe cough, and chronic lung diseases [9]. Besides the impacts on human and animal health; those pollutant gases have an impact on environment. For example, NH3 can contribute to nutrient build up and eutrophication of surface water, acidification, and the promotion of bacterial growth that leads to weathering and corrosive damage of buildings [10–12]. Livestock production systems generate GHGs and are likely to contribute to the global warming [13, 14].
The GHGs have the potential to absorb and emit infrared radiation that increases the earth’s temperature and cause global warming [15]. The principal GHGs are water vapor, ozone (O3), carbon dioxide (CO2), methane (CH4) nitrous oxide (N2O), chlorofluorocarbon, per-fluorocarbon and sulfur hexafluoride; however CH4, N2O and CO2 are the major GHGs emitted from livestock production systems [13, 14]. It is estimated that 3.4 % of the total GHGs emissions in the USA is emitted from livestock [16]. In general, methane is emitted mostly from cattle production systems due to enteric fermentation in rumen and decomposition of manure in the manure treatment and management facilities. Similarly, N2O is produced during alternate aerobic and anaerobic decomposition of livestock manure [1]. Though the reported contribution of CH4 and N2O are only around 9.5 and 5.3 %, respectively, to the total GHG emissions [17]; the global warming potential of these gases are 25 and 298 times of CO2, respectively [17]. On the other hand, CH4 and N2O emission from livestock manure management has increased by 68 and 25 %, respectively, since 1990 [18]. Researchers around the world are seeking technologies and management practices to mitigate emission of these gases from livestock production facilities [19–21]. Among treatment options, diet manipulation is one of the prominent options for minimizing the total gaseous emission (enteric and from manure management) [22–24].
Manure management is one of the major sources of CH4 and N2O emission; however, a larger portion of CH4 (25.9 % of total CH4 emission) is also emitted during enteric fermentation in rumens [17]. Basically, the enteric CH4 production in rumen is affected by cattle feeding practices and feed diet composition [25]. Specifically, the diet composition can affect rumen pH, carbon nitrogen ratio, nutrient composition of manure, odor, and gaseous emissions from the manure system [6, 26]. In ruminal animal diets, carbohydrate and amount of intake influence the production of individual volatile fatty acids (VFAs), which is directly related with CH4 production. Diet with higher sugar and starch components favor propionic acid production resulting in less CH4 production [27]. Carbohydrate has the greatest impact on pH, microbial population, and VFA concentration which influences CH4 production. Similarly, an increase of fat levels in cattle diets increases the energy density of the diet (8.8 kcal g-1) [28], and also help to decrease enteric CH4 production [29].
The addition of supplemental fat in the cattle diets is one of the management practices adopted by farmers [30]. The fat content of commercial beef cattle feed is typically 2–5 % [31]. If the fat content in feed exceeds 6 %, it can cause digestive disturbance, diarrhea, and reduce feed intake [32]. Many researchers have conducted experiments using fat and oil in beef cattle diets and observed its impact on body performance, weight gain, cold tolerance, and gaseous emission from body and manure. Engstrom et al. [33] conducted a feeding trial on feedlot performance and carcass quality with beef cattle in Canada using 0, 2 and 4 % fat from canola oil in diets. They found an increase of 9.8 % in daily weight gain with the addition of 4 % fat in diet during the first 56 days.
The increase of fat level in the diet may affect metabolic changes in the ruminant. It may favor the production of propionic acid, which can reduce CH4 generation. In addition, supplementary fat can also lower the digestibility of fermentable substrates in the rumen, bio-hydrogenate unsaturated fat, and decrease methanogens population in rumen; ultimately reducing CH4 emission [27]. Mathison [34] reported 33 % reduction in enteric CH4 production is achievable by adding 4 % canola oil in a steer diet containing 85 % concentrate. Beauchemin and McGinn [35] carried out an experiment using fumeric acid, essential oil, and canola oil in beef cattle diets to observe their effect on enteric CH4 emission. Their results showed a reduction on CH4 emission using canola oil; though essential oil and fumeric acid did not influence ruminal fermentation or CH4 emission. Similarly, Beauchemin et al. [36] used the fat sources from different oil seeds like sunflower, canola and flaxseed to feed the cattle, and observed significant CH4 reduction in all cases.
