INTRODUCTION
Understanding the impacts of climate change on domesticated animal species, particularly on poultry has been the subject of many researchers. Climate change studies conducted in Korea have shown gradually increasing trend in air temperature over the last decades [1]. A change rate of 0.5°C per decade was reported by the Korean Meteorological Administration, while global temperature was raised 0.2°C per decade [1–3]. The increased air temperature due to climate change may enhance the incidence of heat stress in poultry farms. As heat stress is a critical issue in animal husbandry, a reliable stress measurement method is required to evaluate stress status.
Measuring corticosterone in biological samples such as blood [4–6], feces, soft tissues [7–9], and recent experiments in feathers [4,6,10,11] is a common technique to monitor both acute and chronic stress status in the birds. Among the investigators, blood sampling is the main biological element for measuring corticosterone as a biomarker of stress in chickens [9]. However, blood samples provide a snapshot of corticosterone level at the time of blood sampling [4] or at most a period of 24 hours prior to sampling, which represents acute stress and cannot accurately represent chronic stress. Additionally, when blood sampling from chicken is problematic (e.g., in slaughtered chickens), feather is an alternative tissue for corticosterone extraction, which represents the long term of stress over period of weeks to months and can provide an alternative matrix [4]. Therefore, blood matrix is used for measuring acute stress status while possible fluctuations regarding the effect of capturing the animals on hormone level is inevitable. The feather matrix is accurate for analyzing chronic stress status while the effect of capturing animals on stress level can be ignored as an influential factor. Given this, when possible, feather corticosterone in measuring chronic stress is more reliable and accurate analysis than blood. However, blood and fecal samples required specific storage (i.e., refrigerator, freezer, etc.) before running the analysis while the time lasting the storage and its method can influence the result as the samples degrade over time [9,12]. Taking feather samples from animals is faster and rather easier than taking blood samples. The advantages are including, the reduced handling time, reduced labor, where also it may reduce capturing stress of the birds during sampling. Moreover, hormone changes in the blood can occur very quickly due to the environmental stressors or handling the animals, while the changes will not be instantaneous in the feather corticosterone. For blood sampling, the chicken must be healthy and alive, while for feather sampling, the chicken does not need to be alive, and can be sampled after death. Therefore, the tendency to analyze corticosterone hormone in feathers is more than corticosterone hormone in the blood. Also, the blood corticosterone measurements are vulnerable to parameters such as time of collection, etc. [9,13,14]. Furthermore, the aforementioned matrices, except for feather, require repeated measurements, which are costly and laborious [15,16]. Several studies reported the presence of corticosterone in the feather of chickens as a putative biomarker of chronic stress [4,11,17], because feather contains information about how corticosterone concentration has changed over time. The feather of birds can be stored at room temperature, and having higher accuracy due to its lower variations, and also the fact that the time point sampling and the output indicates the prolonged stress exposure without repeating the measurements [4,9,10]. However, a paucity of consistency in the matrix used and the processing methods and available facilities for hormone extraction can be considered as sources of variations in determination of feather corticosterone concentration (FCC). In our previous laboratory research on the methodological validation of using a bead beater (BB) and surgical scissors (SS) in the hair of Hanwoo cattle, revealed the importance of hair processing method in obtaining constant and reliable hair cortisol [18]. Equally, some literature indicated methods of pulverizing feather into a powder or finely chopping the feather into small pieces for extraction of corticosterone include a) using the SS [4,17], b) the ball mill [9,19,20], and c) the BB [9]. Each of these processing methods has claimed to have some limitations of using, such as long processing time for chopping into small pieces or pulverization of feathers, and homogenization [9,21] that could possibly confound interpretation of the result. For instance, the application of ball mill stated to be costly [18,22]. In addition, it has been noted that the more portion of corticosterone extraction by pulverizing the feather into homogenous dust revealed better accuracy of the methodology and reported to be more reliable technique [21]. Unlike the SS method, the BB method reduces contamination and indicated as a less time-consuming method when multiple samples are processed [23,24]. On the other hand, more pulverizing the matrix such as hair and feather into fine particle will result in a higher amount of extracted hormone, which is desirable and accurate. As validation of feather corticosterone extraction methods may support researchers for saving time and improving the efficiency of sample preparation, the current study was processed. Moreover, sample preparation is the primary step of analytical process and different techniques of feather matrix disruption can be used in the laboratory. Homogenization and pulverization of the sample may result in better extraction of matrices hormone due to the break the structure of tissues that needs validation study. Therefore, to evaluate whether the processing method affects the extracted concentration of corticosterone, this study was designed to validate two main processing methods of feather prior to corticosterone analysis including the BB method versus the SS method in broiler chickens.
