Background
Corn is the most commonly used raw material for energy (carbohydrates) supply in cattle feed in Korea [1]. Until recently, various processing methods including grinding, extrusion, and flaking have been applied to increase the availability of corn. In particular, corn flakes are widely used in cattle feed in Korea owing to increased starch digestibility [2] resulting from flaking and increased energy supply [3] for the generation of marbling adipose tissue [4].
Corn flaking destroys the hull, endodermis, and protein matrix surrounding starch granules in the endosperm via exposure to high temperature, moisture, and pressure, and it induces gelatinization of the normal semi-crystalline structure of starch granules to improve the starch digestibility [5,6].
Until recently, steam chambers are widely used in corn flake production, with general temperatures and retention times of 86°C–102°C [7–9] and 32–75 min [9,10], respectively. However, considering the retention time of corn in the chamber, the steam chamber not only has low production efficiency but also does not have a high rate of corn starch gelatinization. Consequently, pressurized steam chambers incorporating pressure systems have recently been used for corn flake production. The advantages of steam pressure include uniform diffusion of steam, effective heat transfer to starch granules [11], reduction of gelatinization enthalpy [12], and effective starch gelatinization [13].
The use of pressurized steam chambers can increase production costs (electricity, gas, etc.) by increasing the chamber temperature and retention time to increase corn starch gelatinization, similar to the steam chambers. Since 2010, numerous cattle feed companies in Korea have been using pressurized steam chambers [14]. Compared to the conventional steam chamber, the pressurized steam chamber decreases corn flake production times and improves gelatinization [14]. However, no study has investigated the effect of temperature and retention time of the pressurized chamber on the nutritive value of corn flakes.
Hence, this study aimed to investigate the effect of different temperatures and retention times in corn flake production in the pressurized steam chamber on ruminal fermentation characteristics and nutrient degradability. Furthermore, this study aimed to determine the optimal temperature and retention time for corn flake production using the pressurized steam chamber.
Materials and Methods
This study was carried out for 7 months at an experimental farm at Kangwon National University. Three Hanwoo cows (mean body weight, 327 ± 41 kg) and three Holstein cows (mean body weight 421 ± 37 kg), each implanted with a ruminal fistula, were used.
The corn used in this study was imported from South America, its chemical composition being (based on dry matter): 89.23% of dry matter, 7.98% of crude protein, 2.78% of ether extract, 1.48% of crude ash, 11.23% of neutral detergent fiber (NDF), and 1.95% of acid detergent fiber (ADF). The thickness of corn flakes was 3.5 mm, and the dimension of mill roller was φ 610 × 1,220 mm (24″ × 48″ ).
Corn kernels were categorized into 13 groups for corn flake production using the pressurized steam chamber (1.5 atmospheric pressure), based on different temperatures and retention times: T1 (100 °C; 720 s), T2 (105 °C; 700 s), T3 (105 °C; 850 s), T4 (105 °C; 950 s), T5 (110 °C; 400 s), T6 (112 °C; 700 s), T7 (112 °C; 850 s), T8 (112 °C; 950 s), T9 (113 °C; 600 s), T10 (115 °C; 500 s), T11 (116 °C; 700 s), T12 (116 °C; 850 s), and T13 (116 °C; 950 s). The temperature of the pressurized steam chamber, retention time, and the chemical composition of the corn flakes are summarized in Table 1.
| Treatment1) | Chemical composition2)(%) | ||||||
|---|---|---|---|---|---|---|---|
| DM | CP | EE | CF | CA | Star | Gela | |
| T1 | 90.40 ± 0.15 | 7.95 ± 0.14 | 3.13 ± 0.25 | 1.95 ± 0.35 | 1.25 ± 0.07 | 53.39 ± 0.75 | 33.05 ± 2.67 |
| T2 | 91.46 ± 0.31 | 7.11 ± 0.37 | 2.94 ± 0.00 | 2.05 ± 0.76 | 1.27 ± 0.13 | 56.51 ± 0.52 | 30.03 ± 3.06 |
| T3 | 90.14 ± 0.56 | 7.35 ± 0.07 | 3.13 ± 0.01 | 1.45 ± 0.66 | 1.19 ± 0.13 | 54.74 ± 0.57 | 31.34 ± 3.64 |
| T4 | 90.58 ± 0.31 | 7.47 ± 0.11 | 2.79 ± 0.34 | 2.80 ± 1.20 | 1.20 ± 0.01 | 55.46 ± 0.37 | 36.40 ± 2.87 |
| T5 | 90.41 ± 0.54 | 7.46 ± 0.18 | 3.60 ± 0.11 | 3.52 ± 0.02 | 1.20 ± 0.04 | 57.05 ± 0.56 | 34.68 ± 3.72 |
| T6 | 90.97 ± 0.28 | 7.24 ± 0.12 | 3.77 ± 0.19 | 2.26 ± 0.71 | 1.24 ± 0.10 | 54.83 ± 0.03 | 30.54 ± 2.48 |
