INTRODUCTION
Anabolic steroids are used to increase the growth rate of meat-producing animals, such as cattle, sheep, and pigs after severe scrutiny and testing. It is also taken by athletes to gain muscle and improve strength. Out of naturally existing anabolic-androgenic steroid (AAS) compounds, nandrolone (19-nortestosterone) has a more powerful anabolic effect than testosterone [1] and is detectable in boar serum and meat at an unneglectable level [2,3]. Esterified form at C17 position of nandrolone, commercially named nandrolone decanoate (ND), is also chemically synthesized and used for medical purposes to treat muscle wasting, accompanied with human immunodeficiency virus (HIV) infection, and anemia associated with chronic renal failure [4]. However, unregulated and non-clinical use of ND for improving athletic performance and muscle mass is frequently connected with various adverse effects, including endocrinological, cardiovascular, and psychiatric effects [5]. Moreover, a strong association between men’s infertility and the abuse of ND has been reported in previous studies [5,6].
Male fertility is regulated by various intrinsic and/or extrinsic factors, and adequate production of testosterone from the testis is important for maintaining the normal function of the male reproductive system [7]. The testicular testosterone is chiefly synthesized in the Leydig cells within the interstitial compartment of the testis [8]. The sequential reaction of several steroidogenic enzymes, including steroidogenic acute regulatory protein (Star), cytochrome P450 side-chain cleavage (Cyp11a1), 3β-hydroxysteroid dehydrogenase (Hsd3b), cytochrome P450 17α -hydroxylase (Cyp17), and 17β-hydroxysteroid dehydrogenase (Hsd17b), converts cholesterol into testosterone within the Leydig cell [8]. An additional action of cytochrome P450 aromatase (Cyp19) is necessary to aromatize testosterone into estradiol [8]. All of these testicular steroidogenic enzymes are localized in the Leydig cell, and CYP19 is also present in the Sertoli cell and some types of germ cells [8,9]. Thus, structural disruption of the interstitial compartment in the testis could influence the expression of steroidogenic enzymes and the production of testosterone and estrogen from the testis.
The depletion of the interstitial composition and the sloughing of germinal cells within the seminiferous tubules are commonly observed with exposure to ND [10,11]. Such structural disturbance of the rat testis is more severe with a higher dose and a longer period of the ND treatment [12]. A decreased expression of testicular steroidogenic enzymes by ND administration is associated with the severity of depletion of the interstitial compartment, that is, lesser interstitial constituent, the more reduced expression level of steroidogenic enzymes in the ND-treated testis [12]. In fact, previous research has shown that expression of most steroidogenic enzymes declines with a low-dose (2 mg/kg body weight [BW]) ND administration for 12 weeks and a high-dose (10 mg/kg BW) ND administration for 2 or 12 weeks, except for Cyp11a1, which shows a rapid decrease in the expression level at a low-dose ND treatment just for 2 weeks [12]. Other research groups have reported an altered expression of testicular steroidogenic enzymes by ND treatment [13,14]. Together, these findings demonstrate that the destructive effects of ND treatment in the testis result in a decline of testosterone production from the testis and a drop in serum testosterone concentration [11,12].
The cytotoxic and histopathological damages induced by ND administration are found not only in the reproductive system but also in other organs and tissues, including the heart and kidney [15]. Due to such undesirable consequences of ND administration, prolonged use of ND is not common. Many studies have shown the withdrawal effect of ND usage in various tissues, and some have shown that histopathological alterations in the kidney and changes of certain serum lipid profiling induced by ND administration are reversible after its withdrawal [16,17]. However, others have demonstrated that the deleterious effects of ND usage on the histological structure of the heart, kidney, and testis are not completely reversible [15]. Additionally, the harmful impacts of ND on the female reproductive system, including estral acyclicity [18], histopathological changes in ovary, uterus, and fallopian tube [18–20], and aberrant expression of androgen receptor and estrogen receptors α and β [19] in the fallopian tube, are not completely restored within certain recovery periods. Despite contradictions regarding the reversibility of ND-induced harmful outcomes followed by the withdrawal of ND administration, it has not been examined whether the cease of ND usage could reverse aberrant expression of testicular steroidogenic enzymes caused by the exposure to ND.
Thus, the present research has been designed to determine expression changes of testicular steroidogenic enzymes at three different recovery periods (2, 6, and 12 weeks) after ND treatment at 2 or 10 mg/kg BW for 12 weeks. Additionally, the restoration of abnormal histology observed in ND-treated testis has been evaluated by microscopic investigation.
MATERIALS AND METHODS
Male Sprague Dawley rats of 40 days of age, purchased from Samtako (Osan, Korea), were used for the current study. Rats were individually caged with free access to food and drinking water under a controlled environment during the experimental period. The treatment of ND was performed as described in the previous research [12]. Briefly, the ND (Deca-Durabolinâ) obtained from Organon Korea (Seoul, Korea) was diluted to make working solutions with peanut oil. The animals of 50 days of age were randomly divided into three treatment groups, control (peanut oil), 2 mg of ND/kg BW/week, or 10 mg of ND/kg BW/week treatment. Each treatment group was divided into three subgroups according to the recovery period, 2, 6, and 12 weeks, and each subgroup consisted of 5 to 6 rats. All experimental animals were subcutaneously treated with ND for 12 weeks, and body weight was recorded weekly. The present study was carried out in accordance with the guide for the care and use of laboratory animals of the National Research Council in Korea, and it was approved by the Ethics Committee for Animal Use, Eulji University (EUIACUC 20-04).
