INTRODUCTION
Terms such as ‘companion animals’ apply to households with pets, companion dogs and companion cats that are frequently encountered in the surroundings. The word ‘companion’, with which we are already familiar, refers to an animal that lives with humans and was first proposed by zoologist and Nobel Prize winner Konrad Lorenz at an international symposium held in Vienna, Austria in 1983 [1]. Households that raise these pets accounted for 29.7% of the total households in Korea, with 6.04 million households at the end of 2020 [2]. As the number of people raising companion animals is increasing, the relationship between humans and companion animals is further developing.
Most pet owners currently treat their pets as family, colleagues, and friends [3]. Pet humanization, a phenomenon that recognizes companion animals as family members and treats them as individuals with emotions, has been established as a global trend [4]. In Korea, the trend of pet humanization is also spreading, with 88.9% of companion households and 64.3% of general households agreeing to the phrase ‘pets are part of the family’ [2]. A typical example is that the pets do the same things as humans do, such as having birthday parties for dogs and cats, sleeping with the owner in the bed, and others. Companion animals have become an increasingly important part of human life, and therefore, the health and well-being of pets have increasingly attracted interest in recent decades [5].
Dogs and cats have evolved into carnivores with high-protein diets and have relatively simple gastrointestinal tracts (GITs) [5,6]. Cats are carnivores that rely on high-protein animal tissues to meet their unique nutritional requirements in the wild and consume protein-containing feed to meet their nutrients in the case of household felines. They are metabolically adapted to low glucose utilization and high protein metabolism [5,7]. Although dogs share many anatomical and metabolic characteristics with cats, they are metabolically omnivorous and can digest, absorb and metabolize significant amounts of carbohydrates [8].
The gut plays an important role in animal health, and the GIT contains a complex microbial community. A healthy gut is known to affect host physiology and well-being. This microbial ecosystem acts in several ways, affecting both the absorption and metabolism of nutrients and the protective functions of the host. Probiotics are defined as ‘living microorganisms that provide health benefits to the host when administered in appropriate amounts’ [9]. Recently, gut-related probiotic products aimed at pets, particularly dogs and cats, have also gained in popularity among owners [10]. The benefits of using probiotics for pets include their modulation of the immune system, help in reducing stress, protection against infections caused by intestinal pathogens and growth performance development [11]. As dogs and cats become family members, the number of studies about dogs and cats has been increasing. Among their topics, knowledge about the gut microbiome and probiotics in dogs and cats is still expanding. However, published papers on the application of probiotics in companion animals are significantly limited compared to those in humans. The purpose of this review is to describe the current knowledge about the gut microbial communities in dogs and cats in relation to probiotics.
PROBIOTICS FOR COMPANION ANIMALS
Probiotics that are living and beneficial microbiota have been used for companion animal’s health [9]. As people’s desire to have their pets for a long time has increased, interest in probiotics has also attracted more attention [10]. Probiotics provide beneficial health effects to the host animal by altering the gastrointestinal (GI) flora. The GI benefits for dogs and cats include maintaining a balanced and healthy gut microbiome, preventing diarrhea, and managing small intestinal bacterial overgrowth and inflammatory bowel disorders [12]. Since dogs and cats have different dietary needs and digestive systems, their needs for and effects from probiotics differ.
Canines are considered animal models for the study of the human microbiome because of the high structural and functional similarity between the canine and human microbiomes [13]. The study of the dog microbiome can be predictive of the human microbiome. Thus, the study of dogs offers two advantages not only directly for dogs but also for its potentially benefits for humans [14]. Although the beneficial effects of probiotics have been extensively studied in humans and animals, the exact mechanisms of probiotic-based immune modulation are not entirely clear, and the efficacy of probiotic applications varies depending on many different factors [15]. Recent reports of using probiotics in canines are shown in Tables 1 and 2.
