INTRODUCTION
At present, silage corn (Zea may L.) and sorghum-sudangrass hybrids (Sorghum bicolor × S. bicolor L., SSH) continue to be the main summer crops used as feed resources in many countries. Corn is a widely recognized cereal used mainly in food, livestock feed, and industrial raw materials. Corn is also known as the “king of the forages,” having a global annual production that exceeds those of wheat and rice. Silage corn is a high-yield crop that effectively uses nutrients in the soil. Among the forage crops, corn is the crop with the highest nutritional yield. It also has excellent productivity and feed value, and, in particular, has high sugar content, good fermentation quality, and high palatability to livestock [1].
SSH have the highest dry matter (DM) yield among various forages. Compared to corn, they have a smaller leaf area, more secondary roots, and a waxier leaf surface, which help them withstand drought better than corn [2]. They also can effectively increase soil organic matter content in areas with good sunlight [3]. In addition to the two aforementioned crops, proso millet (Panicum miliaceium L.) has also attracted attention because of its short planting cycle and strong growth in saline soil. Proso millet is within the genus Panicum and has high drought and salt tolerance and strong resistance to many diseases affecting corn [4]. Corn is the crop with the highest total digestible nutrients (TDN) yield per unit area. SSH is higher in productivity than corn, but has a disadvantage of lower quality. Proso millet has the disadvantage of low quantity, however, it can be used when corn or SSH cannot be sown due to a short growing period. It has a higher content of crude protein (CP) compared to other crops [5].
Progressively more attention has been given to methods that can produce high-quality silage to improve animal production performance. In recent years, to improve the fermentation quality of silage, various additives have been used to promote or inhibit fermentation, reduce fermentation losses, and improve animal performance, and different types of additives have been shown to have different effects on silage. In the fermentation process, microorganisms, especially lactic acid bacteria (LAB), are most critical. Lactic acid, which reduces the pH, is produced by LAB through fermentation. The low pH limits plant enzymatic activity; therefore, LAB inoculants are crucial in the production of silage [6]. Use of an inoculant is now the predominant technology employed to influence silage fermentation. Kung and Muck [7] found that homo-fermentative inoculants can improve animal performance by 3% to 5%. Tao [8] inoculated silage with LAB and showed that the pH decreased significantly and the quality of the silage obtained was improved in comparison with non-inoculated silage.
Contrary to the promotion of fermentation by LAB, chemical additives such as formic acid can inhibit silage fermentation. Adding formic acid can effectively inhibit fungal and plant enzyme activities in the silage and reduce protein loss [9]. There are reports [10] stating that elephant grass treated with 0.5% formic acid improved the fermentation, intake, and digestibility compared to an untreated control.
Due to the differing nutritional contents of various forages, the effects of additives also differ. Furthermore, there is little information related to a comparison of the effects of different additives on corn, sorghum-sudangrass hybrids, and proso millet. Therefore, this experiment was designed to explore the effects of different additives on the quality of different crop silages.
MATERIALS AND METHODS
Proso millet (“Geumsilchal”), silage corn (“Gwangpyeongok”), and sorghum-sudangrass hybrid (“Turbo-gold”) were collected after harvest from the experimental field of Seoul National University, Pyeongchang Campus (located at 37°32ʹ40ʺ N, 128°26ʹ33ʺ E), during the summer season of 2019. All crops were chopped into lengths of 2–3 cm using a fodder chopper (Richi Machinery, Henan, China). The chopped crop materials were spread flat on plastic film and sprayed with an equal amount of different additives. The additives included Lactobacillus plantarum (1.5 × 1010 CFU/g fresh matter, CMbio, Anseong, Korea) and formic acid (98%, Junsei Chemical, Tokyo, Japan). The silage treatments were as follows: (1) control (distilled water), (2) inoculant LAB (1.0 × 106 CFU/g fresh matter), and (3) formic acid (5 mL/kg). Then, the chopped crop materials were ensiled into 20-L mini silos, which were sealed tightly with lids. Three replications were performed for each treatment. The 27 mini silos were preserved at ambient temperature and with a dark and dry environment for 60 days before opening.