MATERIALS AND METHODS
The experimental procedure and methods were approved by the Institutional Animal Care and Use Committee (IACUC) of Kangwon National University, Chuncheon, Korea (KW-200520-1).
Feathers samples were harvested from the wings of broiler chickens Ross 308 (n = 12 birds) within the age range of four weeks old. The broiler chickens were housed under the same environmental condition with access to commercial feed and drinking fresh water. Feather samples were prepared prior to assaying following Lattin et al. [19]. The feathers (three to five from each individual) were selected from twelve commercial broiler chickens and placed on a piece of clean aluminium foil to eliminate the light effect, stored in a plastic bag. They were labelled then delivered to the laboratory and stored at room temperature for following procedures including removing calamus, pulverization, corticosterone extraction and analysing by corticosterone enzyme immunoassay (EIA) (Fig. 1).

In detail, the samples were unwrapped from the aluminium foil and feathers were cleaned with tissue wipers (Kimtech Science) for removing dust and dirt. Further, feather pulverization was performed by two accessible instruments in our laboratory; 1) the BB (tacoTMPrep, 50/60 Hz 2A, GeneReach Biotechnology, Taichung, Taiwan), which is able to disrupt biological samples (up to 16 samples) within a short period of time through contacting with highly accelerated beads for releasing cellular content and 2) the SS that feather samples is chopped into tiny pieces. Therefore, the calamus part was removed from the feather using the SS. The remaining feather length, weighed (50 ± 0.5 mg) with a digital lab scale and then, cut into small slices and transferred into tubes of the BB (2 mL) containing six stainless steel balls and were loaded into the BB device for 16 min at 50 Hz. And the same feather samples weighed mass (50 ± 0.5 mg) were finely chopped using the SS, each feather was cut into small pieces no longer than 5 mm in length for 2 minutes and put in a 15 mL conical tube. The pulverized feather was suspended with 7 mL methanol (Daejung Chemicals & Metals, Siheung, Korea) into new polypropylene tubes (15 mL Conical Tube, HM Hyundai Micro, Seoul, Korea) and the tube’s cap was sealed with parafilm and inserted in a sonication water bath at room temperature for 30 minutes. A while later, samples were placed in a shaking water bath (50°C, 15 hours). Feather samples and methanol was separated using filtration funnel (#4 Whatman filter paper). 2.5 mL extra methanol was used to rinse the filter paper and tubes. This rinsing procedure was repeated and added to the methanol extraction followed by drying in a 50°C in the incubator to evaporate the methanol. The extract residues were stored in the freezer (−20°C) for final analysis corticosterone hormone. Prior to use of the EIA kit, the dried hormone extracted of feather samples was thawed at room temperature, then added 0.5 mL of assay diluent buffer provided by the EIA kit (Prod. No. K014-H, Arbor Assay, Ann Arbor, MI, USA), vortex completely, and centrifuged at 1,500×g for 15 minutes, and then each sample duplicated into two wells in order to improve the power of the test and reliability of the results. For duplication, 50 µL of supernatant have chosen based on the kit recommendations. It should be noted that 8 standard solutions were used to draw the data curve. The optical density of samples was read using a microplate reader (SpectraMax Absorbance Readers, Molecular Device LLC, San Jose, CA, USA) wavelength of 450 nm (Fig. 1). The concentration of corticosterone obtained from the microplate reader was in pg/mL converted to the unit pg/mg.
The statistical procedure of t-test was performed using SAS software (Version 9.3, SAS Institute, NC, USA) to compare two pulverization methods including BB and SS. The individual broiler chickens were considered as an experimental unit. Statistical differences were considered significant at p < 0.05 and differences among means with 0.05 < p < 0.10 was accepted as representing tendencies to differences.