| Bacterial strains | Amount | Source | Group | Tested for | Result | Reference |
|---|---|---|---|---|---|---|
| Bifidobacterium animalis AHC7 | 2 × 1010 CFU/day | Canine | Young adult dogs with acute diarrhea | Assessment for managing acute diarrhea | Reduced diarrhea compared to placebo group | [22] |
|
Lactobacillus rhamnosus MP01 Lactobacillus plantarum MP02 |
109 CFU/day | Canine | 1 month old puppies | Assessments for preventing gastrointestinal infection in puppies |
Significantly increased Lactobacillus and Faecalibacterium in fecal Significantly increased SCFAs concentration in feces Prevented gastrointestinal infection |
[23] |
| Lactobacillus murinus LbP2 | 5 × 109 CFU/day | Canine | Dogs with canine distemper virus (CDV)-associated diarrhea | Assessment of fecal and mental status | Fecal consistency, mental status and appetite were significantly improved | [24] |
| Lactobacillus johnsonii CPN23 | 2.3 × 108 CFU/day | Canine | Adult female Labrador dogs | Assessment of nutrient digestibility and fecal fermentative metabolites |
Increased crude fiber digestibility Increased concentrations of SCFAs in feces Reduction in fecal ammonia concentration |
[25] |
| Lactobacillus fermentum CCM 7421 | 107–109 CFU/day | Canine | Dogs suffering from gastrointestinal disorder | Assessment of blood samples and composition of the fecal microbiome |
Improved total protein, cholesterol and ALT in blood samples Increased lactic acid bacteria population and decreased clostridia population along with some of the gram-negative bacterial genera Modulate liquid feces to normal consistency (dogs with diarrhea) |
[26] |
| Lactobacillus fermentum AD1 | 3 mL of 109 CFU/mL | Canine | Healthy dogs | Assessment of blood sample and composition of fecal microbiome |
Significantly increased total lipid and total protein in the blood Significantly decreased glucose concentration in the bloodstream Significantly increased the number of lactobacilli and enterococci in the feces |
[27] |
| Bifidobacterium animalis B/12 | 1 mL of 1.04 × 109 CFU/mL | Canine | Healthy dogs | Assessment of blood samples and composition of fecal microbiome |
Significantly decreased concentration of triglycerides and albumin in blood serum Increased ALT and ALP Increased acetic, acetoacetic and valeric acids in feces |
[28] |
| Lactobacillus johnsonii CPN23 | 108 CFU/mL (0.1 mL/kg BW) | Canine | Adult female dogs | Assessment of blood sample profile |
Decreased plasma glucose and cholesterol level Increased HDL/LDL ratio |
[29] |
| Enterococcus faecium DSM 32820 | 109 CFU/day | Canine | Healthy dog | Assessment of blood sample profile | Decreased serum glucose concentration | [30] |
| Lactobacillus acidophilus DSM13241 | 2 × 108 CFU/day | Feline | Healthy adult cats | Assessment for improving intestinal health in cats |
Increased numbers of beneficial Lactobacillus and L. acidophilus groups in feces and decreased numbers of Clostridium spp. and Enterococcus faecalis Decreased fecal pH and plasma endotoxin concentrations resulting in systemic and immunomodulatory changes in treated cats |
[41] |
| Enterococcus hirae | 2.85–4.28 × 108 CFU/day | Feline | Kittens | Assessment for preventing atypical Enteropathogenic E. coli (EPEC) in kittens |
Highly effective at promoting intestinal colonization and fecal shedding of live E. hirae during administration. Ameliorated the effects of atypical EPEC experimental infection on intestinal function and water loss |
[42] |
| Enterococcus faecium SF68 | 5 × 109 CFU/day | Feline | Kittens | Effects of Enterococcus faecium strain SF68 supplementation on immune function | The percentage of CD4+ lymphocytes was significantly higher in the treatment group | [43] |
| Bacterial strains | Amount | Source | Group | Tested for | Result | Reference |
|---|---|---|---|---|---|---|
|
Lactobacillus casei Zhang Lactobacillus plantarum P-8 Bifidobacterium animalis subsp. Lactis V9 |
2 × 109 CFU/g (2 g for young, 4 g for training, 10 g for elderly dogs) |
Lactobacillus casei Zhang (koumiss) Lactobacillus plantarum P-8 (Fermented dairy products in China) Bifidobacterium animalis subsp. Lactis V9 (Feces of a healthy Mongolian child) |
Young, training and elderly dogs | Assessment of nutrition, immunity and composition of fecal microbiome |
Promoted the average daily feed intake of elderly dogs Improved average daily weight gain om all dogs Enhanced the level of serum IgG, IFN-α, and fecal secretory IgA (sIgA), while reducing the TNF-α. Increased beneficial bacteria and decreased potentially harmful bacteria |
[14] |
| Lactobacillus acidophilus D2/CSL | 5.0 × 109 CFU/kg of diet | Gastrointestinal (GI) tract of a healthy adult chicken | Healthy dogs | Assessment of nutritional and fecal status |
Higher body condition score than control group Positive effect on fecal consistency |
[31] |
| Enterococcus faecium SF68 | 5 × 108 CFU/day | Feces of a healthy breast-fed baby | Dogs with diarrhea | Assessment of the effect of administering metronidazole with Enterococcus faecium SF68 to treat diarrhea |
Dual therapy that administrates metronidazole with Enterococcus faecium SF68 improved diarrhea more than administering metronidazole alone Giardia cysts were eliminated from the dual treatment group |
[32] |
| Enterococcus faecium SF68 | 5 × 108 CFU/day | Feces of a healthy breast-fed baby | Healthy dogs | Assessment of blood sample profile |
Mean cholesterol concentration significantly decreased Mean triglyceride concentration significantly increased |
[33] |
| Lactobacillus acidophilus D2/CSL (CECT 4529) | 5 × 109 CFU/kg of food | Conventional foods such as milk, yogurt and dietary supplements | Healthy adult cats | Assessment of the effects on nutritional condition and fecal quality |
Improved fecal quality parameters Increased Lactobacillus count and decreased total coliform bacteria counts |
[44] |
