INTRODUCTION
Colostrum plays a crucial role in transferring maternal immunoglobulins to neonatal calves, as cows are incapable of uterine transfer of immunoglobulins to their calves [1–4]. Consumption of a sufficient quantity of high-quality colostrum during the initial hours of calf life is critical in securing an adequate transfer of colostral immunoglobulins into circulation, contributing to the effective establishment of passive immunization and thus the minimization of morbidity and mortality in neonatal calf [5–7].
Despite the vital importance of colostrum feeding, poor colostrum management, including improper collection, handling, or storage procedures, can expose neonatal calves to pathogens, impairing immunoglobulin absorption and jeopardizing calf health and productivity [8–10]. Occasionally, producers are also unable to supply a sufficient quantity of high-quality, disease-free colostrum, particularly when the transmission cycle for certain infectious pathogens, such as bovine leukosis virus or Mycoplasma bovis mastitis, must be disrupted [11–13]. Poor colostrum quality and inadequate colostrum reserves are predominantly prevalent among Hanwoo calf (Korean native breed)-rearing operations, where calves usually acquire colostrum via nursing. However, there has been a recent trend to immediately separate calves from their dam, resulting in the diminishment of morbidity rates associated with hygienic issues. Hanwoo cows are usually unable to produce a sufficient quantity of colostrum; therefore, an adequate supply of high-quality colostrum is a challenging concern when newborn calves are moved to individual boxes.
These issues have driven the industry to commercialize colostrum replacement products that would enable calf producers to have timely access to colostrum of consistent quality in adequate quantities. However, a major concern with a commercial colostrum replacer is whether the antigen-specific antibodies in the product are available in sufficient quantities and have the necessary efficiency in providing adequate defense against farm-specific pathogens [8,14]. There is less evidence to support the comparative effectiveness of commercial colostrum replacer products versus natural bovine colostrum on calf performance and health, particularly in native species, such as Hanwoo calf that has received little attention.
A literature review has shown conflicting findings regarding the efficacy of commercial colostrum replacers in establishing a sufficient passive immunity transfer in neonatal calves [12,13,15]. For example, Smith and Foster [12] reported that their tested colostrum replacer failed to pass sufficient IgG into calf serum circulation. This necessitates the prior-to-use assessment of any colostrum replacer product, particularly its ability to pass appropriate IgG concentrations into calf circulation. Producers would be able to make more accurate decisions about their colostrum management program if they have access to such information. Therefore, this study aimed to compare the performance, health, and passive transfer of IgG in newborn Hanwoo calves fed a commercially available lacteal-derived colostrum replacer versus natural bovine colostrum.
MATERIALS AND METHODS
Experiments that involved animals were conducted in conformance with the guidelines approved by Konkuk University (approval no. KU17099). The experiment was implemented at the Asia Pacific Ruminant Institute (Icheon, Gyeonggi-do, Korea). A descriptive explanation of calf management has been published previously [16]. In brief, 40 calves were enrolled for the experiment, separated immediately at birth from their dams, and received either natural bovine colostrum (12 males and 8 females) or colostrum-based replacement product (13 males and 7 females). The colostrum replacer was a commercially available product derived from bovine colostrum (Headstart, Saskatoon Colostrum, Saskatoon, SK, Canada). In both groups, colostrum was fed through a bottle within 4 h of birth. A target temperature of about 41°C was set before colostrum feeding. The commercial product was claimed to contain 60 g IgG/225 g dry product and reconstituted according to label directions (225 g in 800 mL water). First-milking colostrum was originated from Holstein cows and collected under aseptic conditions through a vacuum milking system. Prior to colostrum collection, Holstein cows were certified to be healthy and to have received no special treatments. The collected colostrum was tested using a colostrometer, and only the high-quality colostrum with a mean IgG concentration of > 50 g/L [11] was kept for the experiment. The verified colostrum was pasteurized (60°C for 30 min) and stored frozen at –20°C for experimental use. Calves were housed individually in wooden pens (2.0 m long × 1.35 m wide × 1.2 m high) from day 2 of birth. Sawdust was used as bedding, which was replenished when required. Calves received a commercial milk replacer (150 g in 1 L water; Nukamel Yellow, The Dawn, Seoul, Korea) at 8 L/day until 30 days of age, which was gradually decreased from days 31 to 60 (weaning). The nutrient composition of milk replacer powder is shown in Table 1. Milk replacer was fed through the Calf Rail Feeding System (Foerster-Technik, Engen, Germany). Calves that displayed clinical diarrhea were treated using established farm protocols involving the administration of baytril, biodyl, and ketoprofen and an oral solution (Liquid Life-Aid, Norbrook, Newry, UK) containing glucose, glycerin, potassium dihydrogen phosphate, and sodium chloride. Calves with bloody diarrhea were also injected with Ampura-Q (Health Pharma, Dhaka, Bangladesh) according to the veterinarian’s prescription.