| T7 | 90.39 ± 0.65 | 7.20 ± 0.07 | 4.02 ± 0.39 | 1.40 ± 0.02 | 1.20 ± 0.01 | 55.65 ± 0.91 | 28.12 ± 2.34 |
| T8 | 90.32 ± 0.36 | 7.33 ± 0.11 | 3.82 ± 0.08 | 3.50 ± 0.39 | 1.28 ± 0.09 | 55.88 ± 0.52 | 33.43 ± 4.13 |
| T9 | 90.81 ± 0.39 | 7.34 ± 0.28 | 2.97 ± 0.08 | 2.64 ± 0.20 | 1.20 ± 0.11 | 53.95 ± 0.07 | 32.43 ± 1.82 |
| T10 | 90.92 ± 0.25 | 7.00 ± 0.11 | 3.27 ± 0.08 | 2.62 ± 0.10 | 1.24 ± 0.07 | 53.69 ± 0.69 | 37.75 ± 0.86 |
| T11 | 89.57 ± 0.85 | 7.63 ± 0.14 | 3.58 ± 0.39 | 2.20 ± 1.22 | 1.13 ± 0.07 | 56.21 ± 0.32 | 35.45 ± 5.06 |
| T12 | 90.61 ± 0.68 | 7.50 ± 0.18 | 3.70 ± 0.06 | 2.85 ± 0.96 | 1.22 ± 0.04 | 56.37 ± 0.01 | 37.43 ± 1.31 |
| T13 | 90.98 ± 0.45 | 7.61 ± 0.03 | 3.45 ± 0.18 | 1.81 ± 0.44 | 1.21 ± 0.02 | 54.30 ± 0.60 | 31.14 ± 4.62 |
Ruminal fluid was collected from the ruminal fistula of Hanwoo and Holstein cows before feeding in the morning and filtered through 4 layers of cheese cloth, followed by storage at 39 °C in a thermos flask. Thereafter, O2-free CO2 gas was infused for 30 s to eliminate air. The collected ruminal fluid was transferred to the laboratory and allowed to stand for 1 h in an incubator at 39 °C to eliminate feed particles and used as an inoculum for in vitro incubation.
In vitro cultures were established by adding 400 mL of rumen inoculum to 1,596 mL of previously prepared artificial saliva (buffer solution A and B) in accordance with the method of MacDougall [15]. Artificial saliva was prepared using buffer solutions A and B. Before the experiment, buffer solution A (1,330 mL) and B (266 mL) were simultaneously mixed and the pH was adjusted to 6.8. Buffer solution A included 10.0 g of KH2PO4, 0.5 g of MgSO4·7H2O, 0.5 g of NaCl, 0.1 g of CaCl2·2H2O, and 0.5 g of urea per liter of distilled water. Buffer solution B included 15.0 g of NaCO3 and 1.0 g of Na2S·9H2O per liter of distilled water.
Seventy milliliters of the prepared in vitro culture solution was placed in a 100 mL bottle (4 OZ glass bottle) containing 2 g of ground corn flakes, and O2-free CO2 gas was infused for 5 s to eliminate air from the culture bottle. The bottles were then incubated for 0, 3, 6, 9, 12, 24, and 48 h in a shaking incubator (HB-201SLI, Hanbaek scientific co., Bucheon, Korea) at 39 °C.
For in situ analysis, 5 g of sample was placed in a nylon bag (AN-KOM 5 × 10 concentrate bags), and a nylon bag was placed in a mesh for each incubation 0, 3, 6, 9, 12, 24, and 48 h and inserted into the rumen via a fistula before feeding in the morning. The nylon bag was washed until clear water was obtained, and then dried in a 70 °C forced dry oven for 72 h. The dried samples were weighed and used to calculate the degradability of dry matter. The samples were ground to a particle size of 1.0 mm and their chemical composition was analyzed to determine the degradability of starch and crude protein.
The corn flakes used in this study were dried in a forced dry oven (at 60 °C, 48 h), ground using a Wiley mill (Thomas scientific, Model 4, Swedesboro, NJ, USA) and analyzed for moisture, crude protein, ether extract, crude fiber, and crude ash in accordance with the procedures of the Association of Official Analytical Chemists [16]. NDF and ADF levels were determined using a filter bag (Ankom F57, Ankom Technology, New York, NY, USA) in accordance with the method of Van Soest et al. [17].
Starch contents were determined according to the standard feed analysis method of the Ministry of Agriculture, Food and Rural Affairs in Korea [18]. To determine the starch content of corn flakes, 0.2 g of the sample was placed in a 50 mL tube, and the sample was dampened with 200 μL of 80% ethanol, followed by the addition of 5 mL of distilled water and vortex mixing. Thereafter, 25 mL of 80% ethanol at 75 °C was added to the tube, and the tube was allowed to stand for 5 min, followed by centrifugation at 1,250 × g for 15 min at 4°C. The supernatant was discarded and 30 mL of 80% ethanol at 75 °C was added, followed by centrifugation at 1,250 × g for 15 min at 4 °C. The supernatant was dried at 40 °C for 1 h, followed by addition of 5 mL of distilled water and 6.5 mL of 52% perchloric acid. The mixture was stirred for 20 min, and 20 mL of distilled water was added, followed by centrifugation at 1,250 × g for 15 min at 4 °C. Thereafter, 1 mL of the diluted solution and 5 mL of anthrone were mixed, heated at 100 °C for 12 min, cooled to room temperature (approximately 20 °C), and then analyzed using an absorbance analyzer (VERSA max, Molecular Devices, CA, USA) at 630 nm.