| Enterococcus faecium SF68 | 2.1 × 109 CFU/day | A healthy breast-fed newborn baby | Cats | Effects on Enterococcus faecium SF68 in diarrhea | The percentage of cats with diarrhea was significantly lower in the probiotic group when compared with the placebo group | [45] |
| Enterococcus faecium SF68 | 1/4 can of canned food mixed with Enterococcus faecium | A healthy breast-fed newborn baby | Young adult cats | Description of the GI abnormalities associated with the administration of amoxicillin-clavulanate to cats and an assessment of whether feeding with Enterococcus faecium SF68 could ameliorate those abnormalities |
The total diarrhea scores for days 1–11 were significantly lower in the cats fed Enterococcus faecium SF68 compared to the cats fed the placebo Feeding Enterococcus faecium SF68 can lessen some associated clinical abnormalities |
[46] |
| Enterococcus faecium SF68 | 5 × 108 CFU/day | A healthy breast-fed newborn baby | Cats with chronic Feline Herpes virus-1 (FHV-1) infection | Assessment of the effect of feeding Enterococcus faecium SF68 in clinical signs of FHV-1 infection |
Fecal microbial diversity was maintained throughout the study in cats supplemented with Enterococcus faecium SF68, indicating a more stable microbiome in cats receiving Enterococcus faecium SF68 Lessened morbidity associated with chronic FHV-1 infection in some cats |
[47] |
| Proviable®-DC (7 bacterial species) | 5 × 109 CFU of a mixture of seven bacterial species per day | Multistrain probiotic product | Adult cat | Improvement in stool character | Improved diarrhea symptoms after 21-day feeding | [48] |
| Lactobacillus plantarum | 1 × 108 CFU/mL | Mare’s milk | Cats with chronic gingivostomatitis | Assessment of preventive and therapeutic oral pathology |
The administration of the probiotic to the two immunosuppressed cats affected by gingivostomatitis led to an improvement in the time of recurrence The symptoms of chronic feline gingivostomatitis disappeared after two weeks of administration The ulceration, inflammation and pain of the oral cavity decreased, thalism and halitosis disappeared |
[49] |
| Probiotic combination | Lactobacillus casei 4 × 108 CFU, Lactobacillus rhamnosus 3 × 108 CFU, Lactobacillus acidophilus 5 × 107 CFU, Lactobacillus bulgaricus 1 × 107 CFU, Bifidobacterium infantis 4 × 107 CFU, Bifidobacterium breve 5 × 107 CFU, Streptococcus thermophilus 1 × 108 CFU | Multistrain probiotic product | 8-month-old male cats | Management of feline idiopathic cystitis (FIC) using probiotics | Probiotic combination treatment effectively managed this disease due the effect of bactericidal, anti-inflammatory, and immunomodulatory actions | [50] |
| Proviable®-DC (7 bacterial species) | 5 × 109 CFU of a mixture of seven bacterial species per day | Multistrain probiotic product | Healthy cats | Assessment of a multispecies on the fecal microbiome of healthy cats | Increased abundance of probiotic bacteria in the feces of healthy cats | [85] |
GI disorders are one of the most common health problems in dogs [16,17]. Regardless of the cause, most GI disorders present with acute or chronic diarrhea, or, in some cases, vomiting or anorexia [5,18,19]. Many previous studies have shown positive results regarding the treatment of dogs with different types of probiotics [20,21]. Dogs on diets supplemented with 2 × 1010 CFU/day canine-derived probiotic Bifidobacterium animalis AHC7 had a significantly more rapid resolution of acute diarrhea than dogs that received placebo [22]. The administration of Lactobacillus rhamnosus MP01 and L. plantarum MP02, two strains isolated from canine milk, decreased the Faecalibacterium in feces [23]. Supplementing 5 × 109 CFU/day of L. murinus LbP2 in dogs improved their stool output, fecal consistency, mental status, and appetite compared to the control [24]. A total of 15 adult female dogs who were given 2.3 × 108 CFU/day canine-origin probiotic L. johnsonii CPN23 exhibited increased fiber digestibility and concentrations of short-chain fatty acids in their feces and reduced fecal ammonia concentrations compared to the control [25]. Dogs that consumed 107–109 CFU/day canine-derived probiotic L. fermentum CCM 7421 displayed an increased lactic acid bacteria population, reduced Clostridia population and some gram-negative bacterial genera. Additionally, dogs that consumed probiotics showed improved total protein, cholesterol and alanine transaminase in blood samples [26]. Three milliliters of 109 CFU/mL of the new potential probiotic L. fermentum AD1 significantly increased total lipids and total protein and significantly decreased the glucose concentration in the bloodstream [27]. Dogs fed 1.04 × 109 CFU/mL B. animalis B/12 showed a significantly decreased concentration of triglycerides and albumin and increased acetic, acetoacetic, and valeric acid in feces [28]. Supplementing 108 CFU/mL canine-origin probiotic L. johnsonii CPN23 in adult female dogs decreased their plasma glucose and cholesterol levels and increased the high-density lipoprotein and low-density lipoprotein ratio [29]. Dogs receiving Enterococcus faecium DSM 32820 had optimal fecal consistency throughout the experiment, significantly stimulated phagocytic activity and a metabolic burst activity of leukocytes and lower serum glucose concentrations [30]. Heathy dogs receiving 5 × 109 CFU/kg L. acidophilus D2/CSL showed higher body condition scores than the control dogs and there was a positive effect on their fecal consistency [31]. The probiotic feed additive contained three different bacterial strains, namely, L. casei Zhang, L. plantarum P-8, and B. animalis subsp. lactis V9 promoted the average daily feed intake, improved average daily weight gain, increased beneficial bacteria and decreased potentially harmful bacteria [14]. The probiotic E. faecium SF68 improved diarrhea symptoms compared to the control, and Giardia cysts were eliminated [32]. Adding 5 × 108 CFU/day E. faecium SF68 significantly increased the triglyceride concentration and decreased the cholesterol concentration [33].
