INTRODUCTION
As a strategy to improve the meat production chain, it is common to use synthetic growth promoters as anabolic agents to enhance animal performance and carcass characteristics [1]. However, synthetic compounds are becoming unfeasible due to consumer concerns and strict regulations in Europe [2], and natural alternatives are more acceptable for use in animal production. The phytochemicals dietary supplementation could be a strategy to improve animal performance with the same beneficial effect as synthetic growth promoters but without compromising animal welfare, meat quality, and consumer health [2-4]. Recently, some researchers have reported that ferulic acid (FA; a secondary plant compound with bioactive properties) enhances growth performance, carcass characteristics, and meat quality in ruminants by reducing lipid peroxidation and increasing the average daily gain (ADG) and carcass weight [5-7]. Nevertheless, growth performance and carcass characteristics after FA supplementation in lambs have been inconsistent; in addition, the optimal dose or time exposition has not been established [8-11]. On the other hand, some authors suggest that the moderate growth-promoting effect of phytochemicals such as FA, in the case of ruminants, could be related to their antimicrobial activity, which causes a productive improvement due to the modification of ruminal fermentation [4,12]. However, more studies are required to establish the possible mechanisms of action involved.
On the other hand, clinoptilolite (CTL) a naturally occurring zeolite, is classified as safe and is commercially available for use in diets for broilers and pigs, but it is uncommon to use it in beef cattle production [13,14]. A study has shown that supplementing lambs with CTL increased muscle tissue deposition and decreased fat tissue deposition [15]. Additionally, an improvement in feed efficiency and ADG was reported [16,17]; the ion-exchange property of CTL favorably modulates ruminal fermentation, improving energy efficiency, which is reflected in animal growth performance [18]. However, the available results are conflicting; hence, more research is required in order to fully elucidate CTL’s response in ruminants.
There is a lack of studies reporting the effects of FA or CTL on the growth performance and meat quality of lambs. Moreover, the combined effect of both additives has not been studied. Therefore, this research hypothesis was that the simultaneous administration of dietary FA and CTL to hair lambs could synergistically enhance the growth performance, carcass characteristics, and meat quality. This study aims to evaluate the combined effect of the dietary inclusion of FA and CTL on the growth performance, carcass characteristics, meat quality, and chemical composition of finished hair-breed lambs.
MATERIALS AND METHODS
The feedlot performance was carried out during the winter season at a commercial sheep production farm located in northwestern Sonora, Mexico (latitude 28.78°N and longitude 111.40°W). The average temperature and relative humidity during the study were 18 ± 12°C and 49 ± 14%, respectively. The animal slaughter and carcass evaluation were conducted at the Agriculture and Livestock Department (ALD) of the Universidad de Sonora (UNISON), situated 21 km from Hermosillo, Sonora, Mexico. Meat quality, chemical composition, and fatty acid profile were evaluated at the Centro de Investigación en Alimentación y Desarrollo (CIAD), also situated in Hermosillo, Sonora.
All animal management and slaughter procedures were conducted in accordance with the official techniques and standards in Mexico (NOM-051-ZOO-1995, NOM-033-ZOO-1995, and NOM-062-ZOO-1999). Furthermore, the Ethics Committee of CIAD accepted and supervised all of the experimental processes (CEI/002-2/2021).
Twenty-eight Katahdin male lambs with similar initial body weight (IBW; 33.74 ± 3.4 kg) were individually housed in pens (2.5 m × 2 m) for this experiment. Seven pens per group were randomly assigned to one of the four experimental diets as follows: (1) control (high concentrate basal diet; BD); (2) FA (BD with 300 ppm FA; Laboratorios Minkab, Guadalajara, Jalisco, Mexico); (3) CTL (BD with 1% CTL; Zeolex®; Grupo Sanfer, Ciudad de Mexico, Mexico); and (4) FAZ (BD with 300 ppm FA + 1% CTL). All animals were given a compound of vitamins (A, D, and E) at the beginning of the study and were treated with Ivermectin (ivermectin + ADE; Virbamec ADE FUERTE; Virbac Mexico, Zapopan, Jalisco, Mexico; 0.7 mL/animal) for eliminating internal and external parasites. Also, each lamb was weighed and identified with a numbered plastic ear tag before being assigned to the experimental diets.