| Nutrient composition | % of DM |
|---|---|
| DM (%) | 93.8 |
| Crude protein | 24.4 |
| Crude fat | 17.1 |
| Crude ash | 7.52 |
| Calcium | 0.79 |
| Phosphorous | 0.61 |
Fecal samples in diarrhetic calves were submitted to a commercial laboratory to identify pathogenic agents (rotavirus, coronavirus, Escherichia coli K99, and Cryptosporidium parvum) using a commercial kit (Celltrix, Seongnam, Korea). Water was provided in free access in individual buckets. A commercial starter feed (DH Vital Feed, Pyeongtaek, Korea) was freely available in individual buckets from day 2 of calf life. The nutrient composition of starter feed and timothy hay was presented in Table 2. Starter feed consumption was recorded daily. Individual milk replacer consumption was recorded automatically daily through the amount of milk dispensed from the feeding system. Calf weighing was made using a scale (HFS, CAS Scale, Seoul, Korea) on a 10-day basis approximately 2 h after the morning feeding. Body frame dimensions, including heart girth, body weight, body barrel, withers height, and hip height, were also measured at birth and then on a 10-day basis before the experiment ended on day 70 [17]. Blood was collected from each calf via the jugular vein for IgG quantification on days 2, 7, 30, and 60 before morning feeding. The IgG content in serum samples was quantified using a bovine IgG ELISA kit (Koma-Biotech,Seoul, Korea).
Timothy hay (Phleum pratense L.) was chopped to a mean particle size of 2.5 mm and added to starter A at 5% and starter B at 10%.
Calves enrolled in the experiment were blocked by sex and birth weight and then assigned randomly to one of two treatments in a completely randomized block design. Continuous data were subjected to a normality test through Univariate Proc in SAS (SAS Institute, Cary, NC, USA). Any data that did not follow a normal distribution pattern (starter intake, total dry matter [DM] intake, average daily gain, body weight, withers height, and hip height) was logarithmically transformed. Data were analyzed as a completely randomized design using Mixed Proc. The experimental unit was an individual calf. Data collected over time were analyzed as repeated measures. Autoregressive type 1 resulted in the smallest Akaike information criterion and was selected. Calf birth weight was included in the model as a covariate. Tukey’s test was used for means separation. The difference between treatments for days to first diarrhea and days with diarrhea was identified using GENMOD Proc with a Poisson distribution [18]. The threshold level of significance was p < 0.05.
RESULTS AND DISCUSSION
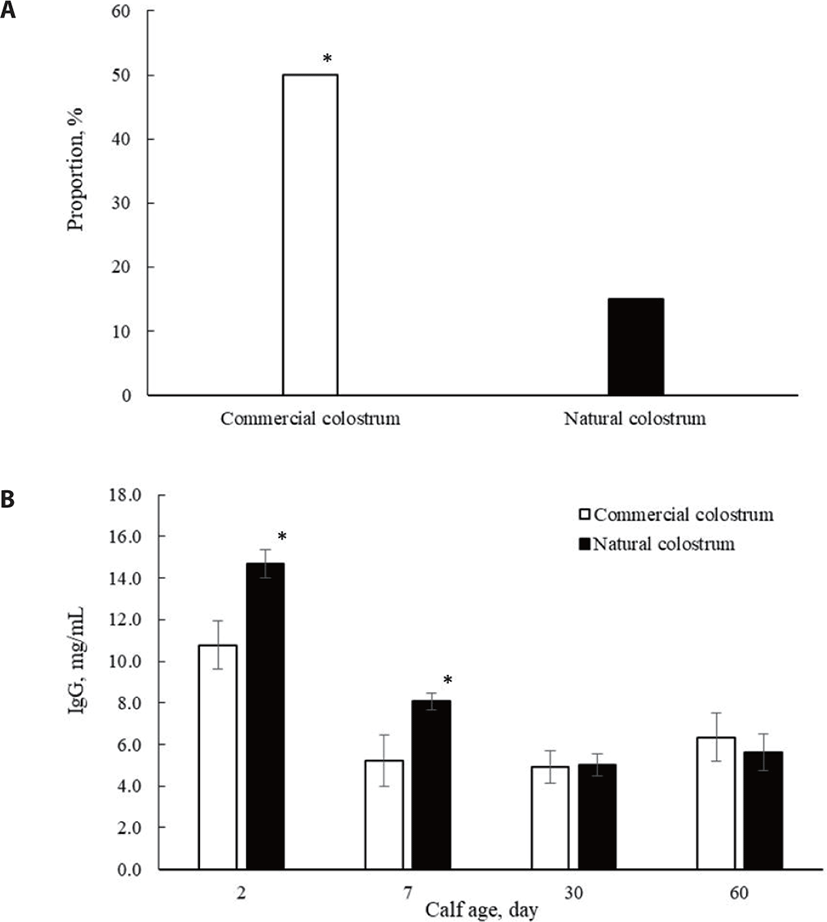
No calf death or exclusion occurred during the experiment. Both groups consumed colostrum at a similar amount of 2.40 ± 0.75 and 2.35 ± 0.91 L (p = 0.70) for commercial and natural colostrum-fed calves, respectively. Failure of passive transfer of immunity (IgG < 10 mg/mL) and mean serum IgG concentration are shown in Fig. 1. On day 2 of calf life, greater circulating IgG was quantified in calves fed natural colostrum versus colostrum replacer (10.8 vs. 14.7 ± 0.92 mg/mL; p < 0.01). Failure to transfer adequate IgG concentration into calf