Corn based distiller’s dried grain with solubles (DDGS) is a by-product from the ethanol industries and widely used in livestock diets. Usually, DDGS contains 12 to 15 % oil on a dry basis; however, partial removal of corn oil is common in the ethanol industry. Typically, 3 to 9 % corn oil levels are found in the commercially available DDGS feedstuffs [37]. In beef cattle diets, DDGS is a major ingredient comprising up to 42 % of the total diets [38]. Besides DDGS; corn grain, corn silage, hay, sunflower meal, and concentrated separator by-product (CSB) are some other common ingredients added to beef cattle diets. The desired fat levels in the diets can be achieved by adjusting the inclusion level of DDGS in the diets. However, to the best of our knowledge no studies have been reported on the effect of various fat levels from DDGS on gaseous emission and manure composition from the feedlot pen surfaces. Therefore, the objective of this study was to investigate the effect of different fat levels in beef cattle diets on manure nutrient composition and GHG emission from feedlot pen surfaces.
Methods
The experimental design and procedures of this study were reviewed and approved by the North Dakota State University Institutional Animal Care and Use Committee (protocol number A13068).
The research was carried out in a research feedlot at the North Dakota State University Carrington Research Extension Center (CREC). The feedlot had 16 pens and each pen with an area of 433 m2 (≈19 m × 23 m). The overall slope of the feedlot was around 3 %. A total of 182 fall-born (n = 92) and spring-born (n = 90) Angus-steer calves in a randomized block design. Steers were blocked by weight (four groups: light, medium light, medium heavy and heavy). After blocking, the steers were allocated to one of 16 pens (11 to 12 steers per pen) and pen was allocated to 1 of 4 dietary fat levels treatment diets (high fat; medium fat, low fat, and control). Initially, the finishing ration was provided to the heavy and medium heavy animals while the growing ration was provided to light and medium light animals. However, after June the same ration (finishing) was provided to all. This study was conducted from June to October of 2013. The information about animal number, blocking groups, feeding strategies, treatment category and weight of animals on each pen on different time period has been provided in Table 1.
During each sampling, the pen surface temperatures were measured using an infrared thermometer (MiniTemp-MT6 Instrument, Carlsbad, CA). Ambient temperature, wind speed, solar radiation, and rainfall were collected from the North Dakota Agricultural Weather Network - NDAWN site, NDSU Carrington Research and Extension center, located 2 km from the study site.
In this study, the effects of four different dietary fat levels (high, medium, low and control) on beef cattle performance, manure composition and gaseous emissions from feedlot pen surfaces were studied. Three different DDGS products sourced from different ethanol plants, were used to obtain different oil levels. High fat treatment group used DDGS purchased from High-water Ethanol, Lamberton, MN; and had 12.96 % corn oil (no corn removal). Medium fat treatment group consisted of DDGS purchased from Blue Flient Ethanol, Washburn, ND; which used 8.05 % corn oil (partial removal). Similarly, low fat treatment group consisted of DDGS purchased from POET, Groton, SD; used 5.47 % corn oil (higher removal). The control diet included sunflower meal used 2.44 % oil. Besides DDGS, other ration ingredients were chopped grass hay, dry-rolled corn grain, corn silage, condensed separator by product and a vitamins and minerals supplements. The diets were formulated to meet the nutrient requirement recommended by NRC [39]. Overall, the fat content of high, medium, low, and control diet (composite diet) were 5.07, 4.12, 3.6, and 3.19 %, respectively in the growing ration and they were 5.48, 4.52, 4.02, and 3.58 %, respectively in the finishing ration. The diet ingredients and the nutrient composition of composite diet is listed in Table 2, and the nutrient composition of each ingredient is listed in Table 3.
