INTRODUCTION
Obesity is one of the biggest health problems in the world today and the number of obese people are increasing in all over the world [1,2]. Obesity is defined as an increase in body weight [3] caused excessive accumulation of fat cells due to adipocyte differentiation [4]. Hence, it is also closely linked to metabolic diseases such as type 2 diabetes (T2D), liver disease, cardiovascular disease (CVD), cancers, hypertension and other disorders which increased these disease occurrence [5,6]. Accumulation of fat cells that cause obesity is by the differentiation of adipocytes called adipogenesis. Peroxisome proliferator-activated receptor γ (PPARγ) and CCAAT/enhancer-binding protein (C/EBP) transcription factor family are key players to regulate the differentiation of preadipocytes by inducing adipogenic related genes including adiponectin (ADIPOQ), leptin, fatty acid synthase (FAS), adpocyte protein 2 (aP2), and acetyl-coA carboxylase 1 (ACC) [7–10].
Laver, an edible seaweed species belonging to the genus Porphya, is commonly grown and consumed in Korea, China, and Japan. Laver is a rich source of vitamins, minerals, polysaccharides, phenolic compounds and mycosporine-like amino acids (MAAs) [11]. The polysaccharides components include laminarin and fucoidan while phenolic compounds present includes epigallocatechin gallate (EGCG) and catechin. Moreover, MAAs present in laver are mycosporine, shinorine, and porphyra-334. Several studies have shown that laver has antioxidant [12], anti-ultraviolet [13], anti-inflammatory [14], and antitumor [15] effects because of its bioactive compounds present. Marine algae, especially seaweeds are a promising source of anti-obesity agent [16] and anti-obesity effects are reported in various kinds of seaweed (brown, red and green) [17]. Also polysaccharides and phenol compounds are also reported to have anti-obesity effects [18,19].
Porphyra dentata (P. dentata) used in this study, is a kind of red algae and belongs to the Bangiaceae and Pyropia genus and an edible red seaweed in eastern Asian countries [20]. P. dentata contains polysaccharides and phenolic compound such as fucoidan, EGCG, and catechin [21]. It was reported that it has anti-inflammatory activity by suppressing nitric oxide production in LPS-stimulated macrophage [21]. As reported, although the components of P. dentata have a potential to regulate adipogenesis, anti-obesity effect on this extract have not been addressed.
The purpose of this study is to evaluate whether the P. dentata extract has inhibitory effects on adipogenesis from preadipocyte to mature adipocyte in 3T3-L1 cells.
MATERIALS AND METHODS
Dulbecco’s Modified Eagle’s Medium (DMEM; high glucose) was purchased from HycloneTM (Logan, UT, USA). Fetal bovine serum (FBS) were purchased from Gibco-BRL (Gaithersburg, MD, USA). 3-Isobutyl-1-methylxanthine (IBMX), dexamethasone (DEX), pioglitazone, insulin, Oil Red O powder, and dimethyl sulfoxide (DMSO) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Formaldehyde Solution for 4% formalin was purchased from Fujifilm Wako Pure Chemical (Osaka, Japan). 2-propanol-GR and ethanol were purchased Merck (Kenilworth, NJ, USA). The 3T3-L1 cells were purchased from American Type Culture Collection (Rockville, CT, USA).
Dried P. dentata was obtained from Mokpo Marine Food-industry Research Center (Mokpo, Korea). The dried P. dentata (50 g) was extracted with 1.5 L of 50% aqueous methanol (MeOH) at room temperature for one day and then filtered. The residues were re-extracted with 0.75 L 50% methanol and then filtered. The combined filtrates were evaporated at 38°C under a vacuum. The 50% MeOH extracts of P. dentata were deposited at −20°C until use in experiment.
3T3-L1 preadipocytes (ATCC®, CL-173TM) were cultured in DMEM (high glucose) supplemented with 10% FBS, 1% penicillin and streptomycin (Welgene, Gyeongsan, Korea) at 37°C in 5% CO2. For experiment, cells were seeded in 6-well plates at a density of 1.0 × 105 cells/well and grown to confluence. Forty-eight hours after confluence (day 0), adipogenesis was induced by adding differentiation medium (DMEM; high glucose containing 10% FBS, 0.5 mM 3-isobutyl-1-methylxanthine; IBMX, 1 μM Dexamethasone; DEX, 1 μM Pioglitazone, 10 μg/mL insulin, 1 μL/mL dimethyl sulfoxide; DMSO) for 48 h. Every two days, the medium was changed with DMEM; high glucose supplemented 10% FBS, 10 μg/mL insulin, 1 μL/mL DMSO until 8 days. The pre-adipocytes were maintained and changed medium for every 48 h with DMEM; high glucose, 10% FBS, and 1 μL/mL DMSO medium. To investigate the effects of P. dentata on adipocyte differentiation, cell culture was treated P. dentata 50% MeOH extract in different concentrations (6.25, 12.5, and 25 μg/mL) in the differentiation medium for every two days, from the beginning to the end of the experiment. After 5 days of treatment with P. dentata 50% MeOH extract. 3T3-L1 adipocyte cells were harvested for Real-time quantitative polymerase chain reaction (RT-qPCR) and after 8 days the 3T3-L1 adipocyte cells were fixed in 4% formalin for Oil Red O staining.