circulation was clearly visible in commercial colostrum-fed calves, with 10 of 20 calves with a blood IgG concentration of <10 mg/mL. In contrast, 3 (of 20) calves failed to absorb an adequate amount of IgG when they received natural colostrum, suggesting the superiority of natural colostrum in securing a successful passive immunization [3,11]. A colostrum replacer is described as a successful product if it can raise serum IgG levels above 10 mg/mL within the first 2 days of calf life [19]. Smith and Foster [12] indicated that natural colostrum versus commercial colostrum led to higher serum IgG levels, supporting the finding that natural colostrum feeding transferred more IgG into calf serum in the early days of calf life. Garry et al. [20] also compared the efficacy of three commercially available colostrum replacer products and reported greater serum IgG levels in natural versus colostrum replacer-fed calves. The success of maintaining an adequate transfer of immunity could not be predicted by the IgG level in the colostrum product because several factors, such as IgG origin and the quantity and type of non-IgG protein, could affect the efficiency of IgG absorption [12,21,22].
The evolution of mean starter intake and total DM intake (for 70 days) in Hanwoo calves fed commercial colostrum versus natural colostrum is shown in Fig. 2. Calves displayed no difference in their starter and total DM intake (starter feed + milk replacer DM) when their colostrum source differed. Likewise, da Silva et al. [23] compared the efficacy of bovine-derived colostrum replacer to maternal colostrum and found a narrow effect on calf starter consumption.
Body weight and daily gains in body weight (from 1 to 70 days of age) in Hanwoo calves fed commercial colostrum versus natural colostrum are shown in Fig. 3. In general, the mean birth weight (30.7 ± 3.15 kg) and daily body gains in this experiment were comparable to those reported previously with Hanwoo calves [16,24,25]. Body weight increased with calf age, with only a small variation observed between calves fed different colostrum sources. Similarly, Jones et al. [14] found no difference in growth rate (from birth to 5 weeks) between calves fed natural colostrum or plasma-derived colostrum replacer. Contrary to these findings, Priestley et al. [26] and Lago et al. [13] reported heavier weaning weight and greater daily gain in calves fed maternal colostrum versus commercial colostrum replacer. Several studies have found a negative relationship between passive transfer failure and calf growth rate [27–29]. However, such an effect was not identified in this experiment. Despite the higher prevalence of failure of passive transfer in commercial colostrum-fed calves, they grew at a rate similar to those fed natural colostrum. Furman-Fratczak et al. [30] found no association between serum IgG and calf growth during the first 6 months of life, supporting the findings of this study. Godden et al. [31] argued that despite the recommended cutoff point in several reports (serum IgG > 10 mg/mL), the lower or higher values might be considered the optimum serum IgG concentration for individual farms to secure efficient calf performance.