RESULTS AND DISCUSSION
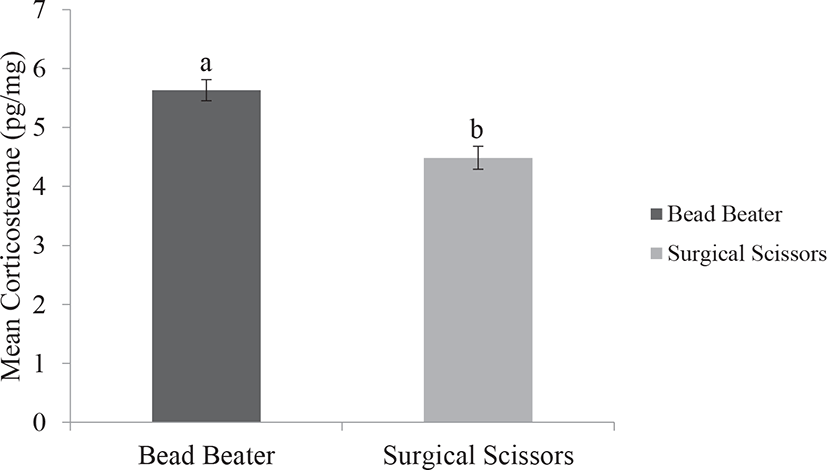
Our results indicated lower standard error and less variation in data output of FCC by using the BB method compared with the SS method. We also observed that using the BB provided significantly higher FCC than those of the SS (p < 0.001). Fast preparation and pulverizing feather samples with the BB has a clear benefit over simply chopping and slicing feathers by the SS. Unlike the SS, the BB is an effective method to break the feather matrix enabling explosion of tissue and outpouring hormone from the follicles. This may increase the hormone extraction to the maximum amount and thus avoiding under detection of the data. Feathers composed of beta keratin proteins and grow in very small follicles in the epidermal skin of birds. In addition, the FCC may be affected by the size, structure, growth rate, and steroid hormone content of feather (Fig. 2) [19,25].
Blood corticosterone is deposited in the keratin structure at the time of feather growth, however, the level of corticosterone dispositions into feather is influenced by feather growth [4,6,10]. Therefore, choosing the feather processing method prior to corticosterone measurement as a putative biomarker of chronic stress is crucial. It is mainly because the results can vary greatly with respect to performing inappropriate feather preparation prior to corticosterone extraction. With similar assumption, but in hair processing, it is found that the pre-processing of hair prior to cortisol extraction will differ greatly due to various hair processing [18,22]. In contrast to feather that is composed of beta keratin proteins, hair is made of alpha keratin and grow in follicles in the dermis skin of mammals [25]. Hair cortisol is a mix of internal and external sources during hair growth. Meyer et al. [22] indicated that the samples need to be finely pulverized to break down the hair’s protein matrix and increase the surface area to increase the portion of cortisol extracted from the hair shaft. Extraction of feather corticosterone indicated to be dependable on the feather particle size and therefore, the higher the amount of powder the higher the concentration of extracting corticosterone [9].
Accordingly, based on two available processing methods that were used in the present study, we depicted that using the BB was relatively easier to perform and more portion of corticosterone could be extracted as compared with the SS (Fig. 3). In addition, the application of the BB showed higher capability to simultaneously pulverized 16–24 samples, in contrast to the SS. Also, using the BB provided homogenized ground feather, which increased the chance of higher portion of corticosterone extraction and thus improved the reliability of the assessment. These results are in agreement with the previous study conducted in the hair of cattle [18]. The greater amount of obtained corticosterone by the BB may be related to the pulverization of thick rachis structure in order to the homogenized and smaller particles of feather compared with simply chopping feathers by using the SS [9,18,22].
Using hair of cows [18], we previously indicated the higher amount of cortisol by the BB which is consistent with the obtained result in this study. It is suggested that pulverized hair samples by the BB method may yield up to 3.5 times higher cortisol with less variation compared to the SS method [26,27]. Application of the SS in finely chopping hair was reported in precedent studies [28–30]. The current experiment showed that using the SS was not an applicable method for small size feathers. Furthermore, feather morphogenesis is more complicated than hair, therefore simply chopping by the SS may not break the thick feather structure compared to the BB (Fig. 4). The length of hair could be cut into pieces for approximately 1mm size with the SS whereas the BB could produce hair with the finest powder possible [18]. Additionally, an extra time-consuming working step was performed by the SS method and it could increase the chance of contaminations with external sources. In contrast, Slominski et al. [31] concluded that using the SS was a more valid method in hair processing than using Bullet Blender. Given these results, the BB is ideally suited versus the SS for breaking feathers matrix and is recommendable as it produces smaller homogenized particle size prior to hormone extraction.

CONCLUSION
Pulverizing feather samples with the BB method had clear advantages over simply chopping feather with the SS method. First, the BB method reduced time for feather sample preparation for hormone extraction because the SS method needed almost 2 times longer time to chop the feather samples compared to the BB method. Secondly, and most importantly, the BB method yielded more corticosterone in feather compared to the SS method due to pulverization and homogenization of feathers, which led to better and more accurate analysis. Therefore, pulverization of feather sample by the BB method is essential to optimize the corticosterone extraction from feather.