The gut microbiome greatly affects the health and disease of the host so maintaining it in good condition is important for the health of the host [34]. Many factors influence the composition of the gut microbiome and aging is one of the greatest impacts [35]. After all, this aging which is defined as the gradual changes that occur after maturation in various organs, resulting in decreased functional capacity in the gut microbiome is thought to be somehow related to the health of the host [36]. Masuoka et al. [34] conducted the experiment with dogs of 5 different age groups (pre-weanling, weanling, young, aged and senile) and analyzed the composition of their intestinal microbiota of dogs in different age groups. As a result, the composition of the dog’s intestinal microbiota changed with age. Lactobacillus and Bifidobacterium were found to decrease as the dog aged. This experiment showed that the gut microbiome of dogs can be changed regarding the age at the level of bacterial groups and species. Further studies are needed to be done to identify whether different probiotics are needed for different phases of life.
Cats have trillions of live bacteria in their bodies, which are mostly in their intestines [21]. Each cat’s bacterial population is different for individuals and can be changed based on diet, health status, and lifestyle choices [37,38]. During times of stress and infection, the microbiome balance can increase the number of bad bacteria, disrupting the system’s balance and potentially causing digestive problems such as decreased appetite, vomiting, diarrhea or stool changes [39,40]. Supplementing probiotics for felines can be one of the best ways to add good bacteria to the cat body [21]. Recent reports of using probiotics in felines are listed in Tables 1 and 2.
Although many studies have investigated the use of probiotics in dogs, studies in cats are relatively scarce. Few studies on probiotic usage in cats have been reported to date, and because of differences in host physiologic characteristics and the diet, the probiotic efficacy in cats cannot be extrapolated from studies in dogs [41]. The purpose of this review paper is to discuss various results about treating cats with different types of probiotics. Kittens receiving 2.85–4.28 × 108 CFU/day E. hirae showed high intestinal colonization and fecal shedding of live E. hirae during administration [42]. Supplementing 2 × 108 CFU/day L. acidophilus DSM13241 as a probiotic in healthy adult cats increased the numbers of beneficial L. and L. acidophilus groups in feces and decreased the numbers of Clostridium spp. and E. faecalis. It also decreased the fecal pH and plasma endotoxin concentrations and resulted in systemic and immunomodulatory changes in treated cats [41]. Kittens fed 5 × 109 CFU/day E. faecium SF68 showed a significantly higher percentage of CD4+ lymphocytes than controls [43]. Healthy adult cats fed 5 × 109 CFU/kg L. acidophilus D2/CSL had better results in terms of their fecal quality parameters and had increased Lactobacillus counts and decreased total coliform bacteria counts [44]. The percentage of cats with diarrhea was significantly lower in the 2.1 × 109 CFU/day E. faecium SF68 group than in the control group [45]. Young adult cats receiving E. faecium SF68 had significantly lower total diarrhea scores for days 1–11 compared to the control. Additionally, feeding E. faecium SF68 could lessen some associated clinical abnormalities [46]. Feeding Enterococcus faecium SF68 in cats with chronic feline herpesvirus-1 (FHV-1) infection showed fecal microbial diversity throughout the study which indicates a more stable microbiome. It also lessened the morbidity associated with chronic FHV-1 infections [47]. Healthy cats with 5 × 109 CFU from a mixture of seven bacterial species per day (Proviable®-DC) showed an increased abundance of probiotic bacteria in the feces. Probiotics also improved diarrhea after 21 days of feeding [48]. Cats with chronic gingivostomatitis that were fed 1 × 108 CFU/mL L. plantarum showed many positive results in gingivostomatitis symptoms. There was an improvement in the time of recurrence, and the symptoms of chronic feline gingivostomatitis disappeared after two weeks of administration. Additionally, ulceration, inflammation and oral cavity pain decreased, and thalism and halitosis disappeared [49]. Giving multistrain probiotic products to 8-month-old male cats with feline idiopathic cystitis effectively managed this disease due to the effects of bactericidal, anti-inflammatory, and immunomodulatory actions [50].
The health and disease of the host are affected by gut microbiota, maintaining the gut microbiota is getting more important as cats get aged. Masuoka et al. [51] conducted the experiment with cats of 5 different age groups (pre-weanling, weanling, young, aged and senile) and analyzed the composition of their intestinal microbiota of cats in different age groups. The results suggested that the composition of the cat’s gut microbiome changed with age, whereas the change was different from that of dogs. Bifidobacterium which predominated in the gut of dogs did not appear to be important in the gut of cats. Instead, enterococci appeared to be the main lactic acid-producing bacteria in cats. Ultimately, the results of this study indicated that the compositions of the gut microbiome between dogs and cats are different and those compositions are changing with aging. Not only are different probiotics might need for dogs and cats but also for regarding aging. Further studies are needed to use different probiotics for different phases of life.
GUT MICROBIOME FOR COMPANION ANIMALS
Microorganisms affect the absorption of nutrients in the host and provide beneficial metabolites in return for using host nutrients [52]. Each intestine harbors a different unique microbial ecosystem due to anatomical and physiological differences [53]. Additionally, each animal harbors a different and unique microbial profile. For example, at the species and strain levels, only a few overlap between individual animals. However, the bacterial phyla, order and genera are shared by most mammals [54].