Note: DDGS = Distiller’s dried grains with solubles; CSB = Concentrated separator by-product; CP = Crude protein; NEm = Net energy of maintenance; NEg = net energy of gain; All feed samples were analyzed by a commercial laboratory; net energy prediction calculations were done as per NRC [58–60]
Note: DDGS Distiller’s dried grains with solubles, CSB Concentrated separator by-product, DM dry matter, CP Crude protein, ADF Acid detergent fiber, TDN Total digestible nutrients, NEm Net energy for maintenance, NEg Net energy for gain. All feed samples were analyzed by a commercial laboratory; net energy prediction calculations are from [58–60]
Air samples from the pen surface were collected for five times during June to October 2013 with a sampling interval of 30 ± 10 days. Air samples were collected using a custom built portable wind tunnel with a foot print area of 0.32 m2 (0.8 m × 0.4 m), Tedlar bag, and Vac-U-Chamber (SKC Inc., Eighty Four, PA) (Fig. 1). Measured air velocity over the foot-print area of tunnel immediate over the manure surface was maintained 0.35 m s-1 that prompted an air flow through the tunnel of 2.75 L s-1 (0.00275 m3 s-1). In each sampling location, a 5 L Tedlar bag was placed inside a vacuum chamber and a uniform air flow rate (2.75 L s-1) was maintained inside the tunnel throughout the sampling period using a DC motor. Additional sampling protocol can be found at Rahman et al. [40]. In each pen, two samples were collected; one from the front end of the pen next to feeding area, and another one from the backside of pen. So, a total of 160 air samples (16 pens × 2 samples/pen × 5 times) were collected and they were brought back to the laboratory for H2S, CH4, CO2, and N2O analysis.
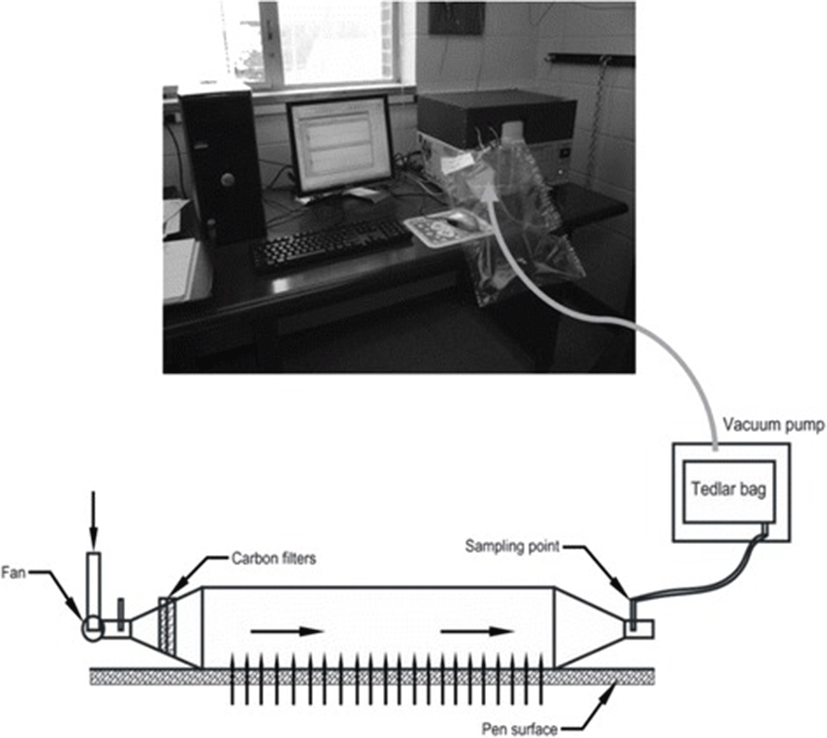
Within 24 h of sampling, they were analyzed for GHGs concentration using a greenhouse gas GC (Model No. 8610C, SRI Instruments, and 20720 Earl St., Torrance, CA 90502), and H2S concentration using a Jerome meter (Jerome® 631-X, Arizona instrument, Arizona, USA). The GC was equipped with a flame ionization detector (FID) to measure CO2 and CH4 and an electron captured detector (ECD) to analyze N2O. GHG was analyzed following the procedure described in Rahman et al. [40].