3T3-L1 cells were seeded in 96-well plates at a density of 7.5 × 102 cells/well containing 200 μL of 10% FBS-DMEM; high glucose. After cell seeding, P. dentata 50% MeOH extract was added by concentration dependent (6.25, 12.5, and 25 μg/mL). After 24 and 48hr after addition of the extract, alamarBlueTM Cell Viability Reagent (ThermoFisher Scientific, Waltham, MA, USA) was added and then fluorescence value was measured by SYNERGY multi-mode reader (BioTek, Seoul, Korea). Viability of cells was measured using alamarBlue assay according manufacturer instructions.
To measure the cell lipid droplets, the 3T3-L1 cells were stained with Oil Red O solution. 3T3-L1 cells were washed twice with PBS and adherent cells were fixed in 4% formalin for 10 min at room temperature. The 4% formalin was discarded and fresh 4% formalin was added and incubated for 1h at room temperature. After 1hr, is was washed with tertiary distilled water. The cells were added with 60% isopropanol and let it stand for 5 min at room temperature. After 5 min, 60% isopropanol was discarded and the cells were allowed dry completely at room temperature. After drying, 1 ml Oil Red O solution was added to each well and incubated at room temperature for 20 minutes. The cells were washed three times with tertiary distilled water and photographed using a Leica Microscopy, DE/Polyvar SC (Leica, Wetzlar, Germany).
Total RNA was isolated from cells using Hybrid RTM (GeneAll Biothechnology, Seoul, Korea) including RiboEXTM treatment of samples to eliminate genomic DNA, protein, and lipid. Quality of RNA was determined by using Nanodrop 2000 spectrophotometer (ThermoFisher Scientific) and RNA gel electrophoresis. cDNA was synthesized from the total RNA using a RevertAid First Strand cDNA Synthesis kit (ThermoFisher Scientific). The real-time PCR was conducted using a CFX96TM Real-Time PCR Detection System (Bio rad, Hercules, CA, USA). The level of target gene cDNA was measured by TB Green® Premix EX TaqTM (Tli Rnase H plus) (Takara Bio, Kosatsu, Japan). All samples were analyzed in triplicate and quantified by the relative standard curve method using the gene expressions of L32 as a housekeeping gene. The sequences of the primer pairs used in this study are listed in Table 1.
Statistical analysis was performed using GraphPad Prism 0.8 (GraphPad Software, San Diego, CA, USA). All the data were analyzed using one-way analysis of variance (ANOVA) with multiple comparisons. Differences between groups were analyzed using t-test and values of p < 0.05 were considered statistically significant. All experiments were performed triplicate and data were expressed as mean ± SEM.
RESULTS
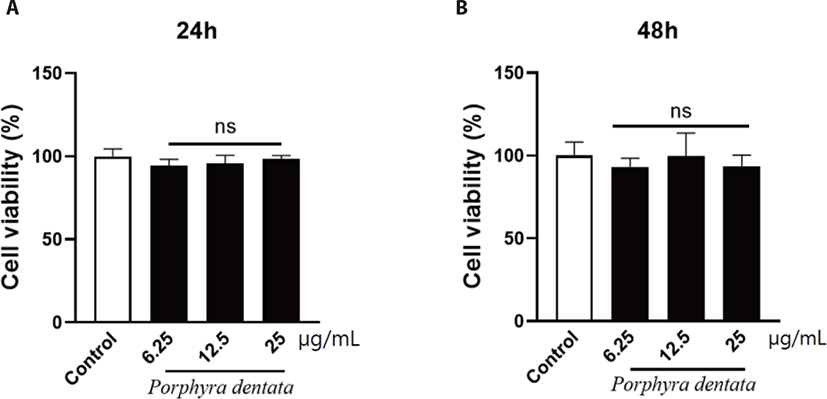
We first performed the alamarBlue assay to test the effect of P. dentata 50% MeOH extract on cell viability. As shown in Fig. 1, P. dentata 50% MeOH extract at 6.25, 12.5, and 25 μg/mL showed no significant effect on cell viability in 3T3L1 mouse preadipocytes after 24 h and 48 h treatment. These results indicate that the P. dentata 50% MeOH extract have no cytotoxicity on cells.