To analyze gelatinization of the corn flakes, 1 g (0.5 mm) of each sample was placed in five 250 mL Erlenmeyer flasks (A1, A2, A3, A4, and B); thereafter, 50 mL of distilled water was added, and the flasks were sealed. A1 and A2 were heated in a water bath at 100 °C for 15 min and rapidly cooled. Thereafter, 5% diastase was added to A1, A3, and B, and the five flasks were placed in a shaker water bath at 37 °C for 90 min. Thereafter, 2 mL of 1 N HCl was added into the flasks, followed by addition of distilled water to 100 mL of the solution. The solution was filtered with a dry filter paper (No. 2), and 10 mL of the filtered solution and 10 mL of distilled water were taken in five revolving flasks (a1, a2, a3, a4, b, and BT), 10 mL of 0.1 N iodine solution and 18 mL of 0.1 N NaOH were added at the same intervals (5 min), the contents of the flask were shaken and mixed, and the flasks were allowed to stand for 15 min. Thereafter, (based on the first revolving flask), 2 mL of 10% H2SO4 was added at 5 min intervals, and the mixture was titrated against 0.1 N Na2S2O3 until the solution became colorless.
The in vitro ruminal pH was measured using a pH meter (Corning 445, Corning, NY, USA) in a 100 mL bottle for each incubation time. Ammonia concentration was measured using automatic analyzer (Quik Chem 8500 series 2, Lachat, CO, USA) after collecting 5 mL of culture solution in a 100 mL bottle for each incubation time.
To analyze volatile fatty acid (VFA) concentration, 10 mL of the culture solution was collected in a 100 mL bottle for each incubation time, followed by addition of 1 mL of 20% HPO3 and 0.5 mL of saturated HgCl2 and centrifugation at 4 °C at 1,250 × g for 15 min. The supernatant was discarded and the VFA concentration was measured via gas chromatography (Shimadzu-17A, Shimadzu, Kyoto, Japan).
Statistical analyses were performed using the GLM Procedure of SAS 9.2 (SAS Institute, Inc., Cary, NC, US). Analysis of variance was performed using the square sum of TYPE III, suitable for the unbalanced data among the four squares resulting from the SAS/GLM analysis. For mean comparison, Duncan’s multiple range test was used to determine the effect of the treatment while the t-test was used to determine the effect of the breed.
To investigate the effects of treatment on pH and ammonia, acetate, propionate, butyrate, and total-VFA concentration in in vitro incubation conditions, the following linear model was used for variance analysis.
Where, Yijkl = individual observations, μ = overall mean, TT = effect of i th treatment (13 conditions), TMj = effect of j th incubation time (j = 0, 3, 6, 9, 12, 24, and 48 h), Bk = effect of k th breed (k = Holstein, Hanwoo), (TT × TM)ij = the interaction effects of i th treatment and j th incubation time, (TT × B)ik = the interaction effect of the i th treatment and the k th breed, (TM × B)jk = the interaction effect of the j th incubation time and k th breed, eijkl = random error.
Ruminal dry matter, starch, and crude protein degradability were analyzed using the following linear model.
Where, Yijkl = individual observations, μ = overall mean, TMj = the effect of the j th incubation time (j= 0, 3, 6, 9, 12, 24, and 48 h), Bk = the effect of k th breed (k = Holstein, Hanwoo), (TT × TM)ij = the interaction effect of i th treatment and j th incubation time, (TT × B)ik = the interaction effect of i th treatment and k th breed, (TM × B)jk = the interaction effect of j th incubation time and k th breed, A(B)ik = the effect of i th animal (6 conditions) overlapped on the k the breed, eijklm = random error.
Results
The results of variance analysis on the effect of the pressurized steam chamber conditions (chamber temperature and retention time), incubation time, and breed on the in vitro ruminal parameters are summarized in Table 2. The conditions (chamber temperature and retention time) of the pressurized steam chamber significantly influenced the in vitro ruminal pH values, and ammonia, acetate, propionate, butyrate, and total-VFA concentrations of corn flakes (p < 0.01). Differences in incubation time and breed also exerted significant effects (p < 0.01) on the in vitro ruminal parameters of corn flakes.
| Items | df1) | Mean square | |||||
|---|---|---|---|---|---|---|---|
| pH | Ammonia | Acetate | Propionate | Butyrate | Total-VFA2) | ||
| Trt3) | 12 | 0.11** | 96.19** | 5,172.05** | 7,264.31** | 1,201.78** | 25,729.68** |
| Time4) | 6 | 59.28** | 3,609.78** | 534,471.83** | 679,900.33** | 273,212.81** | 4,249,018.06** |
| Breed5) | 1 | 0.03* | 18.19NS | 4,012.75** | 24.88NS | 6,425.15** | 139.79NS |
| Trt × time | 72 | 0.12** | 49.22** | 1,240.15** | 1,479.41** | 573.61** | 5,216.24** |
| Trt × breed | 12 | 0.03** | 20.75** | 2,196.52** | 892.98** | 948.34** | 2,512.23** |
| Time × breed | 6 | 0.01NS | 44.89** | 4,382.86** | 1,018.09** | 2,381.00** | 1,812.62** |
The least squares mean for the effects of the pressurized steam chamber conditions (chamber temperature and retention time), incubation time, and breed on the in vitro ruminal parameters are shown in Table 3.