The feeding period was 50 days, ten days for the BD adaptation (12.9% crude protein [CP], 3.2 Mcal/kg feed of metabolizable energy [ME], meeting the recommended nutritional requirements for meat-producing lambs) [19] and the next forty days for the experimental trial. To ensure the daily intake of FA and CTL per lamb, both were mixed with mineral premix and subsequently blended with other ground ingredients to create the corresponding FA or CTL dose (300 ppm or 1%). Finally, the concentrate mixture was incorporated with the remaining ingredients. The ingredients and chemical composition of the experimental diets are indicated in Table 1. Feed was offered ad libitum twice a day at 0800 h and 1600 h in half mixed rations, and the lambs had ad libitum access to fresh water. Additionally, their health status was monitored daily by direct visual examination.
Control, high concentrate basal diet (BD); FA, BD with 300 ppm ferulic acid; CTL, BD with 1% clinoptilolite; FAZ, BD with 300 ppm FA + 1% CTL.
Based on tabular energy values of ingredients from NRC [19].
Animals were individually weighed three times (initial, interim, and final) during the feeding period. From these data, ADG was calculated for the first period (d 0 to 20), the second period (d 21 to 40), and for the whole period (d 0 to 40). Feed intake (feed offered – refusal) was measured daily. At the beginning of the experimental period, dry matter intake was established at 5% of the live weight, and then, the daily feed ration was adjusted to have a minimum refusal (< 10%). Finally, feed efficiency was indicated as the ratio of daily feed intake to ADG.
At the end of the experimental period, the lambs were slaughtered (ALD slaughterhouse) after fasting for 16 h (with access to water). Then, once the lambs were skinned and eviscerated, their carcasses were weighed to determine hot carcass weight (HCW); after chilling the carcasses for 24 h at 4°C, the cold carcass weight (CCW) was measured. Subsequently, following the methodology described byAMSA [20], carcass conformation was evaluated (numeric scale: 1 = bad to 8 = excellent). The cooling loss was also estimated by the difference between HCW and CCW expressed as a percentage. Moreover, body measurements were registered: carcass length, thorax depth, leg length, and leg perimeter [21].
Then, the carcasses were divided along the midline, and each left side was ribbed between the 12th and 13th ribs to measure the back-fat thickness and loin area using a dot squared grid. Finally, half of the carcasses were weighed and split into forequarters and hindquarters to obtain wholesale cuts: neck, shoulder, loin, ribs and flank, plain loin, and leg [22]. Each cut was expressed as a percentage of the half carcass weight.
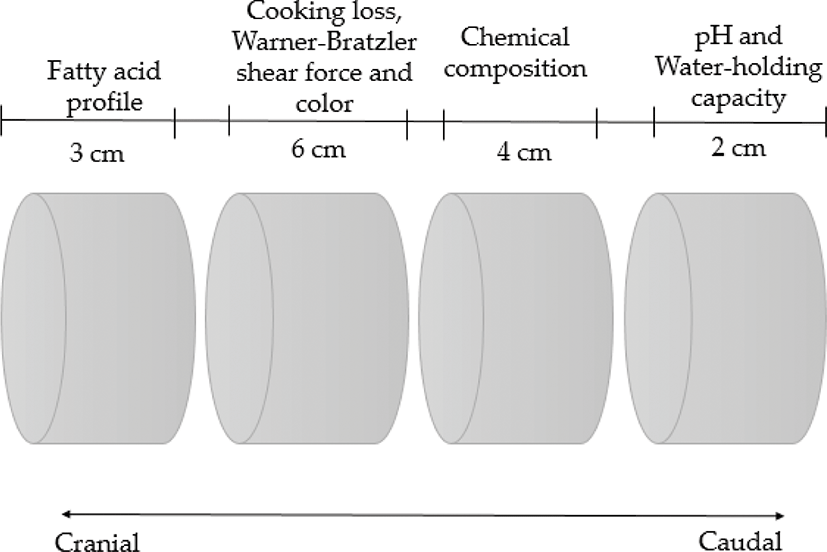
At 24 h postmortem, the pH from the longissimus thoracis (LT) was directly measured between the 12th and 13th ribs with a portable pH meter (model HI 99163, Hanna Instruments, Wilmington, MA, USA) with a puncture electrode. Then, after splitting and weighing each wholesale cut, the LT muscle was dissected from the loin of each left side carcass (located at the top of the 4th and 12th ribs). Subsequently, each muscle sample was vacuum packed, stored, and aged for one week at −18°C in the Meat Science Laboratory of CIAD. At the end of the aging time, the samples were thawed (24 h at 0°C) and sectioned following the diagram shown in Fig. 1 to determine final pH, water-holding capacity (WHC), color values, cooking loss, fatty acid profile, and Warner–Bratzler shear force (WBSF).