Approximately 500-g samples were collected and then dried at 65°C in an air-forced drying oven for 72 h to determine the content of DM. The dried samples were ground to pass through a 1-mm screen (Thomas Scientific, Swedesboro, NJ, USA). CP was measured by the Dumas method, as described by Jean-Baptiste-André Dumas [11], using an Automatic Elemental Analyzer Euro Vector EA3000 (EVISA, Milan, Italy). Acid detergent fiber (ADF) and neutral detergent fiber (NDF) were measured by the method of Van Soest et al. [12] using an ANKOM2000Automated Fiber Analyzer (Ankom Technologies, Fairport, NY, USA). Heat-stable amylase and sodium sulfite were used for the NDF procedure. Water soluble carbohydrate (WSC) was analyzed by modifying the anthrone method proposed by Yemm and Willis [13]. TDN and RFV were calculated by the formula described by Holland et al. [14]. In vitro dry matter digestibility (IVDMD) is an index used to analyze the nutrient digestibility of feed materials in animals [15]. It was determined by the following procedure. First, 0.5–0.6 g of ground sample was weighed into filter bags, which were sealed using a heat sealer (#HS: 100 V–120 V / #HSi: 220 V–240 V). The samples were distributed evenly on both sides of Daisy Incubator digestion jars (Ankom Technologies, Fairport, NY, USA). Then, 1,330 mL of buffer solution A and 266 mL of buffer solution B were added into each jar. Two healthy Holstein steers were selected and cannulated. Their rumen fluid was collected through four layers of cheesecloth before the morning feeding. Then, 400 mL of rumen fluid was added to the buffer solution and samples. The digestion jar was purged with CO2 gas for 30 s, and the lid was secured. The samples were incubated at 39°C for 48 h. Then, the procedure for determining NDF was used to obtain IVDMD.
The spread-plate method [16] was used for microbial analysis. The samples (10 g) were diluted with 90 mL of sterilized saline solution (8.50 g/L NaCl) and shaken for 1 h. Serial dilutions (10−1–10−5) were streaked on de Man, Rogosa, and Sharpe agar medium, plate count agar medium, and potato dextrose agar medium.
Ten gram (10 g) of fresh chopped silage sample was weighed into a 250-mL conical flask and covered with 100 mL of distilled water. The sample was shaken for 1 h with a shaker (Green Sseriker, Vision Scientific, Daejeon, Korea) and stored in a refrigerator for 24 h, during which time the conical flasks were shaken by hand every 2 h. The mixture was then filtered through filter paper (Whatman No. 6, ADVANTEC, Toyo Roshi Kaisha, Ltd, Japan), and the filtrate was used to measure the pH of the silage using a pH meter (AB 150, Fisher Scientific International, Pittsburgh, PA, USA). Subsequently, 1.5 mL of the filtrate was used to analyze the contents of organic acids by high performance liquid chromatography (HPLC) using a system (HP-1100, Agilent Technologies, Santa Clara, CA, USA; Column: Agilent Hi-Plex H, 7.7 × 300 mm, 8 μm (p / n PL1170-6830), Mobile phase: 0.005 M H2SO4, 0.7 mL/min, detector: UV, 55°C, Injection volume: 20 μL) equipped with a refractive index detector. Lactic, acetic, and butyric acid were used to assess the quality of the silages according to the Flieg-Zimmer scale [17]. The buffering capacity (BC) was determined following the method described by Playne and McDonaldet [18]. Ammonia nitrogen (NH3-N) was analyzed via the method described by Broderick and Kang [19].
All data were subjected to analysis of variance using the General Line Model (GLM) of SPSS (SPSS 20.0 program, SPSS, Chicago, IL, USA). The completely random design was used for the effect of varieties on chemical composition, pre0ensiling characteristics and bacterial population, and the split plot design was used for the effect of varieties (main plot) and additive treatment (sub plot) on silage. Mean treatment differences were obtained by Duncan’s multiple range tests with a level of statistical significance of 5%.