| Tteatment1) | PH | Ammonia (mg/dL) | Acetate (mol/dL) | ProPionate (mol/dL) | Butyrate (mol/dL) | Total-VFA (mol/dL) |
|---|---|---|---|---|---|---|
| T1 | 5.72h | 17.83f | 175.38f9 | 103.40e | 72.60bcd | 351.38de |
| T2 | 5.85b | 21.67ab | 203.88ab | 132.78a | 66.83e | 403.48a |
| T3 | 5.91a | 21.17bc | 194.73d | 121.94c | 69.09de | 385.76b |
| T4 | 5.82bcd | 20.15cd | 200.70abc | 111.03d | 71.31cd | 383.04b |
| T5 | 5.77fg | 20.52c | 202.30ab | 121.55c | 66.55e | 390.40b |
| T6 | 5.81cde | 18.65ef | 197.00cd | 109.55d | 76.41ab | 382.96b |
| T7 | 5.83bc | 19.31de | 186.91e | 96.50f | 75.20abc | 358.60cd |
| T8 | 5.84bc | 18.80ef | 179.98f | 95.57f | 71.95cd | 347.51e |
| T9 | 5.78ef | 20.98bc | 200.37bcd | 127.31b | 78.55a | 406.23a |
| T10 | 5.77fg | 22.53a | 206.26a | 125.51bc | 71.33cd | 403.10a |
| T11 | 5.78ef | 18.21ef | 187.35e | 96.78f | 71.03d | 355.16cde |
| T12 | 5.80de | 18.11f | 187.39e | 108.29d | 65.76e | 361.43c |
| T13 | 5.739h | 18.89ef | 173.04e | 97.67f | 57.85f | 328.56f |
Standard error: pH, 0.01; ammonia, 0.40; acetate, 2.10; propionate, 1.63; butyrate, 1.44; total-VFA, 2.85.
The pH value was the highest in T3 (p < 0.01) and lowest in T1. Ammonia concentration was the highest in T10 and lowest in T1. Acetate concentration was the highest in T10; propionate, T2 (p < 0.05); butyrate, T9. Total-VFA concentration was higher in T2, T9, and T10 than in other treatments and lowest in T13 (p < 0.05). In particular, corn flakes in the T2 group displayed higher propionate and total-VFA concentrations, despite a higher pH than that of compared to other treatments.
The least squares mean for the effect of the pressurized steam chamber conditions (chamber temperature and retention time) on the in vitro ruminal fermentable characteristics of corn flakes are summarized in Tables 4 and 5. The pH value was the highest in T3 for Holstein cows (p < 0.05) and in T2 for Hanwoo cows. The pH value was lower in T1 than in other groups, regardless of breed. Ammonia concentration was higher in T10 than in other groups, regardless of breed.
| Treatment1) | pH | Ammonia(mg/dL) | ||||
|---|---|---|---|---|---|---|
| Holstein | Hanwoo | Vd2) | Holstein | Hanwoo | Vd | |
| T1 | 5.70f | 5.73g | −0.03NS | 17.31de | 18.36ef | −1.05NS |
| T2 | 5.77cde | 5.92a | −0.15** | 21.06ab | 22.28ab | −1.22NS |
| T3 | 5.94a | 5.88ab | 0.06* | 21.05ab | 21.29bc | −0.24NS |
| T4 | 5.82bc | 5.83cde | −0.01NS | 21.08ab | 19.22def | 1.86* |
| T5 | 5.76de | 5.78ef | −0.02NS | 20.82ab | 20.22cd | 0.60NS |
| T6 | 5.80bcd | 5.82def | −0.02NS | 18.57cd | 18.72e | −0.15NS |
| T7 | 5.83b | 5.84bcd | −0.01NS | 19.00c | 19.62de | −0.62NS |
| T8 | 5.81bcd | 5.87abc | −0.06* | 19.59bc | 18.01e | 1.58NS |
| T9 | 5.76de | 5.80def | −0.04NS | 19.96bc | 21.99ab | −2.03* |
| T10 | 5.81bcd | 5.73g | 0.08** | 22.16a | 22.91a | −0.75NS |
| T11 | 5.79bcde | 5.78fg | 0.01NS | 17.41de | 19.02def | −1.61* |
| T12 | 5.79bcde | 5.82def | −0.03NS | 16.83e | 19.40def | −2.57* |
| T13 | 5.74ef | 5.73g | 0.01NS | 19.62bc | 18.16ef | 1.46NS |
| Treatment1) | Acetate (mM) | Propionate (mM) | Butyrate (mM) | Total-VFA (mM) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Holstein | Hanwoo | Vd2) | Holstein | Hanwoo | Vd | Holstein | Hanwoo | Vd | Holstein | Hanwoo | Vd | |