Manures on the pen surface were allowed to accumulate until animals were sold out. During a sampling event, the manure sampling was paired with air sampling and relatively fresh manures (freshly excreted or few minutes old) were sampled. The composite manure samples collected from five to seven spots in a pen, bagged and mix in a zip-locked bag, kept in ice cooled cooler in the field and during transportation; and finally stored in a refrigerator at around 4 °C until analysis. Thus, in each sampling day, a total of 16 composite manure (each approximately ~ 800 g) samples were collected. Before analysis, samples were mixed thoroughly again, divided into two sub-samples (sub-sample 1: pH, total nitrogen (TN), potassium (K), total phosphorus (TP) and total carbon (TC); sub-sample 2: volatile fatty acids (VFAs), crude protein (CP) and ammonical nitrogen), and were sent to two analytical laboratory for analysis following the standard laboratory methods (Table 4).
1AOAC Association of Official Agricultural Chemists
In order to estimate the emission rate; the volumetric gas concentration was standardized at standard pressure and temperature (1 atm and 25 °C), and mass concentration of the compound was calculated from calculated volumetric concentration (Equation 1). Flux rates (g m-2 d-1) was calculated using the average airflow through the wind tunnel, mass concentration of the target gas and the surface area covered by the wind tunnel as shown in Equation 2. Finally, emission rate was estimated using the surface area of pen, flux rate, and animal unit (AU) in the pen (Equation 3).
where, Cppm = Volumetric concentration of the target gas (ppm)Cmass = Mass concentration of the target gas (mg m-3)
MW = Molecular weight of the target gas (g mol-1)
24.25 = Volume per mole of an ideal gas at standard temperature and pressure (L mol-1)
where, FR = GHG emission flux rate from pen surface (g m-2 d-1)Vwt = Airflow rate through wind tunnel (m3 s-1)
Awt = Surface area covered by the wind tunnel (0.4 × 0.8 m2)
where, ER = GHG emission rate from pen surface (g hd-1 d-1)
Asc = Surface area of the source (m2)
AU = Animal unit (total weight of animals in pen divided by 500 kg live weight)
The daily mean air temperature, wind speed, solar irradiation, and rainfall at the sampling locations during each sampling period are listed in Table 5. The August sampling time had the highest ambient temperature, while October had the lowest ambient temperature. Likewise, the highest pen surface temperature was noted in August, which equates to the ambient temperature (Fig. 2). Similarly, the lowest pen surface temperature was observed in September. Overall, average pen surface temperatures were very consistent among pens at each sampling time. Besides temperature, solar radiation was also the highest in August, and the lowest in September. During the sampling time, no noticeable rainfall was observed, which might have some effects on gaseous emission from the manure pen surface.
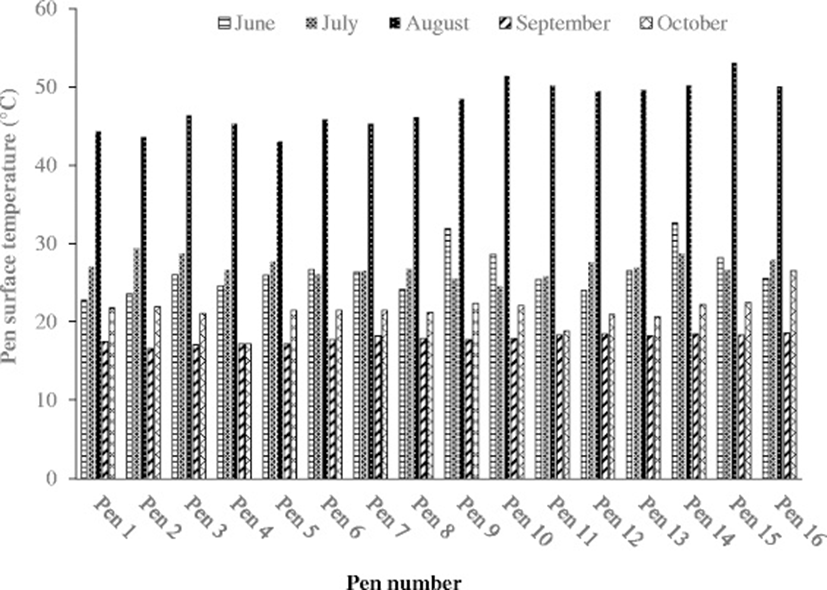
It is known that temperature variation on the pen surface effects gaseous emissions. Usually, higher temperatures enhance CH4 production [41]. The temperature range of 25–30 °C is considered optimum for CH4 production [42]. Surface temperature also influence on N2O emission. Luo et al. [43] reported the highest N2O emissions with moist and warm soil, and the soil exposed to freezing and thawing condition. Lang et al. [44] also observed the higher soil temperature promoted greater nitrification and N2O emissions.