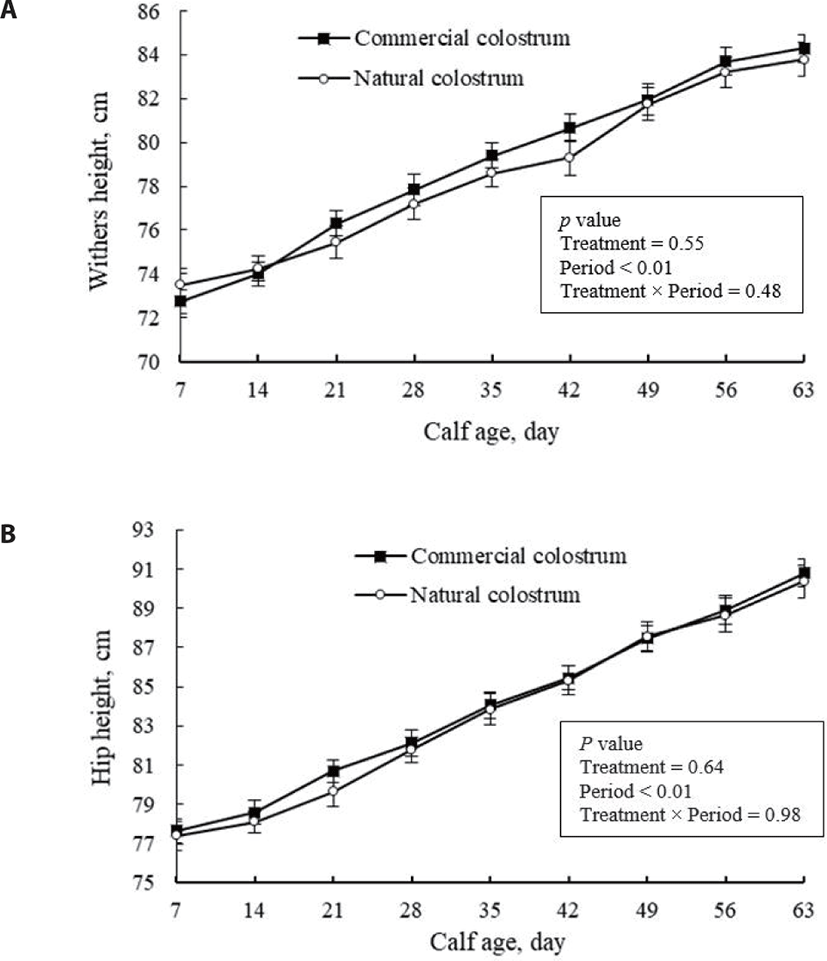
The body dimensions are shown in Figs. 4 and 5. Body frame development increased with developing calf age. However, the colostrum source had no significant effects on these measures, as expected, as it also had no effects on calf solid intake and daily growth rate. Consumption of a sufficient volume of high-quality colostrum triggers anabolic metabolism at the tissue level, promoting postnatal body growth and organ development [4,9]. The lack of significant differences in structural growth parameters among calves suggests the successful efficacy of commercial colostrum replacer in promoting calf growth and development. In support, da Silva et al. [23] and Jones et al. [14] also identified minor differences in heart girth, hip height, and withers height of Holstein calf fed a lacteal-derived colostrum replacer versus maternal colostrum.