The most predominant bacterial gene category in the canine gut is carbohydrate metabolism, such as that related to mannose, oligosaccharide and raffinose metabolism. The fermentation of carbohydrates by colonic organisms such as Bacteroides, Roseburia, Ruminococcus and Lachnospiraceae results in the synthesis of short-chain fatty acids (SCFAs), such as acetate, propionate and butyrate which are sources of energy for the host [20]. SCFAs have beneficial effects on host health, including immunomodulatory effects, anti-diarrheic effects, and a regulatory effect on GI motility. In the case of felines, which are obligate carnivores, consuming raw meat increased Clostridium and Eubacterium, which are known to produce SCFAs [55].
The synthesis of vitamin K and several components of vitamin B are important functions of the intestinal microbiota [56,57]. Vitamin K, which is included in fat-soluble vitamins, plays an important role in prothrombin coagulation factor activity. Therefore, there is a risk of intestinal bleeding in cases of vitamin K deficiency [58]. Vitamin B12 (also known as cobalamin) is important for many aspects of a dog’s health [59]. It is crucial for a healthy nervous system and brain function as well as for the formation and growth of blood cells [60]. Additionally, it is needed to maintain healthy digestion [61]. As a result of a metagenome analysis using dog feces, genes affecting lipoprotein lipase activity in adipocytes were identified in intestinal microbial genes, confirming that microorganisms are also related to lipid metabolism [62].
The microbiome plays an important role in the immune system of the intestinal tract. In particular, early microbial exposure significantly affects gut microbiome formation and immune modulation, which affects susceptibility to intestinal diseases [63]. When comparing animals born through vaginal delivery with germ-free animals through cesarean section, the germ-free animals have fewer and smaller peyer’s patches, mesenteric lymph nodes and CD4+ T cells in the lamina propria of the gut wall [64]. In germ-free animals, a reduction in B cells, macrophages and neutrophils was confirmed [65]. Additionally, in germ-free animals, immunoglobulin was found at a level of 2%, which was significantly lower than that in normal healthy animals [66]. The microbiome also plays a role as a signal indicating health [65]. This characteristic is expected because animals evolved in coexistence with symbiotic microorganisms for a very long time [67]. Microorganisms that coexist with animals communicate directly and effectively with their host’s immune system through metabolites and nutrients [64,65].
The intestine is a major part of the body that influences host health. Numerous microbes form the complex microbial community in the GIT. Disrupting the gut microbiome may cause dysbiosis and lead to several diseases and disorders, such as diarrhea, allergies and obesity [21]. GI disease caused by the dysbiosis of the gut microbial community is also generally observed in dogs and cats [68,69]. Knowing the diversity and taxonomic bacterial distribution of the gut microbiota of healthy dogs and cats is important as a baseline in future studies evaluating GI diseases in dogs and cats [70,71].
Previous studies have focused on the cultivation of intestinal content to characterize and identify the microbiota [72-74]. Most of the bacterial groups cultivated from the canine intestine belonged to Enterobacteriaceae, Bacteroides, Clostridium, Lactobacillus, and Bifidobacterium spp. [75,76]. However, culturing the bacteria to evaluate the complex diversity of the microbiota had limitations. Bacterial species that can be cultivated by using bacterial culture techniques are only a small portion of microbiota composition [77,78], anaerobic bacteria can be easily damaged during sample handling [79,80], the cost used for culture techniques is expensive [80], a great amount of time is used for isolation and cultivation [80,81]. A novel molecular method that uses the 16S ribosomal RNA (rRNA)-enabled the evaluation of the diversity and abundance of bacteria in the sample without culturing [82,83].
The development of next-generation sequencing technologies has helped to characterize bacterial communities and to understand interactions between hosts and bacteria. Using next-generation sequencing, dog and cat organ microbiotas have been described. These microbiota include those of the GIT [70, 84-86], skin [87], oral cavity [88,89], nasal cavity [90], and vagina [91].
All animals, including dogs and cats, harbor numerous microorganisms in the GIT [8]. Dogs and cats have different microbiota compositions and they also differ in the same species [21]. There are lots of factors that can affect microbiota compositions such as age [92-94], breed [8,95,96], diet composition [39,92,94], disease [92,93], environment [92,96,97], food type [93,98] and sex [99,100].
The gut microbiome which is highly related to a healthy life could be affected by dogs’ breed. There was a relationship between GI conditions and dog breeds [101,102]. According to You and Kim’s experiment [103], there was a difference in microbial composition in Poodle and Maltese groups. Also, phylum Fusobacterium was differed by breeds (Maltese, Poodle, and Miniature Schnauzer). From these data, they suggested that there might be differences in the gut microbiome composition depending on the dog breeds. According to Lehtimäki et al.’s experiment [104], living conditions have a significant impact on the skin microbiome in humans and dogs, but not the gut microbiome. Dogs living in rural and urban environments participated in the study. The skin microbiome was more diverse among individuals in rural areas compared to urban areas. This study showed that the living environment had a much greater effect on the skin microbiome than the guts of dogs. Experiments on changes in the gut microbiome of cats according to breeds and living environments have been limited. Further research is needed as with dogs. According to Older et al.’s [96] experiment, breed and living environment played an important role in shaping the cat skin microbiome. In particular, it seems that the hair coat and grooming according to the cat breeds have a great influence on the microbiome of the cat’s skin microbiome.