After the last ND injection in the 12th week, animals were given 2, 6, or 12 weeks of the recovery period. At the end of each recovery period, body weight was measured, and animals were anesthetized by CO2 stunning. The male reproductive tract was isolated, and the testis was quickly separated from the remaining part in cold PBS and weighted. The fixation of testis was achieved by Bouin’s solution for immunohistochemical analysis, and the other testis was rapidly minced into small pieces before freezing in liquid nitrogen. The frozen testicular tissues were kept at −80°C and used for real-time polymerase chain reaction (PCR) analysis.
Total RNA from the testis was isolated using TRIzol reagent (Molecular Research Center, Cincinnati, OH, USA). Homogenized 50 mg of testis tissue in 1 mL of RNA extraction solution was centrifuged at 16,609×g for 20 min, and the total RNA was extracted and precipitated by the addition of chloroform and isopropanol. The total RNA was dissolved in RNA storage solution (Ambion, Austin, TX, USA) and stored at −80°C before constructing the first-stranded cDNA. The concentration of the total RNA was estimated by UV spectrophotometer (Eppendorf, New York, NY, USA), and 1.2% agarose gel electrophoresis was employed to check the quality of the total RNA.
One mg of the total RNA was used to construct the first-stranded cDNA by using iScripTM Reverse transcription Supermix for reverse transcription (RT)-qPCR (Bio-Rad Laboratories, Hercules, CA, USA). The RT reaction was performed at 25°C for 5 min, 46°C for 20 min, and 95°C for 1 min in a sequential manner. The quantitative real-time PCR analysis was carried out in 1 μL of generated cDNA, 7 μL of iQTM SYBR® Green Supermix (Bio-Rad Laboratories, Hercules, CA, USA), 10 pmol of a primer set, and nuclease-free dH2O to make a reaction volume of 25 μL. The Primer 3 software (https://bioinfo.ut.ee/primer3) was used to design the oligonucleotide primers for real-time PCR analysis, and detailed information for primers is shown in Table 1. The molecules examined in this research were Star, Cyp11a1, Cyp17, 3β-hydroxysteroid dehydrogenase/delta-5-delta-4-isomerase type I (Hsd3b1), 17β-hydroxysteroid dehydrogenase 3 (Hsd17b3), and Cyp19. The PCR condition was as follows: a pre-denaturation step at 95°C for 5 min, cycles of a denaturation step at 95°C for 30 s, an annealing step at Tm for 30 sec, and an extension step at 72°C for 30 s, and an extra extension step at 72°C for 10 min. The PCR was performed in PTC-200 Chromo 4 real-time system (BioRad Laboratories), and the size of the PCR product was confirmed by 1.2% agarose gel electrophoresis. Cyclophilin A (Ppia) was included as an internal PCR control.
PCR, polymerase chain reaction; PCR, polymerase chain reaction; Star, steroidogenic acute regulatory protein; Cyp11a1, cytochrome P450 side chain cleavage; Cyp17, cytochrome P450 17a-hydroxylase; Hsd3b1, 3β-hydroxysteroid dehydrogenase/delta-5-delta-4 isomerase type I; Hsd17b3, 17β-hydroxysteroid dehydrogenase 3; Cyp19, cytochrome P450 aromatase; and Ppia: cyclophilin A.
The fixed testis in Bouin’s solution overnight was transferred to 70% EtOH and dehydrated in a series of 90%, 95%, and 100% EtOH. Then, the testis was cleared in xylene and infiltrated and embedded with paraffin. The testis paraffin block was cut into 4 mm thickness for further Hematoxyling and Eosin (H&E) and immunohistochemical staining.
The testis section for H&E staining was deparaffinized in xylene and rehydrated in a series of 95%, 90%, and 70% EtOH and running water. The section was then stained with hematoxylin (YD Diagnostics, Yongin, Korea) for 1 min at room temperature, followed by washing through running water. After immersion in eosin solution (Millipore, Burlington, MA, USA) for 1 min, the section was directly dehydrated in a series of ethanol and cleared with xylene. Finally, it was mounted and placed under light microscopy for histological examination.
For immunohistochemical staining, a deparaffinized and rehydrated section was microwaved in 0.01 M citrate buffer (pH 6.0) for 10 min for antigen retrieval. Inactivation of endogenous peroxidase was performed in 0.3% H2O2/methanol for 15 min, and nonspecific binding of the primary antibody was blocked by incubation with 5% normal goat serum (Millipore) for 30 min at room temperature. Then, the section was treated with the primary antibody in a humidified chamber at 4°C overnight. The preliminary trials were made to select proper concentrations of primary antibodies for immunohistochemical analysis. The present study used dilutions of 1:150 for StAR (orb7014; Biobyt, Cambridge, UK), 1:1,000 for CYP11A1 (ABS235, Millipore), 1:200 for CYP17 (bs-3853R, Bioss, Woburn, MA, USA), 1:400 for HSD3B1 (orb5478, Biobyt), 1:400 for HSD17B3 (orb5476, Biobyt), and 1:100 for CYP19 (bs-0114R, Bioss). After washing in PBS, the section was incubated with biotinylated goat anti-rabbit IgG secondary antibody (DAKO, Glostrup, Hovedstaden, Denmark) of 1:100 dilution for 1 h and then with elite avidin-biotin-peroxidase (Vector Laboratories, Burlingame, CA, USA) for 30 min at room temperature. The positive immuno-staining on the section was detected with a mixture of 3,3’-diaminobenzidine (Sigma, St. Louis, MO, USA), 0.05 M Tris-HCl buffer, and 5% hydrogen peroxide. The section counterstained with hematoxylin was mounted, and the immuno-reactivity of the section was evaluated under light microscopy. For negative control, normal rabbit serum (Millipore) was applied to tissue sections at the same dilutions in the place of primary antibodies. Digitalized images of H&E and immunohistochemistry staining were captured with Olympus-CoolSNAP cf color/OL camera (Olympus America, Melville, NY, USA), using RSImage version 1.1 software (Roper Scientific, Acton, MA, USA). The process of these digital images was carried out in Adobe PhotoShop CS5 software (Adobe Systems, San Jose, CA, USA).