| T1 | 172.06d | 178.70g | −6.64NS | 96.19de | 110.6de | −14.41** | 83.70a | 61.50de | 22.20** | 351.95ef | 350.8e | 1.15NS |
| T2 | 206.90ab | 200.86bc | 6.04NS | 129.83ab | 135.72a | 5.89NS | 66.34e | 67.32e | −0.98NS | 403.06bc | 403.91a | −0.85NS |
| T3 | 199.31bc | 190.15def | 9.16* | 124.24b | 119.64c | 4.60NS | 72.10cd | 66.08cd | 6.02* | 395.64bc | 375.87bcd | 19.77** |
| T4 | 213.10a | 188.31ef | 24.79** | 105.43c | 116.63cd | −11.20** | 73.79c | 68.84bc | 4.95** | 392.32c | 373.77cd | 18.55* |
| T5 | 194.44c | 210.16a | −15.72** | 131.05a | 112.06de | 18.99** | 76.64bc | 56.45e | 20.19** | 402.13bc | 378.66bc | 23.47** |
| T6 | 192.86c | 201.14bc | −8.28* | 110.97c | 108.13e | 2.84NS | 76.50bc | 76.33a | 0.17NS | 380.33d | 385.59b | −5.26NS |
| T7 | 177.68d | 196.14cde | −18.46** | 91.35e | 101.65fg | −10.30** | 81.56ab | 68.84bc | 12.72** | 350.59ef | 366.62d | −16.03* |
| T8 | 179.53d | 180.44g | −0.91NS | 92.16e | 98.98gh | −6.82* | 73.16ed | 70.74abc | 2.42NS | 344.85ef | 350.16e | −5.31NS |
| T9 | 203.65b | 197.10cd | 6.55NS | 128.19ab | 126.43b | 1.76NS | 83.05a | 74.05ab | 9.00* | 414.89a | 397.58a | 17.31* |
| T10 | 204.79b | 207.74ab | −2.95NS | 129.31ab | 121.72bc | 7.59* | 72.36ed | 70.30bc | 2.06NS | 406.45ab | 399.76a | 6.69NS |
| T11 | 176.03d | 198.67c | −22.64** | 99.35d | 94.21h | 5.14NS | 66.02e | 76.04a | −10.02** | 341.40f | 368.91ed | −27.51** |
| T12 | 179.08d | 195.69de | −16.61** | 110.54c | 106.04ef | 4.50NS | 66.26e | 65.26cd | 1.00NS | 355.88e | 366.99d | −11.11NS |
| T13 | 160.63e | 185.45fg | 24.82** | 96.50de | 98.84gh | −2.34NS | 67.58de | 48.11f | 19.47** | 324.72g | 332.41f | −7.69NS |
Acetate concentration was the highest in T4 for Holstein cows and in T5 for Hanwoo cows. Propionate concentration was the highest in T5 for Holstein cows and in T2 for Hanwoo cows (p < 0.05). Total-VFA concentrations were higher in T2, T9, and T10 than in other groups. Total-VFA concentration was the lowest in T13 (p < 0.05), regardless of breed. Overall VFA concentrations were slightly but not significantly higher in T2 than in other groups, regardless of breed.
The effects of the pressurized steam chamber conditions (chamber temperature and retention time) on in vitro ruminal parameters based on incubation time are summarized in Fig. 1. The lowest pH values were recorded at 24 h of incubation in most groups; however, T1 showed the lowest pH value at 12 h of incubation. Propionate concentration was increased with an increase in incubation time in all groups and was higher in T2 than in other groups at 12 h. Total-VFA concentrations were slightly but not significantly higher in T2 than in other groups at 3, 6, and 12 h.
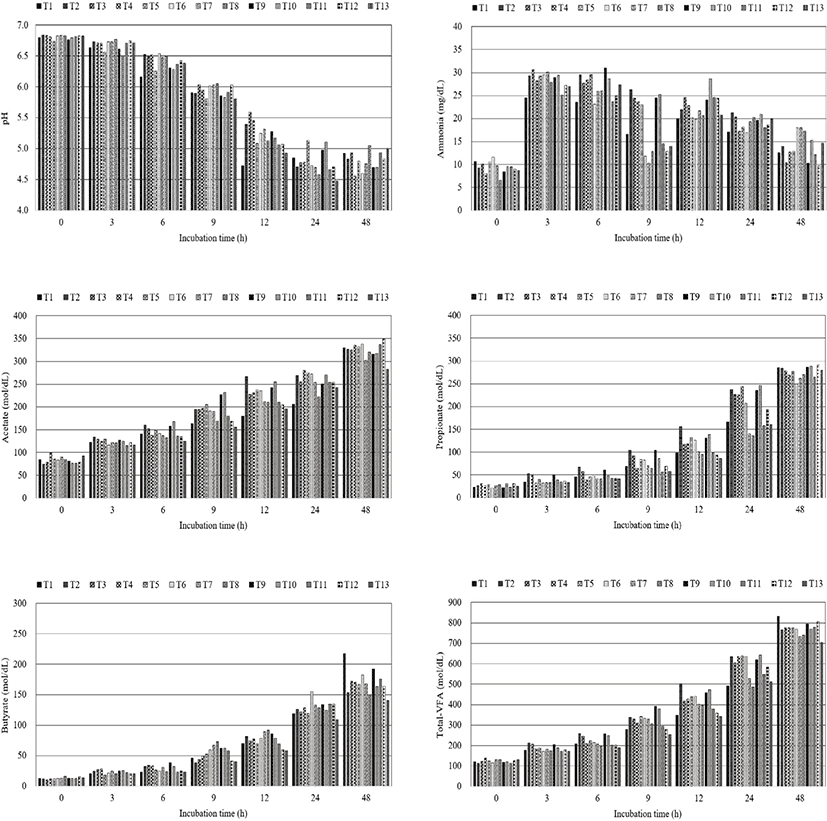
The effects of retention times (700, 850, and 950 s) at 105 °C, 112 °C, and 116 °C on ruminal pH values were less prominent. Propionate concentrations increased with a reduction in retention time at 105 °C, 112 °C, and 116 °C, and total-VFA concentrations displayed a similar tendency. In addition, ruminal pH values and ammonia, acetate, propionate, and total-VFA concentrations tended to decrease with an increase in chamber temperature (105 °C, 112 °C, and 116 °C) at 700, 850, and 950 s.