To evaluate the final meat pH, each steak was homogenized in distilled water with a meat: water ratio of 1:3 (g:mL). Then, the final meat pH was measured using a benchtop digital pH meter previously calibrated with pH 4 and 7 standards (Model HI-2210, Hanna Instruments Digital, Woonsocket, RI, USA). WHC was evaluated following the technique previously described by Sutton et al. [23]. Meat samples were centrifuged for a lapse of time (1,420 g × 5 min, at 5°). The WHC value was calculated by the difference between the initial weight (before centrifugation) and final weight (after centrifugation) of the sample, expressed as a percentage.
LT muscle color analysis was conducted after 30 min of blooming using a Minolta CR-2600 colorimeter with illuminant D65 (10° observer and an 8 mm diameter aperture; Konica Minolta Sensing, Osaka, Japan), where L*, a*, and b* were documented. Chroma (C*; color saturation index) and hue angle (H*) were calculated using the redness and yellowness values, according to the Cassens et al. [24] methodology. Five measures were taken directly from the LT muscle surface in distinct locations from each sample.
To evaluate the cooking loss and WBSF, LT samples were weighed raw, and after being cooked until the internal temperature of the steak reached 71°C (an electric skillet was employed; Cook Master 3222-3, Oster, Mississauga, Ontario, Canada), they were immediately weighed again. Next, the cooked samples were chilled, first, at room temperature (22°C–27°C), and then, at 4°C for 24 h. Finally, from each steak, six cores of 3 cm long and 1.27 cm2 in cross-section were cut parallel to the muscle fibers and used to evaluate WBSF with a WBSF device (TA. XT Plus Texture Analyzer texturometer, Texture Technologies, Scarsdale, NY, USA). The WBSF value was indicated in kg. The percentage of cooking loss was determined as the ratio of the raw weight to the cooked weight.
Moisture, ash, fat, and protein contents of the LT muscle were determined as described by the AOAC standardized procedures [25]. To evaluate the fatty acid profile, lipid extraction was carried out following an adaptation to the Bligh and Dyer [26] procedure with the following modifications: 2 g of meat was mixed with 10 mL of methanol and homogenized (Ultra-Turrax T25, IKA, Staufen, Germany) for 1 minute at 1,450×g. Then, 20 mL of chloroform was added, homogenized again for 2 minutes at 1,450×g, and filtered with Whatman no. 41. Later, three milliliters of KCl (0.88%) was added, and after vigorous shaking, the top layer was poured out. Finally, 4 mL of distilled water and methanol were added to assist separation before the sample was shaken again; the top layer containing the non-lipid fractions was removed, and the lower phase was used for esterification.
Fatty acid methyl esters (FAMEs) were prepared following an adaptation of the standard method described by Li and Watkins [27]. First, samples were placed in a water bath (40°C) with a constant nitrogen gas flow until all solvents were evaporated. Then, 4 mL of NaOH (0.5 N in methanol) was added; later, tubes (previously capped) were placed in a water bath (90°C) for 5 min, and after cooling at room temperature, 5 mL of boron trifluoride (BF3) was added to the samples and heated in a water bath for 5 min again. Next, when the samples were cooled, 4 mL of hexane was added and then heated for 2 min in a water bath (90°C). Finally, 0.5 g of anhydrous sodium sulfate and 1 mL of saturated NaCl solution were added, and 1 mL of the top lipid phase was carefully collected into a 2 mL gas chromatography sample vial containing 1 mL of hexane.
FAMEs were analyzed using a gas chromatograph (Hewlett Packard model 6890, Waldbronn, Germany) equipped with a flame ionization detector and a capillary column (Agilent J&W DB-23 [0.25mm internal diameter x 60 m; 0.25 μm film thickness]). The chromatographic conditions were as previously described by González-Ríos et al. [5]. Identification of each FAME was based on the retention times compared with those from a known standard (tridecanoic acid; 13:0, Sigma-Aldrich, St Louis, MO, USA). Data are expressed as the percentage of total fatty acids identified. Nutritional ratios (polyunsaturated fatty acid [PUFA] / saturated fatty acid [SFA], monounsaturated fatty acid [MUFA] / SFA, and n-6 / n-3) were calculated as well as the total amounts of PUFA, MUFA, and SFA.