We have carried out a real-time PCR analysis of the triplicated RT reaction and real-time PCR for each sample. The mean value of each target molecule was normalized to the value of Ppia and then used for statistical comparison among experimental groups in each recovery period. The expression level of the target molecule was presented in a relative ratio to its of Ppia, as an arbitrary unit. The body weight of each animal was recorded on the first and last day of the treatment and before the sacrifice. The testis weight of each animal was expressed in a relative value of wet testis weight, normalized to body weight.
Data in figures were presented as means ± SEs. Statistical comparison among means of experimental groups in each recovery period was evaluated by one-way analysis of variance (ANOVA), followed by Duncan’s post-hoc test. If the p-value was lower than 0.05, it was considered significant.
RESULTS
There was no significant difference in body weight among experimental groups before ND treatment, but the treatment for 12 weeks resulted in significant decrease in body weights in all treatment groups (Fig. 1). The reduction of body weight was more significant in the 10 mg ND-treated group than the 2 mg ND-treated group F(ig. 1). The recovery period of 2 weeks after the withdrawal of ND treatment was not sufficient to influence the body weight (Fig. 1A). However, the body weight of the 2 mg ND-treated group was not significantly different from the control group, even though the body weight of the 10 mg ND-treated group was significantly lower than other groups (Fig. 1B). The change of body weight at 12 weeks of recovery period was similar to the one seen at 6 weeks of recovery period (Fig. 1C). There was no significant difference in relative testis weights among experimental groups in 2- and 12-weeks recovery periods, except for the 6-week recovery period, showing a significantly heavier testis weight in the 10 mg ND-treated group (Fig. 1D).
Histological changes in the testis after the withdrawal of ND treatment at different recovery periods are shown in Fig. 2. At 2 weeks of recovery period, the space between seminiferous tubules in the testis of the control group was found to be compactly filled with the interstitial constituent, including Leydig cell, while visible interstitial component in very tiny size was sporadically found in the testis of 2 mg or 10 mg of ND-treated group (Fig. 2, top row). Compared with the testis of the control group for the 6-weeks recovery period, the interstitial compartment of the 2 mg ND-treated group became filled with cellular components, even though a few dispersed interstitial elements were observed in the testis of 10 mg ND-treated group (Fig. 2, middle row). For the 12-week recovery period, the testicular interstitium of the 2 mg ND-treated group was comparable with the control group, and cellular components between seminiferous tubules of the 10 mg ND-treated group were more cumulated than those in the 6-week recovery period but still noticeably less than the control and 2 mg ND-treated groups (Fig. 2, bottom row). Moreover, severe germ cell sloughing within the seminiferous tubules was not notable in the testis of all ND-treated groups in all recovery periods (data not shown).
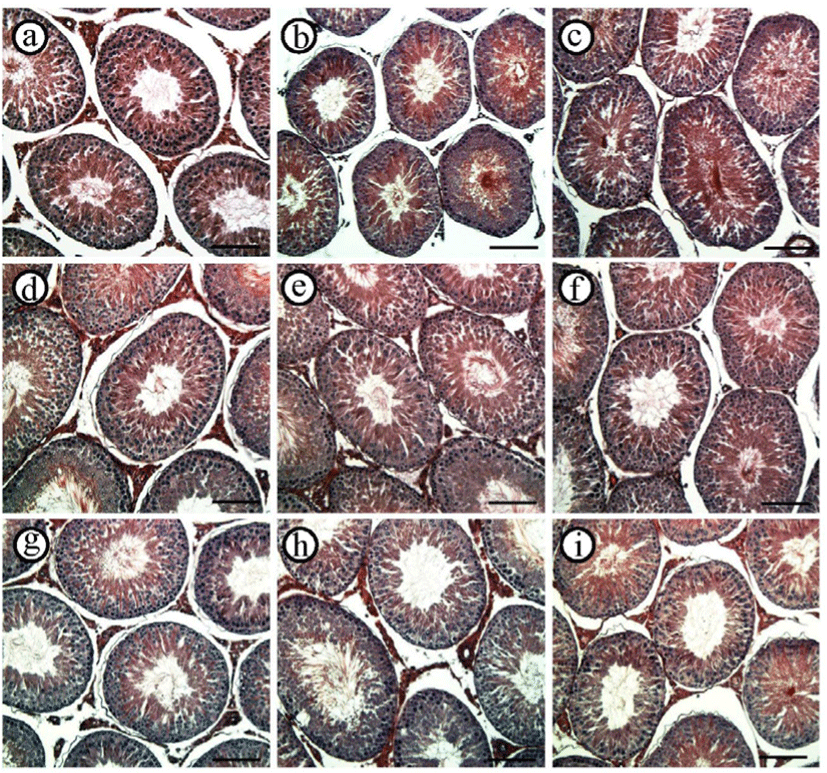
The 2-week recovery period was not sufficient for returning to the normal expression level of Star in the testis of ND-treated groups (Fig. 3A). The expression level of Star in the testis of the 2 mg ND-treated group was significantly lower than that of control, and the level of Star transcript in the testis of 10 mg ND-treated group was significantly lower than that of the 2 mg ND-treated group (Fig. 3A). However, for the 6-weeks recovery period, the transcript level of Star in the testis of the 2 mg ND-treated group was comparable with that of the control, although the abundance of Star transcript in the testis of the 10 mg ND-treated group was still significantly less than in other experimental groups (Fig. 3A). The expression levels of testicular Star of 2 mg and 10 mg ND-treated groups were returned to that of the control (Fig. 3A).