The effect of fat levels in the diet on GHG emission and manure composition were compared using the Generalized Liner Model (GLM) procedure in SAS software (SAS 9.3, 2002-2010). Randomized complete block design was chosen for each sampling event (months) with animal weight as a block (light and heavy) for four treatments (control, low, medium and high). However, during analysis no significant difference of treatments were observed separating the animals on weight basis. Therefore, a comparative study of different treatments were carried considering the animal types as a single block. All significance tests were evaluated at P = 0.05. The null hypothesis was that the means value of GHGs concentrations, emission flux, emission rates, manure nutrient and VFAs concentrations were equal within and among treatments and sampling time.
Results and discussion
Overall, no significant differences in manure composition were observed among treatments in most of the sampled months, but significant differences on some manure composition were observed over the sampling period (Table 6). Likewise in August, the moisture content, crude protein (CP), and TN were also significantly lower in manure from pens with cattle fed high fat diets compared to other treatment groups.
*Values followed by the same letter in row are not significantly different at P ≤ 0.05; wb = wet basis
However, when the analysis was simply carried out on time basis (comparison among months), significant difference on most of the parameters of manure composition were observed (Table 7). Manure pH was significantly lower in August as compared to other months. Similarly, moisture content of manure was significantly lower in October as compared to June, July and September as shown in Table 7. Ash content of manure was the highest in June and the lowest in August. Crude protein, TP, and ammonical nitrogen NH3-N content in manure were the lowest in June and the highest in August. Total carbon (TC) in manure was significantly lower in August as compared to other months. In 2012 summer, Borhan et al. [45] had also measured the nutrient composition of the manure in the same feed lot under similar condition and the values of nutrient parameter were almost comparable with this study.
* Values followed by the same letter in row are not significantly different at P ≤ 0.05; CP Crude protein, TN Total nitrogen, NH3-N ammonical nitrogen, TP Total phosphorus, K Potassium, TVFA Total volatile fatty acids, DM Dry matter
No significant differences in any of VFAs concentration were observed among treatments during the study period except for two months. In July, isovaleric acid was significantly higher in manure from pens with cattle fed the low fat diets than the control. Likewise, in September butyric acid was significantly higher in the manure from pens with cattle fed the medium fat diets compared to the control (Table 8). Similarly in August and September, the total volatile fatty acid (TVFA) content were significantly lower in the high fat group than the others (Table 8), which may contribute to lower CH4 emission. However, when the analysis carried out on monthly basis, the lowest acetic acid concentration and the highest propionic acid concentrations were observed in August (Table 9). Likewise, the TVFA content of manure was significantly higher in July and August compared to other months (Table 9), which is likely due to temperature effect on VFA production. Due to higher TVFA, comparatively higher CH4 emission can be expected during July and August.