The results of the variance analysis of the effects of the pressurized steam chamber conditions (chamber temperature and retention time) on ruminal dry matter, starch, and crude protein degradability of corn flakes are summarized in Table 6. The conditions of the pressurized steam chamber (chamber temperature and retention time) significantly influenced ruminal dry matter, starch, and crude protein degradability of corn flakes (p < 0.01) depending on the breed or incubation time.
| Items | df1) | Mean square | ||
|---|---|---|---|---|
| Dry matter | Starch | Protein | ||
| Treatment2) | 12 | 357.78** | 408.13** | 833.33* |
| Time3) | 6 | 31,456.52** | 87,195.32** | 3,719.82** |
| Breed4) | 1 | 1,211.99* | 6,641.62NS | 574.43** |
| Trt × time | 72 | 18.05NS | 45.31** | 15.83NS |
| Trt × breed | 12 | 179.54** | 158.35** | 457.94** |
| Time × breed | 6 | 116.33NS | 109.63** | 49.45** |
The least squares mean of the effects of the pressurized steam chamber conditions (chamber temperature and retention time) on ruminal dry matter, starch, and crude protein degradability of corn flakes are summarized in Table 7. The dry matter (p < 0.05) and starch degradability were significantly higher in T1 than in other groups. Crude protein degradability was the highest in T1 (p < 0.05) and lowest in T7.
| Treatment1) | Dry matter (%) | Starch (%) | Protein (%) |
|---|---|---|---|
| T1 | 43.41a | 43.51a | 37.16a |
| T2 | 36.46cd | 38.79de | 26.77g |
| T3 | 37.92c | 37.51fg | 30.62cde |
| T4 | 39.50b | 39.99c | 29.78cde |
| T5 | 36.81cd | 39.25cd | 27.28g |
| T6 | 36.09d | 37.38fg | 27.47fg |
| T7 | 36.30cd | 38.42dfe | 26.75g |
| T8 | 36.96cd | 38.10ef | 28.39efg |
| T9 | 37.45c | 36.71g | 29.32de |
| T10 | 37.19cd | 36.48g | 29.12ef |
| T11 | 39.89b | 41.30b | 33.55b |
| T12 | 39.51b | 42.43ab | 33.87b |
| T13 | 36.81cd | 37.52fg | 31.09c |
The results of least squares mean of the effects of the pressurized steam chamber conditions (chamber temperature and retention time) on the ruminal dry matter, starch, and crude protein degradability of corn flakes based on breed are summarized in Table 8. In both Holstein and Hanwoo cows, dry matter and starch degradability were higher in T1 than in other groups. Among Holstein cows, crude protein degradability was significantly higher in T1 than in other groups (p < 0.05). Regardless of the breed, T1 displayed the highest dry matter and starch degradability, and crude protein degradability was slightly but not significantly greater in T1 than in other groups.
| Treatment1) | Dry matter (%) | Starch (%) | Protein (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Holstein | Hanwoo | Vd2) | Holstein | Hanwoo | Vd | Holstein | Hanwoo | Vd | |
| T1 | 45.96a | 40.85a | 5.11** | 46.11a | 40.91a | 5.20* | 40.43a | 33.90ab | 6.53** |
| T2 | 36.45d | 36.46bcd | −0.01NS | 41.03def | 36.55cd | 4.48** | 27.80ef | 25.74d | 2.06NS |
| T3 | 37.98cd | 37.86b | 0.12NS | 38.36g | 36.65d | 1.71* | 30.58c | 30.66c | −0.08NS |
| T4 | 39.34bc | 39.67a | −0.33NS | 40.75def | 39.22b | 1.53NS | 29.49de | 30.08c | −0.59NS |
| T5 | 40.40b | 33.22f | 7.18** | 44.09b | 34.41ef | 9.68** | 31.18cd | 23.38e | 7.80* |
| T6 | 36.55d | 35.63cd | 0.92NS | 39.56fg | 35.20de | 4.36** | 27.66e | 27.28d | 0.38NS |
| T7 | 36.59d | 36.00cd | 0.59NS | 41.49de | 35.34de | 6.15** | 26.30f | 27.20d | −0.90NS |
| T8 | 37.25d | 36.68bc | 0.57NS | 38.62g | 37.58c | 1.04NS | 26.37f | 30.40c | −4.03* |
| T9 | 40.00b | 34.90d | 5.10* | 40.21defg | 33.21f | 7.00* | 32.82bc | 25.83de | 6.99** |
| T10 | 40.88b | 33.50ef | 7.38** | 41.34de | 31.61f | 9.73** | 33.71b | 24.52e | 9.19** |
| T11 | 40.19b | 39.59a | 0.60NS | 43.19c | 39.42b | 3.77** | 32.73bc | 34.37ab | −1.64NS |
| T12 | 39.47bc | 39.54a | −0.07NS | 44.86ab | 40.00ab | 4.86** | 31.44bcd | 36.30a | −4.86** |
| T13 | 36.93d | 36.70bc | 0.23NS | 39.82efg | 35.23de | 4.59** | 30.09de | 32.09bc | −2.00NS |
The effects of pressurized steam chamber conditions (chamber temperature and retention time) on the ruminal dry matter, starch, and crude protein degradabilities of corn flakes are summarized in Fig. 2. Dry matter degradability was the highest in T1 throughout the incubation period; its degradability at 9 h similar to that in other groups at 12 h. Starch degradability was the highest in T1 from 9 to 48 h. Crude protein degradability was higher in T1 than in other groups from 3 to 48 h.