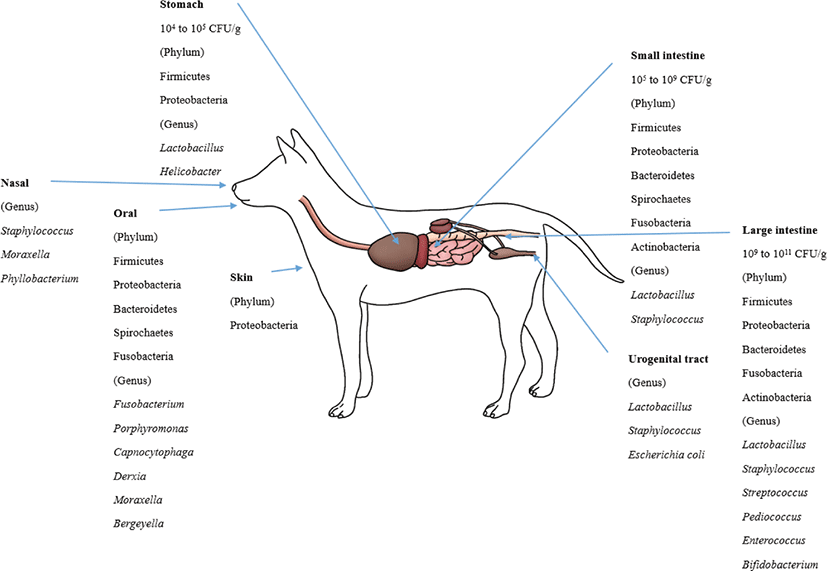
The bacterial count in the stomach is between 104 and 105 CFU/g [105]. In the duodenum and jejunum, the bacterial counts are generally low (105 CFU/g) but can reach 109 CFU/mL in some dogs and cats [106]. The ileum contains an increasing number of diverse microbiota, mostly at 107 CFU/mL. The bacterial counts in the colon are between 109 and 1011 CFU/g [38,73].
The healthy canine stomach has a comparably low number of total bacteria. Most belonged to Proteobacteria (99.6%), and few belonged to Firmicutes (0.3%). The dominant species are Helicobacter and Lactobacillus spp. [85]. Using 16S rRNA sequences, four phyla (Firmicutes, Fusobacteria, Bacteroidetes and Proteobacteria) predominated in the small intestine [70]. The duodenum of healthy canines consisted of six phyla. Firmicutes predominated followed by Proteobacteria, Bacteroidetes, Spirochaetes, Fusobacteria and Actinobacteria [37,107]. Healthy dog microbiota in the jejunum were evaluated, and the most predominant phylum was Proteobacteria (46%), followed by Firmicutes (15%), Actinobacteria (11.2%), Spirochaetes (14.2%), Bacteroidetes (6.2%) and Fusobacteria (5.4%) [84]. The ileum microbiota of healthy dogs predominantly consists of Fusobacteria, Firmicutes and Bacteroidetes [70].
Lactobacillales was present in all parts of the intestines (22% in the duodenum and 10% in the jejunum). Enterobacterales were more frequently detected in the small intestine than in the colon. Clostridiales were highly abundant in the duodenum (40%), jejunum (39%), ileum (25%) and colon (26%) [70]. Facultative anaerobic Lactobacillus strains predominated in the jejunal microbiota, and L. acidophilus was the most abundant among them [108]. In the jejunal samples, facultative anaerobic and anaerobic bacteria were similarly detected, while anaerobic bacteria predominated in the fecal samples. The number of bacteria in the jejunum was 102 to 106 CFU/g, while the number in the feces was 108 to 1011 CFU/g. Despite the lower number in the small intestine, some microbial groups were more prevalent in the small intestine than in feces: staphylococci, 64% versus 36%; non fermentative gram-negative rods, 27% versus 9%; and yeasts, 27% versus 5% [73].
Firmicutes, Bacteroidetes, Fusobacteria, Proteobacteria and Actinobacteria were the most abundant phyla in the fecal microbiota of healthy dogs [70, 86, 109]. However, Fusobacteria (39.17%) were dominant, followed by Bacteroidetes (33.36%) and Firmicutes (15.81%) in healthy adult Miniature Schnauzer dogs [86], while the abundances of Fusobacteria, Bacteroidetes and Firmicutes were similar (approximately 30% each) in six Hound dogs [109].
Clostridia was the most predominant bacterial class in the dog fecal microbiota [109]. At the genus level, Lactobacillus was the most predominant, followed by Bifidobacterium, Enterococcus, Streptococcus and Pediococcus, in the dog fecal microbiota [110]. Many Lactobacillus spp. including L. casei, L. salivarius, L. rhamnosus, L. mucosae, L. fermentum, L. reuteri, L. animalis, L. acidophilus and L. johnsonii were the most frequently isolated ones from the feces. L. reuteri, L. animalis, and L. johnsonii were the most predominant species in dogs [110-112]. Weissella confuse, Pediococcus acidilactici, Enterococcus spp. and B. animalis ssp. lactis were also frequently isolated from dog feces [111-113]. At the fungal kingdom-phylum level, Ascomycota, Basidiomycota, Glomeromycota, and Zygomycota were detected in dog feces [109].