The observation from STAR immunohistochemistry in the testis was similar to the findings from real-time PCR analysis (Fig. 3B and Table 2). Strong immuno-localization of STAR was observed in the Leydig cells of the control group in all recovery periods (Fig. 3B and Table 2). No immuno-reactivity of STAR was found within the seminiferous tubules of all experimental groups (Fig. 3B and Table 2). At the 2-weeks recovery period, weakly positive immuno-staining of STAR was detected with the testicular interstitial compartment of 2 mg and 10 mg ND-treated groups (Fig. 3B and Table 2). The interstitial components of the 2 mg ND-treated group at 6-weeks recovery period showed clear immuno-staining of STAR, and weak immuno-reactivity of STAR was also found in the testis of 10 mg ND-treated group (Fig. 3B and Table 2). The immunolocalization of STAR in the Leydig cell was detected in the testis of 2 mg and 10 mg ND-treated groups at the 12-weeks recovery period (Fig. 3B and Table 2). No immuno-reactivity of STAR was detected in negative control (data not shown).
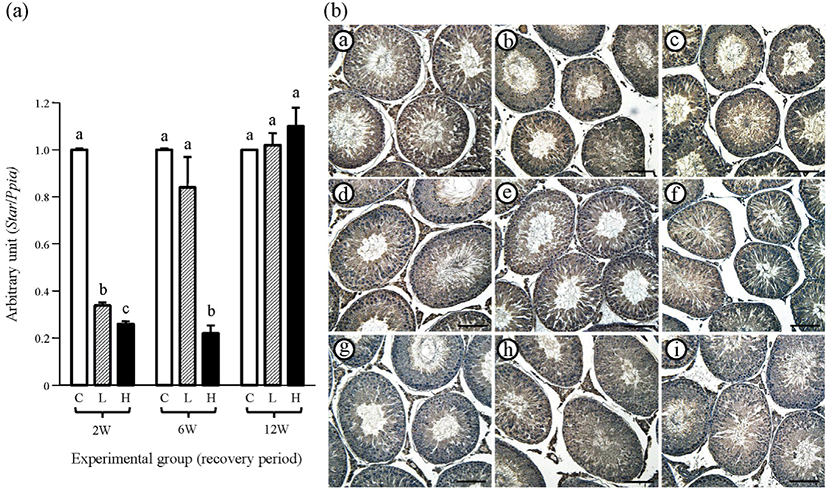
w, weeks (recovery period); C, control (peanut oil); L, 2 mg ND/Kg body weight/week-treated; H, 10 mg ND/Kg body weight/week-treated; +: positive; +/−: weakly positive; −, negative; STAR, steroidogenic acute regulatory protein; CYP11A1, cytochrome P450 side chain cleavage; CYP17, cytochrome P450 17a-hydroxylase; HSD3B1, 3β-hydroxysteroid dehydrogenase type 1; HSD17B3, 17β-hydroxysteroid dehydrogenase 3; CYP19, cytochrome P450 aromatase.
The expression level of testicular Cyp11a of the 2 mg ND-treated group at the 2-weeks recovery period was significantly lower than that of the control group, and the Cyp11a transcript level of the 10 mg ND-treated group was even lower than that of the 2 mg ND-treated group (Fig. 4A). However, at 6-weeks recovery period, a significantly higher level of Cyp11a transcript than that of the control group was detected in the testis of the 2 mg ND-treated group, even though the amount of Cyp11a mRNA of 10 mg ND-treated group was significantly lower than those of the control and 2 mg ND-treated groups (Fig. 4A). Even at 12-weeks recovery period, the expression level of Cyp11a of the 2 mg ND-treated group was significantly lower than that of the control group but significantly higher than that of the 10 mg ND-treated group (Fig. 4A).
As expected, positive immuno-staining of CYP11A1 was restricted in the Leydig cell, and the interstitial compartment of all control groups showed strong reactivity of CYP11A1 (Fig. 4B and Table 2). No immuno-reaction of CYP11A1 in the testis of 2 mg and 10 mg ND-treated groups was observed at 2-weeks recovery period (Fig. 4B and Table 2). At 6-weeks recovery period, control and 2 mg ND-treated groups showed strong immunolocalization of CYP11A1 in the Leydig cell, while visible immuno-reactivity of CYP11A1 in the testis of 10 mg ND-treated group was not detected (Fig. 4B and Table 2). However, all experimental groups at 12-weeks recovery period exhibited positive immuno-staining of CYP11A1 in the Leydig cell (Fig. 4B and Table 2). No immuno-reactivity of CYP11A1 was detected in negative control (data not shown).