RESULTS
The chemical compositions and characteristics of the raw materials are shown in Table 1. Proso millet had the highest DM and WSC contents. There was no significant difference in CP content among the three crops (p > 0.05). The lowest ADF and NDF contents were detected in corn. These were significantly lower than those of proso millet and sorghum-sudangrass hybrid (p < 0.05). The pH of corn (5.80) was significantly lower than those of proso millet and sorghum-sudangrass hybrid (p < 0.05). Furthermore, corn had the lowest BC and the highest LAB and TM (total microorganism) counts, which were significantly higher (p < 0.05).
SSH, sorghum-sudangrass hybrid; DM, dry matter; CP, crude protein; ADF, acid detergent fiber; NDF, neutral detergent fiber; IVDMD, in vitro dry matter digestibility; NH3-N, ammonia nitrogen; TN, total nitrogen; WSC, water soluble carbohydrate; BC, buffering capacity; FW, fresh weight; LAB, lactic acid bacteria; TM, total microorganisms.
The effects of additives on the chemical composition of silage differed depending on the crop (Table 2). The DM content of SSH was significantly lower than those of corn and proso millet (p < 0.05), and there was no significant difference among additive treatments of SSH and proso millet (p > 0.05). The DM loss of proso millet was higher than those of corn and SSH, and Lactobacillus plantarum (LP) treatment decreased the DM loss in corn significantly (p < 0.05).
Different lowercase letters in the same column indicate significant differences among additives (p < 0.05) and different uppercase letters in the same column indicate significant differences among crops (p < 0.05).
DM, dry matter'; CP, crude protein; ADF, acid detergent fiber; NDF, neutral detergent fiber; IVDMD, in vitro dry matter digestibility; TDN, total digestible nutrients; RFV, relative feed value; C, control; FA, formic acid; LP, Lactobacillus plantarum; SSH, Sorghum-sudangrass hybrid; V, variety; A, additive.
Low CP content was observed in the control groups. However, the CP content was affected by the treatments and was higher in the LP-treated silage (p < 0.05). Among these, the CP content in the LP treatment of proso millet was 5.50 g/kg higher than that of the control group, and the values for corn and SSH were 5.10 and 20.40 g/kg higher, respectively. Among the three crops, the effects of the different additives on the CP content of corn were not significant.
For proso millet, the contents of NDF and ADF in the LP treatment were significantly lower than in the control group (p < 0.05). SSH treated with additives also showed lower values, but these differences were not significant (p > 0.05). LP and formic acid (FA) addition significantly (p < 0.05) improved the IVDMD of proso millet silages, but no no differences in IVDMD were found among the corn and SSH silage groups.
The estimated TDN content is related to the ADF content. For proso millet, the lowest TDN content was detected in the control group. However, the TDN content of the control group corn was higher than that of the additive group without significant difference (p > 0.05).
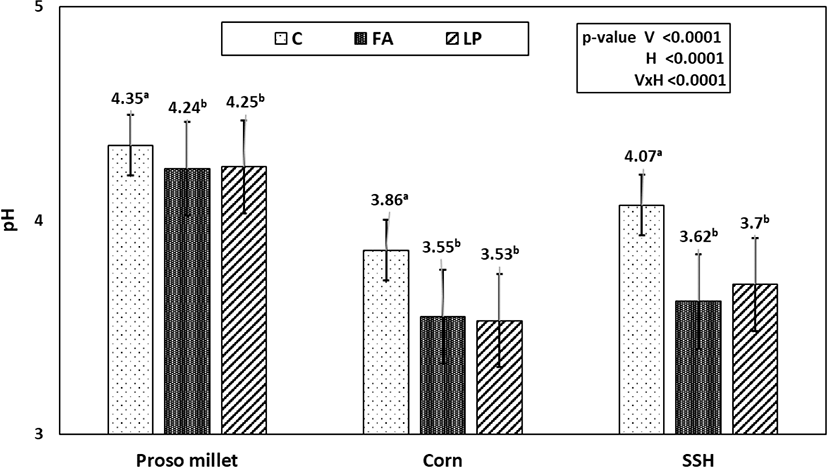
Compared with the pH values of the fresh crops, the pH values of all silages were significantly lower (Fig. 1). Moreover, the pH values of the additive-treated groups were significantly lower than those of the control groups (p < 0.05). Corn silage showed the lowest pH, and proso millet had the highest.