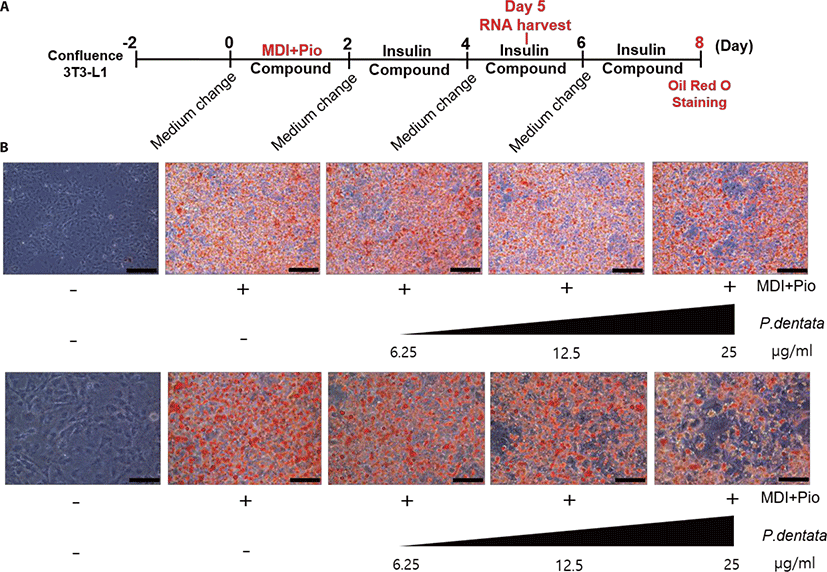
To investigate the effect of P. dentata extract on 3T3-L1 preadipocytes adipogenesis, we treated P. dentata extract with various concentrations for 8 days and stained the lipid droplets with Oil Red O during adipocyte differentiation (Fig. 2). Oil Red O staining assay revealed that P. dentata dramatically reduced lipid accumulation in a concentration dependent manner. These results indicate that P. dentata 50% MeOH extract suppressed adipocyte differentiation and lipid droplets formation in 3T3-L1 preadipocytes.
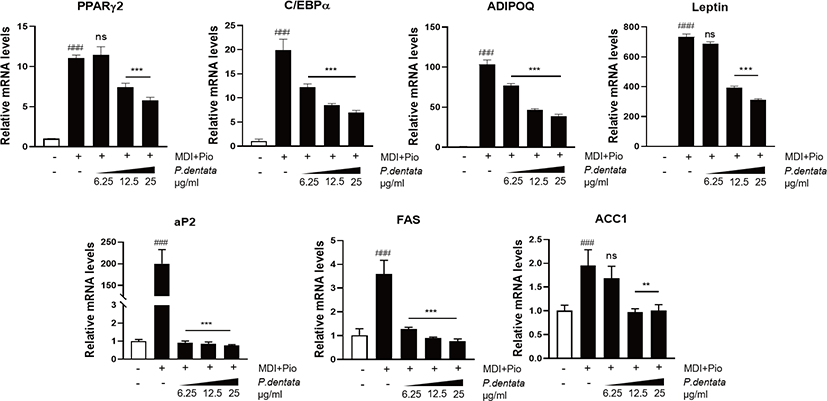
Next, we performed RT-qPCR analysis to examine the mRNA expression of adipogenic specific transcription factors such as PPAR-γ2, C/EBPα, and their target genes such as ADIPOQ, Leptin, FAS, aP2, and ACC1 after the treatment of P. dentata 50% MeOH extract. The extract decreased the PPAR-γ2, C/EBPα, as well as ADIPOQ, Leptin, FAS, aP2, and ACC1 mRNA expression. Gene expression of the PPAR-γ2, C/EBPα, and their adipogenic related genes following P. dentata treatment was significantly lower compared with that of differentiated control adipocytes treated MDI (methylisobutylxanthine, dexamethasone, insulin) plus pioglitazone. P. dentata 50% MeOH extract significantly downregulated the expression of adipogenesis associated genes in a dose-dependent manner (Fig. 3).
DISCUSSION
The 3T3-L1 mouse preadipocytes have been widely used for screening the effective agents to regulate the adipogenesis. The adipogenesis was determined by Oil Red O staining to show the amount of lipid droplets by specifically staining neutral triglycerides with high levels of adiopocyte-related genes expression in 3T3-L1 cells [22].