The changes of testicular Cyp17 expression at different recovery periods after the stop of ND treatment are shown in Fig. 5A. The expression level of Cyp17 of the 2 mg ND-treated group for the 2-weeks recovery period was significantly lower than that of the control group but higher than that of the 10 mg ND-treated group (Fig. 5A). A surge in the expression level of Cyp17 was observed in the 2 mg ND-treated group at the 6-weeks recovery period, while Cyp17 mRNA abundance of 10 mg ND-treated group was significantly lower than control and 2 mg ND-treated groups (Fig. 5A). At the 12-weeks recovery period, the Cyp17 transcript level of the control group was significantly higher than two ND-treated groups, which were statistically insignificant (Fig. 5A).
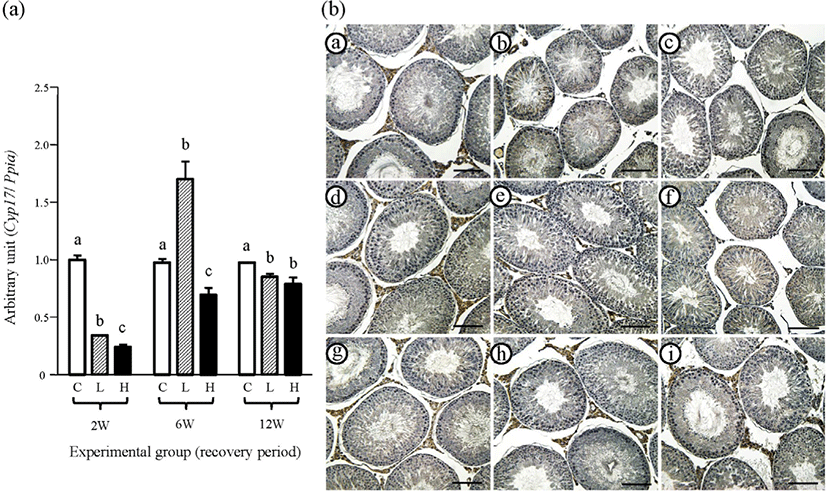
The immunolocalization of CYP17 was limited in the Leydig cell, but not in other testicular cell types (Fig. 5B). At 2-weeks recovery period, strong immuno-staining of CYP17 was detected in the interstitial compartment of the control group (Fig. 5B and Table 2). However, a weak immuno-reactivity of CYP19 in some interstitial cells was observed in the 2 mg ND-treated group, while there was no specific reactivity of CYP19 in the 10 mg ND-treated group (Fig. 5B and Table 2). Strong immuno-staining of CYP19 in the Leydig cell was clearly noticed in the control and 2 mg ND-treated groups at the 6-weeks recovery period (Fig. 5B and Table 2). The positive immunolocalization of CYP17 in the testicular interstitial compartment of the 10 mg ND-treated group was visible at the 12-weeks recovery period, with the control and 2 mg ND-treated groups also having strong CYP17 immuno-stained Leydig cells in the testis (Fig. 5B and Table 2). No immuno-reactivity of CYP17 was detected in negative control (data not shown).
The transcript level of Hsd3b1 of the control group at the 2-weeks recovery period was significantly higher than in the two ND-treated groups, and there was no significant difference in the expression level of Hsd3b1 between 2 mg and 10 mg ND-treated groups (Fig. 6A). A similar finding among experimental groups was observed at the 6-weeks recovery period (Fig. 6A). The expression level of Hsd3b1 of the 2 mg ND-treated group at the 12-weeks recovery period was not significantly different from that of the control group, although the Hsd3b1 transcript level of the 10 mg ND-treated group was lower than those of the control and 2 mg ND-treated groups (Fig. 6A).
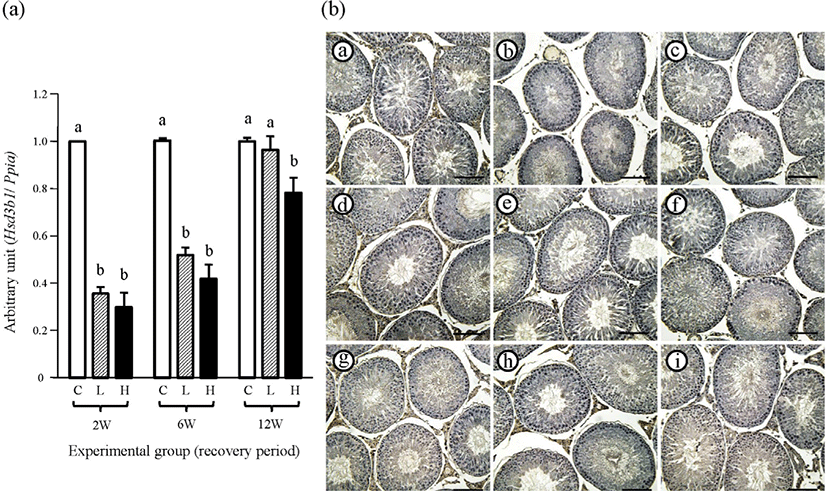
The intense immuno-reactivity of HSD3B1 in the testis of the control group at all recovery periods was restricted in the Leydig cell (Fig. 6 and Table 2). The weak immuno-stained interstitial components of HSD3B1 were very occasionally observed in the testis of 2 mg or 10 mg ND-treated group at the 2-weeks recovery period (Fig. 6 and Table 2). At the 6-weeks recovery period, strong immuno-reactivity of HSD3B1 in the Leydig cell was visible in the testis of 2 mg or 10 mg ND-treated group (Fig. 6 and Table 2). Additionally, more HSD3B1-stained cells were detected within the interstitial compartment of the 2 mg or 10 mg ND-treated group (Fig. 6 and Table 2). No immuno-reactivity of HSD3B1 was detected in negative control (data not shown).