The WSC concentrations of the silages were higher (p < 0.05) in the FA-treated silage than in the control and LP-treated silages, and the residual amounts of WSC were in the order of C < LP < FA (Fig. 2). As can be seen from Fig. 3, the NH3-N/total nitrogen (TN) contents of the additive-treated groups were much lower than those of the control groups, and proso millet silage had higher NH3-N/TN than the corn and SSH silages.
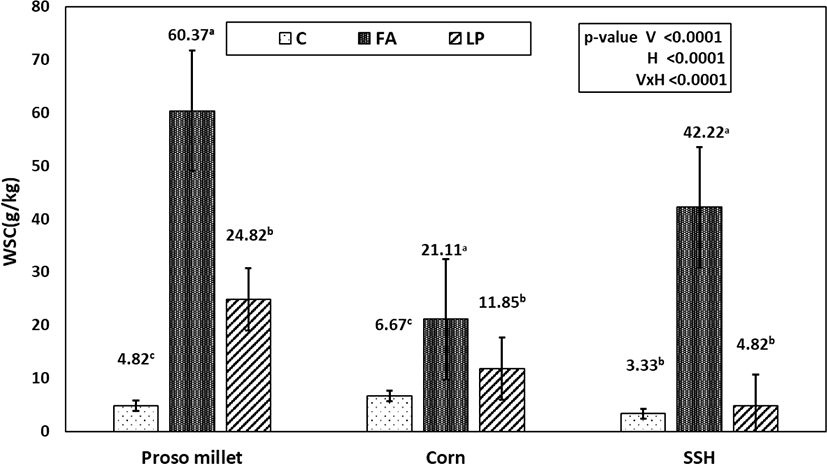
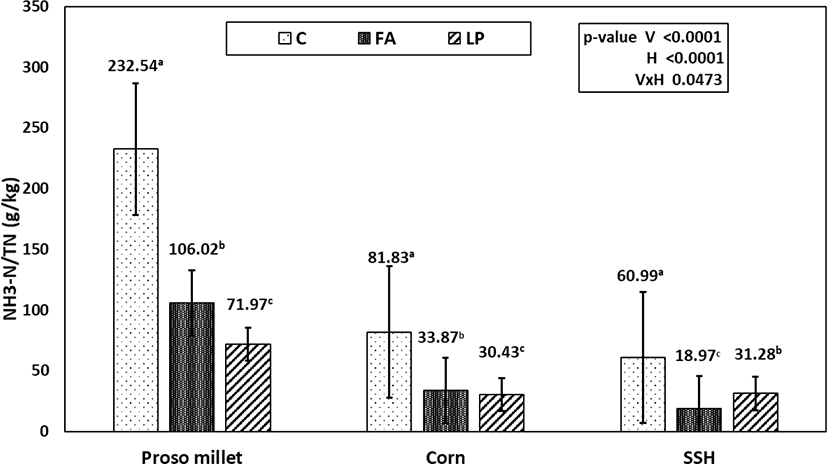
Table 3 shows the organic acid contents of the additive-treated silages. The additives had significant (p < 0.05) effects on the lactic acid and acetic acid contents and the lactic acid/acetic acid ratio (LA/AA). For the three crops, the LP treatment gave the highest lactic acid concentration, followed by the control and FA (p < 0.05). The lactic acid contents of the three LP-treated crop silages showed little difference (i.e., proso millet, 76.67 g/kg; corn, 70.42 g/kg; SSH hybrid, 78.37 g/kg). As can be seen from Table 3, among the treatments of the three crops, the FA treatment group had the highest acetic acid content, which was significantly higher than those of the control group and the LP treatment group (p < 0.05). The SSH had the highest content of acetic acid, followed by corn and proso millet. In all treatments, butyric acid was only detected in the control of millet and the FA treatment of SSH (2.27 and 5.91 g/kg, respectively).