In the skin microbiota, the most predominant phyla and families were Proteobacteria and Oxalobacteriaceae [87]. At the oral microbiota phylum level, Bacteroidetes (60%) was the most predominant, followed by Proteobacteria (20.8%), Firmicutes (11.4%), Fusobacteria (4.7%) and Spirochaetes (1.7%). At the genus level, the oral microbiota consisted of Porphyromonas (39.2%), Fusobacterium (4.5%), Capnocytophaga (3.8%), Derxia (3.7%), Moraxella (3.3%) and Bergeyella (2.7%) [88]. In the nasal microbiota of healthy dogs, Moraxella spp. was the most abundant species, followed by Phyllobacterium spp., Staphylococcus spp., and Cardiobacteriaceae [90]. The most frequently isolated bacteria from the dog’s vaginal tract were Lactobacillus, Escherichia coli and Staphylococcus pseudointermedius [21,91].
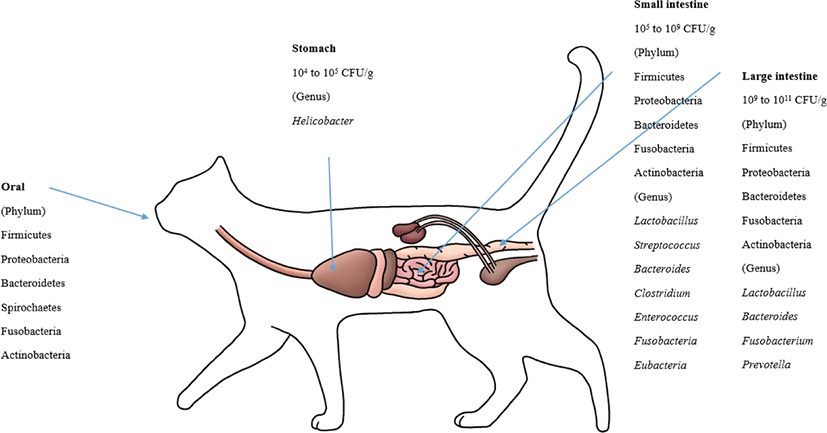
The feline GIT has different bacterial species than other animals. Helicobacter is known to reside in the stomachs of cats [114]. For the microbiota composition of the GI (stomach, duodenum, jejunum, ileum, and colon) contents, which were collected from 5 healthy felines, Firmicutes (68%) predominated, followed by Proteobacteria (14%), Bacteroidetes (10%), Fusobacteria (5%) and Actinobacteria (4%). At the order level, Clostridiales (54%) prevailed, followed by Lactobacillales, Bacteroidales, Campylobacterales, and Fusobacteriales [37]. Based on several studies, it is known that Bacteroides spp., Clostridium spp., Enterococcus spp., Streptococcus spp., Fusobacteria spp., and Eubacteria spp. are present in the small intestines of felines [69,115]. Representative lactic acid bacteria present in the GIT of felines include L. acidophilus, L. salivarius,L. johnsonii, L. reuteri and L. sakei, which are typical intestinal lactic acid bacteria found in animals, including humans, although the amount varies by individual [21,37]. The major phyla in the feline fecal microbiota were Firmicutes, Bacteroidetes, Actinobacteria and Proteobacteria [69,109,116]. These four phyla make up more than 99% of the fecal microbiota [69]. Handl et al. [109] reported that Firmicutes was the most prevalent phylum in the fecal microbiota, followed by Bacteroidetes and Actinobacteria; however, Tun et al. [116] reported that Bacteroidetes was the most predominant phylum, followed by Firmicutes and Proteobacteria. Bacteroides, Fusobacterium, and Prevotella were the most predominant genera in the feline fecal microbiota, which indicated that these genera play a major role in the feline intestine [117]. Fungi, archaea and viruses compose a minor part of intestinal microbial communities. Ascomycota was the only phylum of fungi detected in cats [109,116].
Malassezia spp. were the most prevalent fungi in feline skin mycobiota. M. restricta and M. globosa were the most predominant fungal species in all cat breeds [96]. M. pachydermatis is known as a yeast that is present in the skin microbiome, yet it can also act as a pathogen that can cause dermatitis [118]. The phylum level of the oral microbiota is generally conserved between cats. These phyla are predominated by Proteobacteria (75.2%), followed by Bacteroidetes (9.3%), Firmicutes (6.7%), SR1 (2.7%), Spirochaetes (1.8%), Fusobacteria (1.3%), and Actinobacteria (0.6%) [89]. The composition of the canine and feline microbiota is shown in Figs. 1 and 2.
SAFETY ISSUES OF PROBIOTICS AND THE GUT MICROBIOME IN COMPANION ANIMALS
The Food and Agriculture Organization of the United Nations and World Health Organization (FAO/WHO) defined probiotics as “live microorganisms, which when administered in adequate amounts, confer a health benefit on the host” [9]. Tremendous scientific evidence for the efficacy of probiotic candidates has been available for decades, but insufficient information about their safety is available. While known to be safe in general, a few adverse effects associated with probiotics use have been documented in patients [119,120]. Moreover, there is a lack of information on the inherent characteristics of each probiotic strain that may be associated with health risks [121].