The transcript level of testicular Hsd17b3 of 2 mg or 10 mg ND-treated group was significantly lower than that of the control group at the 2-weeks recovery period, and no significant difference in Hsd17b3 mRNA level between the two ND-treated groups was observed (Fig. 7A). At the 6-weeks recovery period, the abundance of Hsd17b3 mRNA of the control group was still significantly higher than in the two ND-treated groups, which were not different (Fig. 7A). However, the expression level of testicular Hsd17b3 of the 2 mg ND-treated group was not significantly different from that of the control group, even though the Hsd17b3 transcript level of the 10 mg ND-treated group was lower than in other groups (Fig. 7A).
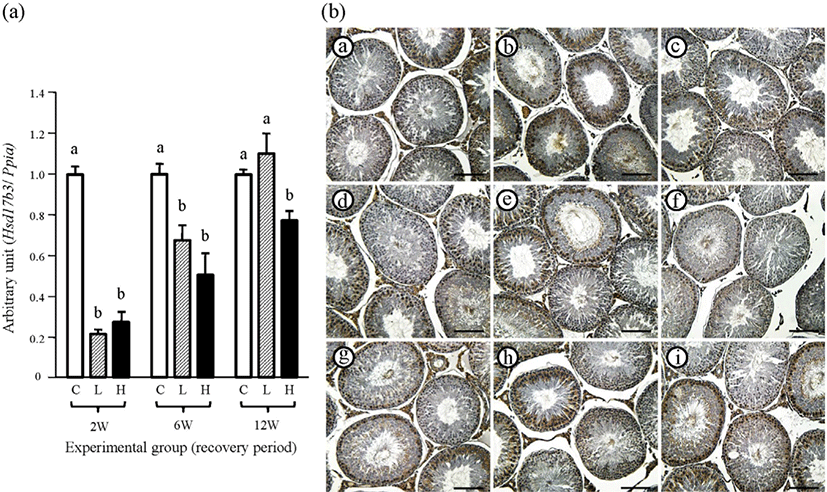
The immuno-reactivity of HSD17B3 in the testis of the control group was localized in the Leydig cell and specific types of germ cells, but not in the Sertoli cell (Fig. 7B and Table 2). Additionally, the immuno-intensity of HSD17B3 in specific germ cells was not visibly changed by the ND treatment (Fig. 7B and Table 2). At the 2-weeks recovery period, there was no immuno-reaction of HSD17B3 in the Leydig cell of the two ND-treated groups (Fig. 7B and Table 2). The intense immunolocalization of HSD17B3 in the Leydig cell was observed in control and 2 mg ND-treated groups at the 6-weeks recovery period. However, immuno-intensity of HSD17B3 in the Leydig cell of 10 mg ND-treated group was weak (Fig. 7B and Table 2). Positive immunoreactivity of HSD17B3 in the Leydig cell at the 12-weeks recovery period was visible in the testes of all experimental groups (Fig. 7B and Table 2). No immuno-reactivity of HSD17B3 was detected in negative control (data not shown).
The changes of testicular Cyp19 transcript level at different recovery periods after 12 weeks of ND treatment are shown in Fig. 8A. The transcript levels of Cyp19 in the testes among all experimental groups at the 2-weeks recovery period were not significantly different (Fig. 8A). Additionally, there was no significant difference in testicular Cyp19 transcript level among all experimental groups at both 6- and 12-weeks recovery periods (Fig. 8A).
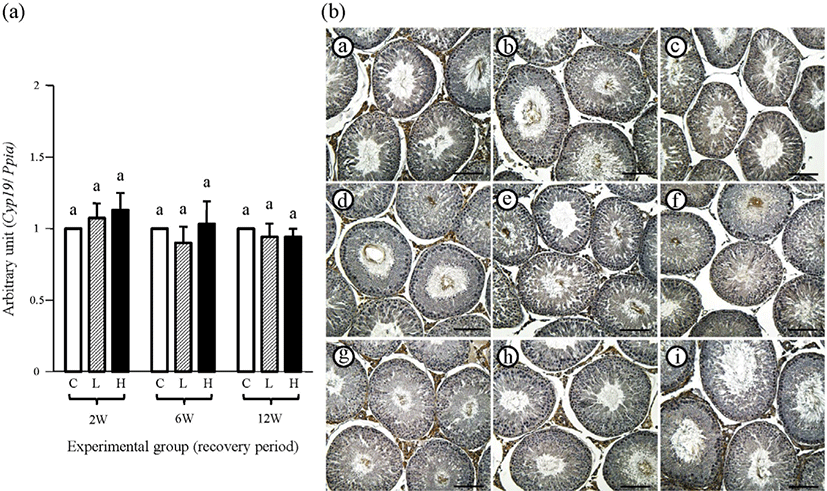
Immunohistochemical localization of CYP19 in the testis was found in the Leydig, Sertoli, and certain types of germ cells in the control group (Fig. 8B and Table 2). At the 2-weeks recovery period, there was no visible immunoreactivity within the interstitial compartment of the 2 mg or 10 mg ND-treated group, even though the Sertoli and ger cells were weakly immuno-positive for CYP19 in all experimental groups (Fig. 8B and Table 2). Relatively intensive immuno-staining of CYP19 in the Leydig cell of the 2 mg ND-treated group was found at the 6-weeks recovery period (Fig. 8B and Table 2). Also, weak immunolocalization of CYP19 in the Leydig cell at the 6 weeks-recovery period was detected in the 10 mg ND-treated group (Fig. 8B and Table 2). As at the 2-weeks recovery period, the Sertoli and certain germ cells at the 6-weeks recovery period were weakly positive for CYP19 (Fig. 8B and Table 2). At the 12-weeks recovery period, the Leydig cells of all experimental groups were immuno-stained for CYP19, and the Sertoli and specific germ cells were weakly positive for CYP19 (Fig. 8B and Table 2). No immuno-reactivity of CYP19 was detected in negative control (data not shown).