Data acquired from the study were analyzed with the NCSS statistical program (version 2007, NCSS, Kaysville, Utah, USA). Data from feedlot performance, carcass characteristics, wholesale cuts, meat quality, chemical composition, and fatty acid profile were analyzed as a 2 × 2 factorial under a complete randomized design. The model considered fixed effects of dietary supplementation of FA and CTL and their interaction (CTL × FA). For feedlot performance variables, IBW was included as a covariate in the model. Differences among means were compared using the Tukey–Kramer test. Significances were considered when P < 0.05, and a trend was declared when 0.05 < p < 0.10.
RESULTS
Table 2 presents the influence of dietary supplementation with FA and CTL on hair-breed lamb feedlot performance. No variable throughout the feeding trial or by period was affected by the FA × CTL interaction (p > 0.05). Neither IBW nor final body weight differed between experimental diets (p > 0.05). Additionally, ADG, feed intake, and feed conversion were not affected (p > 0.05) during the 40 d trial period. Lambs supplemented with CTL tended to increase their daily feed intake through each period by 7.5% (d 0 to 20; p = 0.088) and 7.6% (d 21 to 40; p = 0.054), while feed conversion tended (p = 0.065) to be higher in the second period; however, this trend was not shown during overall feeding period (d 0 to 40; p > 0.05). Moreover, from d 21 to 40, dietary FA decreased lamb daily feed intake by 7% (p < 0.05), but this reduction did not affect their feed conversion (p > 0.05).
Regarding the carcass characteristics, no significant FA × CTL interaction (p > 0.05) was shown (Table 3). Neither FA nor CTL had an effect on HCW, CCW, dressing weight, fat thickness, or loin area (p > 0.05; Table 3). However, the cooling loss value of the carcass from lambs supplemented only with CTL tended to be 47% lower (p = 0.072). In addition, dietary FA did affect the carcass conformation (p < 0.05); nevertheless, the conformation score was lower for this group (7.4%; Table 3). No FA × CTL interaction or FA main effect (p > 0.05) was presented for any of the body measurements (Table 3). On the other hand, while CTL tended (p = 0.057) to increase leg length (4%), other variables (carcass length, thorax depth, and leg perimeter) remained unaltered (p > 0.05). Wholesale cuts yield are presented in Table 4. Dietary FA, CTL, or their interaction (FA × CTL) did not impact (p > 0.05) any wholesale cut yield of the hair-breed lambs: neck, shoulder, loin, ribs and flank, plain loin, or leg.
Neither the FA × CTL interaction nor the additives themselves had any impact on pH 24 h, WHC, cooking loss, or WBSF (p > 0.05) (Table 5). The final pH was unaltered (p > 0.05) by FA or CTL supplementation, while their interaction (FAZ treatment) tended (p = 0.095) to decrease the final FAZ sample pH (pH = 5.67; unpublished raw data from the study). Regarding color variables, dietary FA did not have any effect on the L*, a*, b*, or C* values (p > 0.05; Table 5). On the other hand, dietary CTL improved (p < 0.05) the L*, a*, and C* values, whereas it tended to increase yellowness (b*; p = 0.070). The hue angle (H*) remained unaltered for all treatments (p > 0.05).
The chemical composition of the lamb meat is shown in Table 6. In general, the chemical composition of the lamb meat was not influenced (p > 0.05) by the additives themselves or by their interaction in terms of moisture, ash, fat, or protein contents. Table 7 presents the influence of dietary supplementation with FA and CTL on the fatty acid profile of the intramuscular fat of hair lambs. Palmitic (C16:0), stearic (C18:0), and oleic (C18:1ɷ9c) acids represent the greatest proportion of the fatty acid profile of the lambs’ LT muscle in all treatments. FA supplementation did not have any effect (p > 0.05) on the fatty acid profile of the lamb meat, whereas the CTL and FA x CTL interaction modified the content of some fatty acids (Table 7). CTL increased (p < 0.05) the percentages of C10:0, C14:1, and C15:0 (18, 40, and 22%, respectively) in comparison to animals not supplemented; meanwhile, C14:0 decreased (p > 0.05) 13.30% by CTL supplementation. Additionally, CTL tended to reduce C16:0 (p = 0.068; 5.4%) and C20:1ɷ9 (p = 0.060; 26.3%). Regarding C18:0, C18:2ɷ6t, and C20:3ɷ6, the FA x CTL interaction increased (p < 0.05) the content of the aforementioned fatty acids while it tended to increase C17:0 (p = 0.098) and C20:3ɷ6 (p = 0.097). No FA × CTL interaction or FA main effect (p > 0.05) was presented for any of the sums of fatty acids and nutritional indices of lamb meat (Table 8). However, while CTL tended to increase the sum of PUFAs (p = 0.053; 12.04%) and n-6 (p = 0.066; 11.87%) and the PUFA/SFA ratio (p = 0.070; 21.05%), the other sums and nutritional indices remained unaltered (p > 0.05).