The European Food Safety Authority (EFSA) recommended the qualified presumption of safety (QPS) status for microorganisms used in feed and food production in 2003. Based on the QPS guidelines, microbes that produce toxins or possess virulence factors that may contribute to their pathogenicity cannot be used as probiotics. In addition, it must be ensured that there are no acquired genes encoding antimicrobial resistance (AMR). The existence of knowledge, including a history of use, ecology, industrial application, clinical reports, and a public database, is considered important evidence for evaluating the safety of microbial species [122]. QPS list includes several taxonomic units for bacteria, yeasts, and viruses [123], of which Lactobacillus and Bifidobacterium are representative because their reasonable certainty of no harm has been supported by an extensive record of safe use [124].
With the recent focus on the beneficial effects of probiotics in companion animals and their relationship to gut microflora and health, probiotic products are being increasingly marketed in the form of feed additives, dietary supplements, and probiotic-containing foods [125]. Although there have been no reports of adverse events when probiotics are administered to small animals, safety concerns remain to be addressed [126]. The microorganisms used in feed additives require safety verification for target animals, manufacturers, and owners/consumers. In particular, Enterococcus, known as canine and feline intestinal commensal bacteria, have been used as probiotics for small animals, but their use is restricted in some countries because of the risk of host infection by AMR gene transfer [125,127]. Rinkinen [128] demonstrated that some Enterococcus faecium strains promoted the adherence of the zoonotic pathogen Campylobacter jejuni in the intestines of canines. Notably, the administration of E. faecium strain SF68 (deposited as strain NCIMB 10415), which originated from infant feces in 1968, reduced the occurrence of diarrhea in dogs and cats housed in animal shelters [45], and it has been verified that the strain may not cause any safety concerns for companion animals and their owners [129-131]. Due to these conflicting outcomes, stringent safety evaluations are required in a strain-specific manner with regard to probiotic use.
Host specificity is considered an important criterion for selecting probiotic candidates, primarily due to differences in physiological structure, immune systems, and microbial composition [132,133]. However, most commercial probiotics used as feed additives originate from humans and are verified by human-based methods and criteria [134]. The clinical results from Weese and Anderson [135] showed that Lactobacillus rhamnosus GG, a commercial probiotic strain isolated from a healthy human GIT, may not be suitable for use in canines because of its short persistence. In addition, in an in vitro test, probiotic strains of canine origin inhibited the adhesion of enterotoxigenic Clostridium perfringens to canine jejunal chyme more efficiently than non-canine strains [128]. Recent studies have focused on strains isolated from the intestines of healthy dogs and cats to demonstrate their impact on pathogen inhibition, attenuation of inflammatory status, and modulation of the gut microbiome [136,137]. Host specificity has been discussed with respect to probiotic efficacy in most publications but must also be addressed from a safety perspective. Furthermore, clinical outcomes for the safe use of probiotics in target animal species should be documented. Given the host specificity and broad diversity of potential probiotic candidates for small animals, the availability of novel species with no record of use requires attention [133]. For a complete characterization, documentation of genetic and biochemical properties and long-term clinical trials are needed. Additional efforts are required to standardize safety assessment methods for novel species to be considered QPS or as having a generally recognized as safe status based on the opinions of regulatory bodies and expert panels [138].
Quality control issues associated with probiotics relate to products intended for animals as well as humans [21]. The global market for probiotics for companion animals is growing, but insufficient quality control regulations create serious problems for the safety of consumers and target animals. Some investigators have disclosed that many commercial animal probiotics or pet foods that claim to contain probiotics did not contain microbial species listed on the label or even contain other species. Moreover, bacterial viability, a key concept to stipulate probiotics, was inconsistent with labeled values expressed in colony-forming units [139-141]. The current low level of quality control may lead to exposure to unknown health risks not only for the animals but also for the owners and the environment. Recent advances in meta-omics technologies (metagenomics, metatranscriptomics, metaproteomics, and metabolomics) have promoted a correct evaluation of the quality of probiotic products. For the multistrain product VSL#3, Mora and colleagues [142] successfully identified microbial taxa with metagenomics and viability by flow cytometry and confirmed reproducibility using metaproteomics. Metagenomic approaches have also enabled the analysis of genes related to safety concerns in a culture-independent manner. Stringent oversight by regulatory bodies, high manufacturer awareness, and the development of rigorous evaluation methods by researchers are needed for the safe selection and production of probiotic candidates intended for both companion animal and human use.
CONCLUSION
Although not enough research has been conducted on the probiotics and gut microbiome thus far, because the number of companion animal people and the companion animal market are growing, research related to the companion animal microbiome is also growing. Recently, in-depth research has been performed to identify the functionality of probiotics and the gut microbiome of companion animals and to make them with various materials, ranging from feed, snacks, supplies, and treatments for the diseases of companion animals. The current evidence suggests that specific probiotic strains and/or their defined combinations may be useful in canine and feline nutrition, therapy and care. However, probiotics and the gut microbiota used in the present study are of human origin; thus, the companion animal-specific health benefits are not unclear. Therefore, the most important step is to secure pet-originated microorganisms for their health claims. Moreover, detailed in vivo designs and trials using companion animals are needed to identify and characterize newly isolated pet-originated microbiomes with an impact on health maintenance in both dogs and cats. Corroborations of these health-promoting effects and microbiological safety issues should be assessed regarding potential probiotics and the gut microbiome for animal health and welfare.