DISCUSSION
Our previous research has shown that exposure to ND with even low doses for a long period results in histological disruption and abnormal expression of steroidogenic enzymes in the testis [12]. Therefore, the effect of the withdrawal of ND administration on the testis has been examined at histological and transcript expression levels. The findings from the current research are summarized as follows: 1) the changes of body and testis weights induced by the ND treatment are returned to normal levels, 2) the occurrence of germ cell sloughing within seminiferous tubules is visibly reduced, 3) the depletion of interstitial composites by prolonged ND exposure is alleviated as the recovery period progresses, 4) expression levels of testicular steroidogenic enzymes at longer recovery periods tend to return to normal levels, even though there are some variations in enzymatic molecules, and 5) the degree of immunohistochemical intensity detected within the interstitial compartment is strongly associated with the recurrence of cellular components, most likely Leydig cell, as the recovery period is getting longer.
The long-term usage of ND in a normal state results in decreased body and testicular weights [12,15,21], even though the administration of ND for clinical treatment mostly induces body weight gain [4]. In the present research, a decrease in body weight after ND treatment for 12 weeks is in line with our previous study’s findings [12]. As the recovery period progresses, the body weight of the 2 mg ND-treated group becomes comparable with that of the control group. However, the 12-weeks recovery period is not enough for the 10 mg ND-treated group to completely regain body weight to the level of the control group, even though the difference in body weight between the 10 mg ND-treated group and other groups diminishes at a longer recovery period. It is considered that a longer recovery period than the ND-exposed one is necessary to make up for normal body weight. Unlike the change of body weight after the withdrawal of the ND treatment, the testis weight of the ND-treated group quickly returns to the control level at even the 2-weeks recovery period. Nonetheless, a transient surge of testis weight of 10 mg ND-treated group at the 6-weeks recovery period is not clearly explainable at this point. This phenomenon might be acceptable if the rate of testis weight gain in 10 mg ND-treated group between the 2-weeks and 6-weeks recovery period is faster than the rate in body weight gain.
The histological aberrance in the testis induced by the exposure to ND is frequently detected [11,12,15,22]. The germ cell sloughing following the detachment of germ cells from the seminiferous epithelium is often accompanied with ND administration by not only injection [11,12] but also feeding [22]. The loss of germ cells from the seminiferous epithelium is associated with endogenous testosterone level reduction [23]. In fact, the ND treatment results in a drop in serum and testosterone concentration [11,12]. Moreover, the incidence of germ cell sloughing after the cease of ND treatment has not been remarkable. Thus, it is speculated that even the 2-weeks recovery period is sufficient to allow the regeneration of the interstitial Leydig cell population, which produces an adequate amount of testicular testosterone to suppress the germ cell sloughing.
Another evident histological change observed in the ND-treated testis is the depletion of interstitial components, including Leydig cells [11,12]. Such reduction of Leydig cell population is strongly associated with the decrease of serum or testicular testosterone level by the ND treatment [11,12]. Direct quantitative comparison of the number of cellular components within testicular interstitium at the final injection of ND and at the 2-weeks recovery period after the withdrawal of ND treatment is impossible at this point. However, light microscopic evaluation of the testis has observed sporadic and clear Leydig cell population within the interstitial compartment of the testis at the 2-weeks recovery period, which was not the case right after the final ND injection (observations not described in the results). Even though the space between seminiferous tubules becomes filled with more interstitial components as the recovery period becomes longer, the testis of 10 mg ND-treated group at the 12-weeks recovery period still has a loose interstitial compartment. Based on these findings, it is supposed that the complete return of abnormal testicular histology caused by the ND exposure requires a longer recovery period than the exposure period. The withdrawal effect of ND on other tissues has been examined from other researches, and the permanent irreversibility of abnormal characteristics induced by the ND treatment has been suggested [15,16,18–20]. Thus, the results indicating that a longer recovery period guarantees a complete reverse of testicular histological aberrance derived by the ND treatment are not conclusive. Further and longer period-course research is proposed to determine the effect of withdrawal of the ND treatment on the testicular histology in detail.
The expression and activity of testicular steroidogenic enzymes are suppressed by long-term and/or high-dose ND treatment [12,21]. Our previous research has demonstrated that the treatment of 10 mg ND/week for 12 weeks results in significant decreases in transcript and protein levels of testicular steroidogenic enzymes, including STAR, CYP11A1, CYP17, HSD3B1, and CYP19 [12]. The reduction of HSD17B activity has also been detected in the testis of 10 mg ND/week treatment for 8 weeks [21]. As a serial action of these enzymes is required for the synthesis of steroid hormones in the testis, a disruption in expression and/or activity of these enzymes in the testis could alter the production of testicular testosterone. Indeed, the drop in testosterone concentration in serum or testis has been detected with the ND treatment [12,21]. In the present study, the expression level of testicular Cyp19 at the 2-weeks recovery period after the withdrawal of ND treatment has been restored to the control level. However, expression levels of other testicular steroidogenic enzymes of ND-treated groups at the 2-weeks recovery period are still significantly lower than those of the control group.