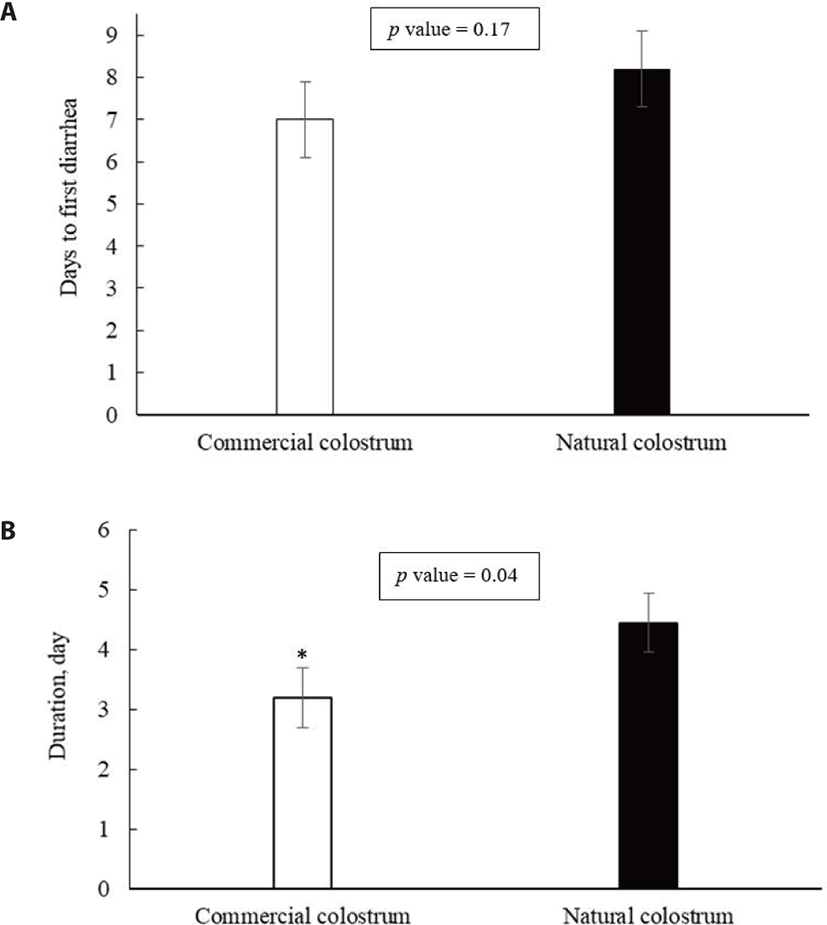
The onset and duration of diarrhea in calves fed commercial versus natural colostrum are shown in Fig. 6. Although colostrum source had no significant effects on days to the first occurrence of diarrhea (average, 7.6 ± 3.30 days; p = 0.17), the number of days with diarrhea was greater in calves fed natural colostrum (3.2 vs. 4.5 ± 2.57 days; p = 0.04). Diarrhea is the main cause of mortality and morbidity in calf-rearing operations worldwide, and it is caused primarily by viral (rotavirus or coronavirus), bacterial, or parasitic pathogens [32]. Diarrheic calves in this experiment were often diagnosed with rotavirus infection. Typically, calves are highly susceptible to rotavirus diarrhea during their first few weeks of life; this is why passive immunity via colostrum is an important defensive strategy for prevention of rotavirus infection and disease [33–35]. Several studies have identified that the transfer of serum IgG antibodies into the intestine provides a complementary role in protection against rotavirus diarrhea in neonatal calves [33,36]. The shorter duration of diarrhea in commercial colostrum-fed calves might provide evidence about the efficacy of this product in protecting Hanwoo calves against rotavirus diarrhea. Lago et al. [13] reported that calves fed commercial colostrum replacer tended to exhibit a lower incidence of diarrhea but a higher number of days with diarrhea than those fed maternal colostrum. Consumption of high-quality colostrum during the first days of calf life aids in establishing adequate passive immunity, which is especially important in minimizing the prevalence of extreme neonatal calf diarrhea, the leading cause of neonatal calf death [9,37]. Colostrum immunoglobulins facilitate the development of local neonatal immunity against a variety of pathogens involved in diarrheal disease in calves [38,39]. This might provide additional evidence about the efficacy of the colostrum replacer product in transferring the passive immunity, as the colostrum source had a small effect on the prevalence of diarrhea in Hanwoo calves. Contrary to our finding, Priestley et al. [26] reported that maternal colostrum was superior to a plasma-derived colostrum replacer in diminishing the prevalence of calf diarrhea. Immunoglobulins derived from blood plasma are less effective than lacteal-derived colostrum replacers in preventing the prevalence of neonatal calf diarrhea. An abundance of hormones, growth factors, and cell-modulating factors in lacteal-originated colostrum can contribute to calf gut health and development that is associated with a lower rate of diarrhea incidence in neonates [9,40,41].
CONCLUSION
Although feeding commercial colostrum increased the risk of failure to transfer adequate immunoglobulins into calf circulation, calves consuming commercial or natural colostrum exhibited no significant differences in their daily gains in body weight and body frame evolution and solid feed intake. Despite the lack of significant effects on the onset of diarrhea, the duration of diarrhea was longer in natural colostrum-fed calves. These findings provide evidence that the commercial colostrum replacer evaluated in this study might be seen as an alternative to natural colostrum in Hanwoo calf-rearing operations, where the timely supply of high-quality colostrum is still a challenge. Additional experiments are needed to identify potential differences in long-term productivity and health of Hanwoo calves fed a colostrum replacer product.