*Values followed by the same letter in row are not significantly different at P ≤ 0.05
*Values followed by the same letter in row are not significantly different at P ≤ 0.05
During anaerobic decomposition of manure; acetic, propionic, butyric and valeric acids are the common VFAs produced by micro-organisms. Acetic acid is the major VFA responsible for CH4 production from anaerobic biomass which accounts more than two third of CH4 production [46]. Propionic and butyric acids are considered as the inhibitory agents in anaerobic process [47]. Higher concentration of propionic usually inhibits the CH4 production in the case of anaerobic digester [48]; however, some researchers have mentioned that it’s the effect rather than cause for the inhibition of CH4 production [49, 50]. The ratio of acetic acid and propionic acid is another important factor for determining the CH4 production rate. Higher acetic acid (>800 mg L-1) as well as propionic acid and acetic acid ratios greater than 1:4 is taken as the indicator for failure of anaerobic processes [51]. However in this study, the ratio of propionic acid to TVFA was <1:4 (Tables 8 and 9), which was an indicator of anaerobic process on the pen surface.
Overall, no significant difference in GHGs emissions were observed from the feedlot pen surfaces with beef cattle fed four levels of fat (control, low, medium, high) in the diet (Table 10). However, some variations on GHG emission were observed when the measurement were compared between months. In July and September, the highest CO2 efflux was observed from pen surface with cattle fed medium fat diets. The increased fat source in the diets is likely to increase dietary energy, suppress methanogens decreasing CH4 emissions (both enteric and from manure) as well as reduce nitrogen emissions from manure [27, 52]. No significant difference in the total nitrogen and ammonium nitrogen between the treatments also support less variation of N2O emission between treatments.
*Values followed by the same letter in row are not significantly different at P ≤ 0.05; where, FR flux rate from pen surface (g m-2 d-1), ER emission rate from pen surface (g hd-1 d-1)
The effect of fat on gaseous emissions depends on many factors; such as type of fat, amount of fat in feed, and environmental condition. Though the literatures [35, 36] showed that the addition of fat effects enteric CH4 production, this study showed that different fat levels from DDGS may not greatly influence the CH4 production from the pen surface area. The emissions from the pen surface area are most likely to influence from environmental factors. The environmental conditions were almost similar in all the pen surfaces; therefore, very little variation in gaseous emissions might have been observed under the different treatments conditions. In addition, the reduction of CH4 concentration using supplementary fat may not be applicable for corn oil in DDGS; or the application rate of corn oil used in this research may not be sufficient for significant reduction on gaseous emission from pen surface.
When the gaseous emission were compared between different months, significant differences in the gaseous parameters were observed. The CH4 emissions were significantly higher during September and October from the pen surfaces as compared to June, July, and August. Higher emissions of CH4 were expected due to higher temperatures in July and August [41]. Though the CH4 concentration was observed higher in August and July compared to June; the concentration in September and October were even higher than July and August. This could be due to the accumulation of manure on the pen surface that provide anaerobic conditions for CH4 emission. Nitrous oxide emissions were significantly lower during September and October and higher during June, July and August (Table 11). The higher temperature during June, July and August could be a reason for higher N2O emissions [44]. Similarly, the dry and wet condition of the pen surface due to rain in summer may provide alternate aerobic and anaerobic condition on the pen surface, thus variation of N2O emission was observed. The wet conditions of pen surfaces favors anaerobic conditions in manure, resulting in denitrification. Dry conditions favor aerobic conditions in manure resulting in nitrification. Nitrous oxide is produced during both nitrification and denitrification processes [53]. The significantly lowest nitrous oxide and carbon dioxide fluxes during October are most likely due to prevailing dry surface and ambient condition (Table 10).
*Values followed by the same letter in row are not significantly different at P ≤ 0.05
In comparing the results with the previous study; in 2011, Rahman et al [40] simply measured GHG emission from the same feedlot pen surface and they found that CH4, CO2 and N2O emission were 38, 26, and 17 g hd-1d-1, respectively, during the 2011 summer period. Similarly, in 2012, Borhan et al. [45] studied the effects of two dietary crude protein levels (12 and 16 %) in the GHG emission on the similar conditions. They found that CH4, CO2 and N2O emission ranged from 40–61, 31–43, and 50–116 gAU-1d-1 (0.8–1.1, 593–431, and 1–1.9 g m-2d-1), respectively, during the summer months. They noticed no significant differences on gaseous emission due to different protein diet levels.