In the present study, we demonstrated that P. dentata extract dramatically inhibited lipid accumulation during adipocyte differentiation with decreasing PPARγ2, C/EBPα, ADIPOQ, leptin, FAS, aP2, and ACC1 expression. One of the alternative splicing forms of PPAR, PPARγ2, is a lipid-activated transcription factor which specifically expressed in adipose tissue [23]. In response to fatty acids, PPARγ2 leads to fat accumulation in adipocytes by modulating target genes involved in lipid metabolism [24]. However, PPARγ2 does not function alone but cooperatively with transcription factors in the C/EBP family to induce adipocyte differentiation [9,24]. The C/EBPs belong to the basic-leucine zipper class of transcription factors and has several forms including C/EBPα, C/EBPβ, C/EBPγ, and C/EBPδ [25]. The temporal expression of these factors during adipocyte differentiation indicates a cascade whereby early induction of C/EBPβ and C/EBPδ leads to induction of C/EBPα, which C/EBPα induces expression of many adipogenic related genes directly [26]. In this study, the mRNA expression of PPARγ2 and C/EBPα decreased significantly after treatment of P. dentata extract compared with that in differentiated control cells. It has been reported that PPARγ2 and C/EBPα cooperates to increase adipogenic genes using a positive feedback loop between them leading to adipogenesis [27]. ADIPOQ, leptin, and aP2 investigated in this study, are adipokine which cytokine secreted by adipose tissue and in obesity [28,29]. ADIPOQ is an adipocyte-specific factor, which adipocyte-derived hormone, it is abundantly produced and secreted by adipose tissues and widely recognized for its anti-inflammatory effects [30]. Leptin is also adipocyte-derived hormone that circulates in proportion to fat mass and acts as a negative regulator of energy homeostasis [31]. aP2 called fatty acid binding protein 4 (FABP4) is one of the only genes characterized by sufficient regulatory sequences to direct adipose-specific expression in vivo [32,33]. Having played an important role as adipocytes differentiation marker, as it can lead to the development of increasingly large adipocytes by leptin resistance and contribute to the accumulation of excessive fat masses found in obese states [34]. These adipokine levels were increased during differentiation from preadipocytes to maturation adipocytes [34,35]. The other adipogenic related genes, FAS and ACC1 are lipogenic enzymes. FAS is the key enzyme in lipogenesis, catalyzing the reactions for the synthesis of long-chain fatty acids [36]. ACC1 is a multi-subunit lipogenic enzyme that catalyzes the irreversible carboxylation of acetyl-CoA to produce malonyl-CoA for the biosynthesis of fatty acids [37]. Plants have been used as traditional natural medicines for healing many diseases [38] and many studies have shown that plant-derived foods have the potential to reduce obesity [39]. A plant belonging to the genus Porphyra, called laver, also consumed mainly as processed food or used a source of health-enhancing substances and this group have a unique active substances which provide health benefits [39,40]. Porphyra species contain biological active compounds, including polysaccharides, carotenoids, phenolic compounds, and MAAs. These compounds have been reported to have antioxidant [41], anti-inflammatory [42], anti-cancer [43,44], prevention of nervous system [45], and bone disease [46]. In particular, fucoidan, carotenoids, and phenolic compounds (EGCG) inhibited lipid accumulation in 3T3-L1 cells.
Previously, Kim et al. [22] indicated that Pyropia yezoensi (P. yezoensis) methanol extract, one types of laver contain high MAAs content (120 mg/g dried extract) reduces the contents of accumulation lipid determined by Oil Red O staining in a dose-dependent manner [22]. However, they demonstrated that treatment with high concentration (5 mg/mL) of the P. yezoensis methanol extract inhibited adipogenesis with decrease of preadipocytes proliferation via oxidative stress and proapoptotic effects. Our result indicates that P. dentata 50% MeOH extract at low concentration of ~25 μg/mL significantly suggest anti-adipogenesis in a dose-dependent manner with no cytotoxicity in 3T3-L1 cells. Hence, further study is needed to identify whether bioactive compounds (polysaccharides, carotenoids, phenolics, etc.) contained in laver contribute suppression of lipid accumulation in adipocyte.
In conclusion, P. dentata extract inhibited the accumulation of lipid droplets in concentration-dependent manner. Moreover, P. dentata extract inhibits the expression of adipogenic related genes involved in the adipogenesis from preadipocytes to mature adipocytes in 3T3-L1 cells. Especially, in all experiments, lipid droplets formation and gene expression are inhibited in a concentration-dependent manner (6.25, 12.5, and 25 μg/mL) of P. dentata extract. Thus, the result revealed that P. dentata has an effect of anti-obesity that inhibits adipogenesis. Since pharmacological effects of the Porphyra species are proven [47], and P. dentata belonging to that species is also consumed as food, it has the potential to be used as a dietary supplement and medicinal food item to suppress obesity.