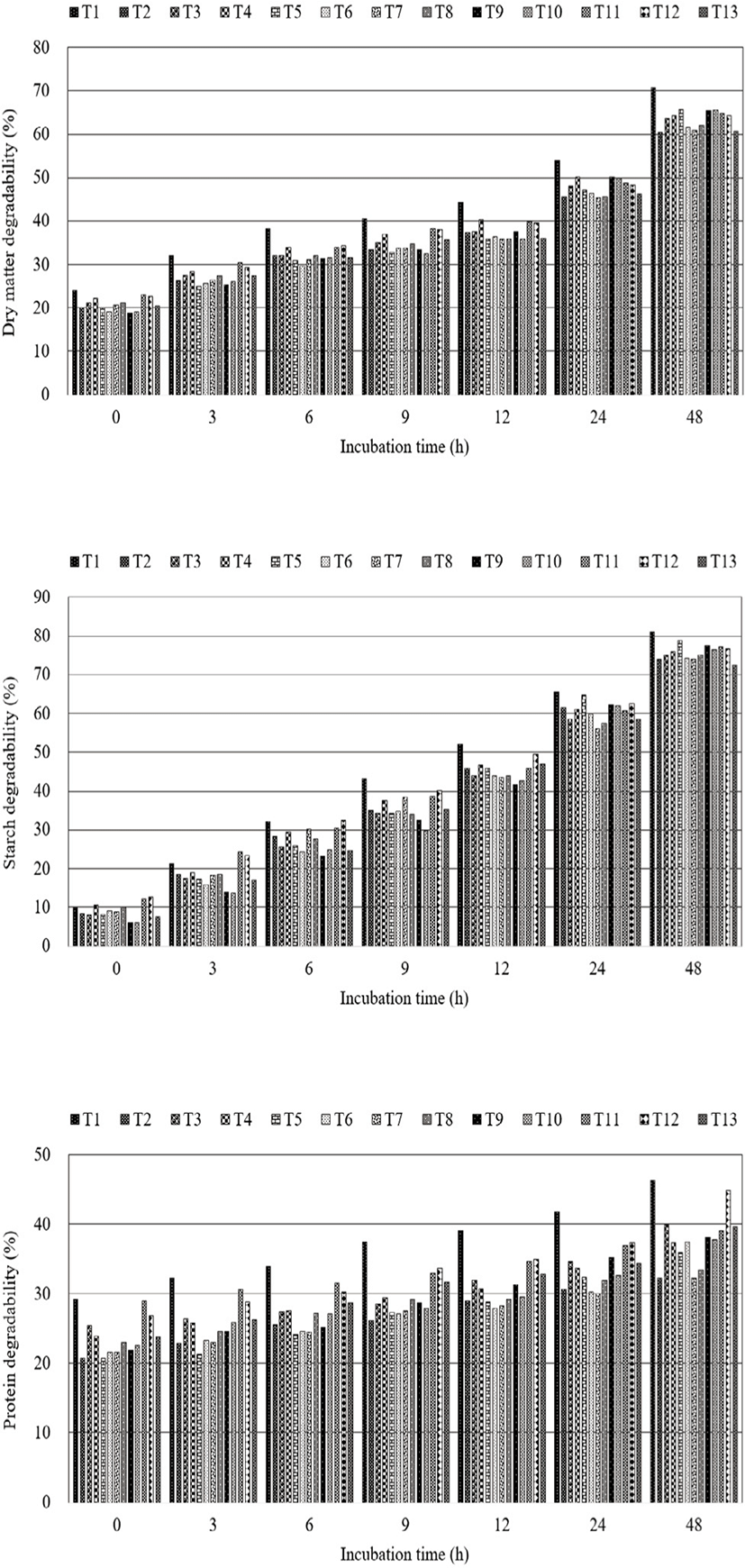
On increasing the retention time (700, 850, and 950 s) at 105 °C and 112 °C, dry matter degradability of corn flakes tended to increase; however, the opposite trend was observed at 116 °C. In addition, notwithstanding the lack of statistical significance, higher chamber temperatures (105 °C, 112 °C, and 116 °C ) at 700, 850, and 950 s tended to improve the nutrients degradability of corn flakes; however, this tendency was inconsistent.
Discussion
Flake treatment for corn improves the efficiency of ruminal fermentation [19,20] and the degree of efficiency may vary depending on conditions such as chamber temperature, retention time, etc. [10]. In the present study, the conditions of the pressurized chamber (chamber temperature and retention time) had a direct effect on the ruminal fermentable characteristics of corn flakes. Ratnayake and Jackson [21] reported that chamber temperature and retention time could affect moisture absorption, molecular motion, and physical changes in starch and gelatinization of corn. Therefore, conditions in the pressurized chamber (chamber temperature and retention time) may be important factors that have been suggested to improve the degradability of corn flakes in the rumen.
Ruminal pH is influenced by starch content, starch digestibility, VFA and lactic acid [2]. In the present study, the lowest ruminal pH was obtained in corn flakes produced at 100 °C–720 s; however, a direct association between the lowest pH and VFA concentration was less. Though we did not measure the lactate concentration in the present study, the above mentioned results could be due to the effects of lactic acid [22,23]. In the present study, the highest starch degradability was obtained at 100 °C–720 s, which might explain the decrease in ruminal pH owing to increased lactic acid production. A similar finding was reported by Firkins et al. [24], who found that corn starch is rapidly degraded in the rumen, producing a large amount of lactic acid and causing a drop in ruminal pH.