Further analsysis was carried out to see the interaction of diet and time on GHG emisison. The results reveal that all CH4, CO2 and N2O emisison (concentration and emission rate) varied significantly (p < 0.05) over the sampling period; however, diet did not have any interaction with time for the effect on GHG emissions (Table 12).
| Parameters | Interaction | ||
|---|---|---|---|
| Time | Diet*Time | ||
| CH4 g m-2 d-1 | <0.01 | 0.68 | |
| CO2 g m-2 d-1 | <0.01 | 0.43 | |
| N2O g m-2 d-1 | <0.01 | 0.37 | |
| CH4 g AU-1 d-1 | 0.03 | 0.41 | |
| CO2 kg AU-1 d-1 | <0.01 | 0.97 | |
| N2O g AU-1 d-1 | <0.01 | 0.48 |
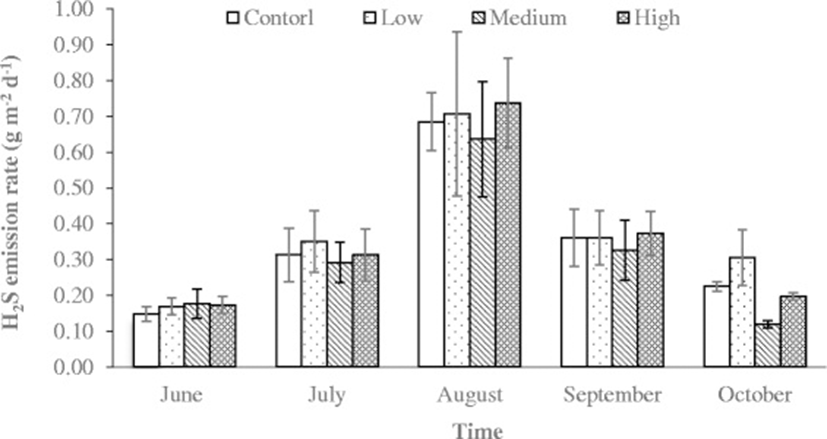
Hydrogen sulfide concentration was very low (<80 ppb) at the pen surfaces throughout the measurement period. Other researchers have also reported the concentration around 50 ppb in feedlot [54]. There was no significant difference in H2S emission rate among dietary treatments. However, variations in H2S emission rates were observed during different sampling periods (Fig. 2). The H2S emission rate was fairly low (<0.18 g m-2 d-1) in the first month since pen surfaces had a thin layer of manure on the surface. The H2S concentration gradually increased over time and reached up to 0.7 g m-2 d-1 in August (Fig. 3). However, as the temperature started decreasing (Fig. 2), the H2S emission rate also declined gradually (Fig. 3). This study shows that H2S emission rate measured on the feedlot pen surfaces were correlated with temperature change (R2 = 0.49), and manure accumulation (Figs. 2 and 3). Other researchers have also observed very low emission rate of H2S from feedlot. Wood et al. [55]) reported the emission rate 103 μg m-2 min-1. Similarly, Baek et al. [56] and Koziel et al. [57] reported an the H2S emission rate as 1.88 μg m-2 min-1, and 1.39 μg m-2 min-1, respectively.
Conclusions
In this study the effect of four dietary fat concentrations (3 to 5.5 % in the composite sample) feed to beef cattle was evaluated in term of manure nutrient composition, VFA concentration, hydrogen sulfide and GHG (CH4, CO2, and N2O) emissions. The study was conducted over a 5-month period from June to October for five ~28-day sampling periods. Overall, the fat levels in the diets showed no or little effect on the manure compositions, VFA, and H2S and GHGs emissions. However, some variation in the above mentioned parameters was observed among different measurement periods. In this research, the variation of fat levels from 3 to 5.5 % in cattle diets did not reflect any significant difference on GHGs and H2S emission from beef cattle feedlot pen surfaces, as well as on manure composition. It can be concluded that addition of fat to animal diet may not have any impact on gaseous emission and manure compositions.