In our study, the highest LA/AA ratio was observed for the LP-treated silage of proso millet (4.12). Similarly, the LA/AA ratio of the LP treatment group was also higher for corn and SSH. By contrast, in the treatments of the three crops, the LA/AA ratio of the FA treatment group was significantly lower than those of the control group and the LP treatment group (p < 0.05).
As shown in Table 4, we found that the additives had a significant effect on the numbers of silage microorganisms (p < 0.05). Although here were no differences between crops, LAB counts were highest in the silages treated with the LP additive. The FA-treated silages had fewer LAB than either the control or the LP-treated silages. In all FA treatment groups, the total microorganism and mold numbers were significantly lower than those of the control and LP groups (p< 0.05). The numbers of LAB and total microorganisms did not differ significantly among the species. The corn silage had significantly lower mold than the proso millet and SSH silages (p < 0.05).
DISCUSSION
It has been reported that an initial WSC content between 60 and 80 g/kg DM is sufficient to produce high-quality silage [20]. Thus, all forage crops in this study contained sufficient WSC (136.7–170.0 g/kg) for fermentation into good-quality silage. Chemical changes are inevitable during ensiling due to the conversion of soluble carbohydrates into organic acids and the degradation of fresh crop fibers and proteins. For the proso millet and SSH, the DM contents in the groups with additives were higher than that of the control group. Correspondingly, the DM losses of the proso millet and SSH with additives groups were lower than that of the control group [21]. The use of FA as an additive can effectively restrict the fermentation of silage, thereby reducing the loss of DM [22]. The addition of LP to silage can produce a large amount of lactic acid, which can reduce the pH of the silage in a short time, effectively inhibiting the growth of undesired microorganisms and also reducing DM losses [23]. Thus, in most experiments, the addition of FA should inhibit the growth of undesirable microorganisms and also reduce losses. However, Rooke et al. [24] showed that silage after the addition of FA cannot completely inhibit the growth of yeast that can consume WSC in the silage, accompanying the loss of DM, which is consistent with our experimental data for corn.
The additive treatments of the three silage crops had higher CP contents than the control groups. The highest CP content was detected in the LP-treated groups which can be explained by the fact that the LP application induced rapid acidification, thereby suppressing protein degradation by undesired microorganisms [25]. The FA treatments also showed enhanced preservation of CP content. This may be due to the restriction of fermentation, deamination, and decarboxylation of proteins after the addition of FA [24,26]. The proso millet treated with additives had significantly decreased contents of NDF and ADF compared with the control group (p< 0.05). This conclusion is the same as the conclusion of Baytok et al. [27]. The NDF and ADF contents of the SSH treated with additives decreased slightly. When LP is added to silage, LAB can effectively degrade the composition of the cell wall, thereby reducing the fiber content [8]. Desta et al. [28] reported that the addition of FA directly reduces the pH value by quickly acidifying raw materials, which significantly reduces the contents of cellulose and hemicellulose in grass silage. The NDF in the corn control group was significantly lower than that in the additive groups (p< 0.05), but the ADF content did not differ significantly. This may have occurred because the additive treatment inhibited the activity of plant enzymes and reduced degradation of the cell wall. The IVDMD increased with the addition of additives, possibly because the use of additives significantly improves the quality of silage fermentation, inhibits unfavorable microbial fermentation, and especially inhibits protein digestion and hydrolysis, thereby increasing the IVDMD [29]. Li et al. [30] also found that treatments with organic acid could increase the IVDMD.