Irregular concentrations of ammonia observed in this study could be due to changes in the efficiency of ammonia nitrogen utilization for microbial protein degradation and microbial protein synthesis [25]. The low ammonia concentration at 100 °C–720 s, 116°C–750 s and 116°C–850 s seems to be due to the improved efficiency of microbial protein [26]. Ferraretto et al. [27] reported that an increase in ruminal starch degradation improved microbial protein synthesis. Bach et al. [28] noted that microbial protein synthesis was increased owing to carbohydrate fermentation in excess of protein degradation. Xin et al. [29] also reported that decrease in ammonia concentration was caused by increased microbial protein synthesis in vitro studies.
Starch, a major component of corn flakes, is converted to VFA, especially propionic acid, by the rumen microbes and lactic acid is produced as an intermediate degradable product [30]. In this study, the concentrations of propionic acid and total-VFA were higher at 105°C–700 s, 113°C–600 s, and 115°C–500 s; however, concentrations of propionic acid and total-VFA tended to decrease as the chamber temperature or retention time increased. This finding could be potentially attributed to lactic acid (not converted to VFA) and starch retrogradation. Starch retrogradation is a phenomenon in which the gelatinized starch changes into a crystalline or regular arrangement during the cooling process [31] and the digestibility is reduced due to the formation of resistant starch [32]. In addition, it has been reported that the production of resistant starch increases in accordance with the increase in heat effect. Dundar and Gocmen [33] found that resistant starch production was significantly higher at 145 °C than at 140 °C, and Lee et al. [34] reported that the formation of resistant starch in corn starch was higher at a high temperature (121°C) than at a lower temperature (100 °C). In the present study, the lowest VFA production at 116 °C-950 s, which is the highest chamber temperature and retention time, is also supported by previous observations.
Factors affecting the quality of corn flakes include temperature, capacity, time, moisture, and pressure [35]. Among these factors, the chamber temperature and the retention time are important factors that determine the gelatinization temperatures (initial, maximum and final), degree of gelatinization, retrogradation of corn flake starch and the quality of corn flakes. In the present study, the condition of the pressurized steam chamber (chamber temperature, and retention time) had a significant effect on ruminal dry matter, starch and crude protein degradability; therefore, as mentioned in the previous research reports [3,36], temperature and retention time of the pressurized steam chamber are contributing factors that determine the ruminal utilization of the corn flakes.
During the production of corn flakes, the rise in chamber temperature has been known to increase the absorption and swelling of moisture [37], thereby affecting starch gelatinization and facilitating action by enzymes [38]. In addition, Schwandt [9] noted that the temperature of the chamber was a factor that significantly increased the processing of starch by the enzyme, and Lee et al. [39] reported that as the heating time increased from 5 to 30 minutes, the gelatinization was increased, affecting flake product characteristics. In the present study, the degradability of dry matter and starch were high at 100 °C–720 s, which is the lowest chamber temperature, and these degradabilities were not consistent with the retention time at the same temperature condition. Taking these into consideration, it can be inferred that the increase of exposure time to heat (temperature) and the effect of pressure during the use of a pressurized steam chamber are influential factors in starch retrogradation or resistant starch production. Pressure treatment on starch has been shown to have effects such as effective heat transfer, enthalpy reduction for gelatinization, initial gelatinization temperature, and reaction promotion [11,13], thereby improving amylase-degrading ability [40] and increasing starch gelatinization [41]. On the other hand, the similar tendency of dry matter and starch degradability pertaining to the condition of the pressurized steam chamber (chamber temperature and retention time), is considered to be related to the high starch content (more than 70%, dry matter basis) of corn [42]. In the present study, the crude protein degradability in 100 °C–700 s was higher than other high temperature conditions. This finding corroborates with previous studies [43,44], which reported that long-term heat treatment at high temperature for cereal grains caused not only protein denaturation but also decreased ruminal degradability.
Hungate [45] reported that the types and ratios of rumen microorganisms can vary depending on factors such as feed type, age, breed, and weight. In the present study, the effects of the pressurized steam chamber conditions on corn flakes varied according to the breed (Holstein and Hanwoo). These results can be attributed to the differences in body weight, rumen volume and rumen microbes (number, type, ratio, etc.) of Holstein and Hanwoo cows.
Collectively, the results of this study show that the corn flakes produced at 100°C–720 s and 105°C–700 s had higher propionic acid, total-VFA, and nutrients (dry matter and starch) degradability. Whereas, the conditions of the pressurized steam chamber (chamber temperature and retention time) were the main factors influencing the feed value of corn flakes in this study; however, the effect of pressure and resistant starch also influenced the feed value of corn flakes. Therefore, it is necessary to study these additional factors under the same conditions used in the present study.
Conclusion
In this study, we investigated the rumen fermentable characteristics and nutrients degradabilities of thirteen corn flakes produced by the different temperatures (100°C–116°C) and retention times (700–950 s) of the pressurized steam chamber; therefore, the temperature and retention time of the pressurized steam chamber are important factors affecting the quality of corn flakes. When we consider the input cost (electricity cost, the operation cost of equipment, etc.) and the production time, it is considered that the temperature of 105 °C or higher and the retention time of 720 s or more are not effective to improve the quality of corn flakes. Thus, considering the ruminal VFA and starch degradability, the proper temperature of the pressurized steam chamber for enhancing the feed value of corn flakes is 100 °C–105 °C and the retention time is 700–720 s.