At the 6-weeks recovery period, expression levels of Star in the 2 mg ND-treated group and Cyp19 of 2 mg and 10 mg ND-treated groups have been returned to those of the control group. Moreover, expression levels of Cyp11a1 and Cyp17 of the 2 mg ND-treated group are significantly higher than those of the control group. Such transient increases in testicular Cyp11a1 and Cyp17 transcripts in the 2 mg ND-treated group are unexpected. As no similar phenomenon has been observed in other steroidogenic enzymes, such remarkable changes in the transcript levels in the 2 mg ND-treated group seem to be restricted in the expression of Cyp11a1 and Cyp17 but not of other enzymes. Detailed research is suggested to resolve the existence of specific regulatory mechanisms in the expression of Cyp11a1 and Cyp17 in the 2 mg ND-treated group at 6-weeks recovery period.
Providing the 12-weeks recovery period after the ND treatment allows for restoration to the normal transcript level of Hsd3b1 and Hsd17b3 at the 2 mg ND-treated group and Star and Cyp19 in all ND-treated groups. However, expression levels of other molecules in ND-treated groups have not been reached to those in the control group. Such differential expression responsiveness to the same stimulus depending on the molecule is frequent. For example, treatment with the same dose and duration of ND results in differential expression patterns in testicular steroidogenic enzymes at mRNA and/or protein levels [12]. Additionally, a differential expression of androgen and estrogen receptors has been detected in the ampulla of the female reproductive tract at a certain recovery period after the withdrawal of ND treatment [19]. Thus, it is reasonable to consider that different expression patterns of testicular steroidogenic enzymes to the withdrawal of ND treatment could be due to the divergence of expressional regulation, partly depending on the molecule.
Direct measurement of testicular testosterone concentration and quantitative analysis of steroidogenic enzymes at the protein level has not been achieved in this research. Thus, the change of endogenous testosterone synthesis during the recovery period after the withdrawal of the ND treatment could not be provided. However, this issue could be interpreted by observations acquired from immunohistochemical and histological analyses. Except for the immuno-staining within seminiferous tubules, positive immuno-reactivities of all molecules have become stronger and/or more prevalent in the interstitial compartment as the recovery period progresses. This phenomenon is supported by a histological finding that a more compact interstitial compartment is filled with cellular components in the testis at the 12-weeks recovery period, compared to the 2-weeks recovery period. Besides, the transcript and protein levels of certain molecules are usually closely related, as shown in our previous study [12]. Overall, it is supposed that testosterone production in ND-treated testis would gradually increase along with the reappearance of Leydig cells within the interstitial compartment, as the recovery period progresses. Despite such weak points of the current research, this is, to our knowledge, the first report showing a comprehensive analysis of expression changes of testicular steroidogenic enzymes after the ND treatment’s withdrawal.
An interesting finding is the immunolocalization of HSD17B3 within seminiferous tubules, along with Leydig cells in the interstitial compartment. It is generally considered that testicular HSD17B3 is only localized in the Leydig cell of the testis [24], and a clear positive immuno-reactivity of HSD17B3 in rat Leydig cell has been observed in the current study. However, a strong immunolocalization of HSD17B3 in rat testis has also been detected in certain germ cell types, presumably spermatocytes, of certain spermatogenic stages. However, the specific localization of HSD17B3 in germ cells has not yet been defined. The function of HSD17B3 is responsible for the conversion of androstenedione to testosterone [24]. Thus, the existence of HSD17B3 in specific germ cells could be relevant to the production of testosterone within the seminiferous tubule, which involves local regulation for maintaining the structure and function of cells residing within the germinal epithelium. A detailed molecular examination is required to reveal the existence and function of HSD17B3 in the seminiferous tubule.
The AAS usage does not guarantee complete restoration of spermatogenesis and testosterone production, diminished by AAS [25]. The AAS-induced hypogonadism is not fully reversible after the withdrawal of AAS in some cases [26]. Additionally, although the hypogonadism and azoospermia induced by AAS abuse are reversible after steroid withdrawal, it requires a longer recovery period than expected [6]. Moreover, incomplete recovery of structural aberrance caused by the ND exposure is frequently found in various tissues [15,16,18]. Similar outcomes have been observed in the present research, showing that the same recovery duration with the ND-treated period is insufficient for the complete reversal of ND-induced changes at histological and molecular biological levels. Even though nandrolone and/or AAS-like substances are most likely contracted by intramuscular injection, exposure to nandrolone and its derivatives could be accomplished by the consumption of pork, containing non-negligible levels of nandrolone and testosterone metabolites [27,28]. Mendiola et al. [29] have reported a negative relationship between semen quality and a frequent intake of meat products. Thus, these findings suggest that repeated and lasting exposure to nandrolone and/or AAS-like elements, even at a very low dose, could cause permanent obstruction in the testis, eventually affecting sperm production and further fertility. In conclusion, the complete recovery of ND-induced unfavorable outcomes in the testis would not be accomplished simply by the withdrawal of ND administration. Even at this point, it is not clear whether the aberrance caused by the exposure to ND is completely reversible. The time-course analysis for the more extended period is suggested to determine if a complete recovery of testicular damages occurred by ND usage is possible. Besides, the results obtained in this study pave the way for clinical trials and are useful for studies related to the effect of meat on animal’s growth and development.