In the process of ensiling, microbial fermentation produces organic acids, causing the pH of the silage to decrease. Adding FA as an acid to silage directly reduces the pH of the silage, whereas adding LP increases the number of LAB, which then produce more lactic acid and thus lower the pH. This finding has been verified in other studies [31]. WSCs are considered to be important substrates for LAB growth during proper fermentation, and WSC is consumed continuously as fermentation proceeds. As in our study, treatment of silage with FA additives results in increased residual WSC concentration, indicating that partial inhibition of fermentation results in WSC not being consumed continuously. In comparison with the control group, the WSC content of the LP treatment was also higher. This may have occurred because the large amount of LAB consumed WSC while reducing the pH of the silage, thus inhibiting the growth of undesirable microorganisms and thereby reducing their consumption of WSC. This also indicates that the LP-treated group reached a steady state quickly, allowing a portion of the WSC to be retained. Meeske et al. [32] also reported that bacterial additives have no effect on WSC during fermentation, which indicates that WSC has the same utilization rate in control and LP-treated silage. Protein hydrolysis in silage leads to an increase in soluble N and NH3-N during ensiling [33]. Well-preserved silages should contain less than 100 g NH3-N/kg TN [6]. As can be seen from Fig. 3, except for the control and FA-treated silage of proso millet, the remaining treatments met the requirements of well-preserved silage (18.97–81.83 g/kg). For the three crops, the NH3-N/TN contents of the additive-treated groups were much lower than those of the control groups. This is consistent with the conclusion of Contreras-Govea et al. [34]. The lower NH3-N/TN in the silage indicated inhibition of proteolysis during fermentation, and therefore the efficiency of nitrogen synthesis by rumen microorganisms was improved [35].
In all of the crops, use of the LP additive increased the concentration of lactic acid compared to the control group. It may be that the lactic acid acidification in the LP silages was enhanced by the addition of exogenous LP, which favored homo-fermentation of silage and thus resulted in higher production of lactic acid [36]. Contreras-Govea et al. [34] also found that inoculant containing LP increased the concentration of lactic acid in maize silage. Many reports have indicated that addition of FA to silage limits the fermentation of silage, thereby reducing the content of lactic acid in the silage [37,38]. This conclusion is consistent with our experimental data. As can be seen from Table 3, the use of FA can increase the content of acetic acid in silage. This result shows that the addition of organic acid can provide a favorable environment for the growth of hetero-fermentation bacteria and thus more acetic acid. The same results were observed during ensiling of maize after the addition of FA [38]. The highest content of acetic acid occurred in the SSH, possibly because the BC value of the sorghum-sudangrass was higher, which prolonged the fermentation time, resulting in a large amount of acetic acid production. Butyric acid is produced by clostridia, and its presence is related to the degree of silage spoilage. For proso millet, its presence may have occurred because the activity of clostridia in the untreated silage was not restricted. The presence of butyric acid in the SSH may have been due to the high moisture content of the SSH, which creates an environment suitable for the growth of clostridia and thus leads to the production of butyric acid. This is in agreement with the result that clostridial fermentation occurs particularly when an ensiled crop has low WSC and DM contents at high temperature [39]. As Jones et al. [40] demonstrated, the LA/AA ratio is an efficiency indicator of homo- or hetero-fermentation. In our study, the highest value of 4.12 (p < 0.05) was observed in the LP-treated silage of proso millet. This indicates that the LP additive used made the fermentation more homo-fermentative.
The FA-treated silages had fewer LAB than either the control or LP-treated silages. The same result was found by Da Silva et al. [41] and Tyrolová et al. [38]. With addition of FA, the total number of microorganisms and the number of molds were relatively reduced. This occurred because FA treatment can inhibit the growth of undesirable microorganisms. The mold content of forage crops is one of the factors affecting their fermentation quality. Molds are aerobic microorganisms present in silage that can cause spoilage during ensiling [42]. Among all treatments, the corn groups had the lowest amounts of mold, possibly because the low pH of the corn silage effectively inhibited the growth of undesirable microorganisms.
CONCLUSION
In conclusion, both additives improved the fermentation quality and nutritional composition of the main summer forage crops. Latic acid inoculant increased lactic acid content and LA/AA ratio. FA inhibited the fermentation of crops. The FA inhibited the silage fermentation. Thus, the lactic acid content was reduced and the acetic acid content was increased, and the average pH was the lowest at 3.80. Proso millet is a cash crop, and its potential for forage is sufficient; however, use of additives to improve silage quality is recommended.