DISCUSSION
The findings of the present study did not show any additive or synergistic effect in the supplemented animals that could support our hypothesis; therefore, the discussion focuses on the individual impact of each supplement.
The mechanism of action of FA has not been fully elucidated. Based on previous studies with this compound, it has proposed different mechanisms of action involved in the growth-promoting effect of FA: 1) FA could enhance animal performance by reducing their oxidative stress [28]. 2) FA has a similar action mechanism to beta-agonists (βAAs) due to the similarities of their molecular structures [29,30]. 3) FA acts as a modulator of ruminal fermentation [12,31].
In our current study, we have no clear information to explain why FA supplementation did not improve feedlot performance traits, carcass characteristics, or wholesale cuts of the finished lambs. In agreement with our results, other studies reported that dietary FA administration did not affect the growth performance of hair lambs [8-10,32]. Contrary to our results, Peña-Torres et al. [30] observed that feeding FA (300 ppm and 600 ppm) enhanced the ADG of lambs; however, in that study, the lambs had a lower body weight and age than the animals in our study. This growth-promoting effect reported in the last study can be related to the high growth rate of the lambs at younger ages, which is usually the period when lambs have continuous growth without reaching their inflection point in the growth curve [33]. Nevertheless, an anabolic effect has been shown in other species, such as pigs and beef cattle [7,34]. Further research on beta-adrenergic receptor (β-AR; subtypes β1, β2 and β3) gene expression or receptor binding affinity evaluation is needed to elucidate these inconsistencies between species.
Based on the aforementioned β–AR gene expression, Valenzuela-Grijalva et al. [29] studied the change in pigs’ skeletal muscle mRNA abundance after FA supplementation. β2-AR mRNA expression was increased by FA intake. Baxa et al. [35] reported a similar effect in finishing steers by zilpaterol hydrochloride supplementation. It is worth mentioning that the β2-AR subtype is predominant in bovine and porcine skeletal muscle [36]. On the other hand, Ekpe et al. [37] found an increase in the receptor density of β1-AR in sheep under feed restriction and observed a trend toward cold stress. Previous studies have reported a low plasma concentration of endogenous catecholamines in lambs under thermoneutral conditions. Thus, there might be a lower activation of skeletal muscle β-receptors, which results in less receptor binding affinity [37,38]. These observations suggest that in pigs and beef cattle, FA stimulates β2-AR to promote a growth effect, while in lambs, FA behaves differently.
Based on the above, the lack of effects on the feedlot performance of lambs supplemented with FA can be explained. However, the lambs in our study significantly decreased their feed intake without affecting their performance, which could represent an advantage in meat production development because feeding is an element that elevates production costs. In contrast with our results, other studies with lambs found no effect of FA or feruloyl oligosaccharides on feed intake [8-10,32]. Additionally, published reports have studied the effect of supplementing lambs with different phytochemicals or plant extracts; their results have shown that feeding these compounds either increased or did not affect the feed intake of fattening lambs [39]. In our study, a decrease in feed intake was only present in the second period (days 21 to 40), and this reduction did not affect the lambs’ weight gain. In this regard, prolonged feeding of FA could positively modify the ruminal environment and, consequently, some ruminal fermentation patterns, nutrient digestion, or energy utilization [12,31].
On the other hand, dietary supplementation of fattening lambs with CTL tended to increase feed intake in each experimental period. Similarly, several studies have reported that CTL supplementation increased the daily feed intake of livestock [13,40,41]. Otherwise, the lambs’ weight gain in our experiment was not affected by CTL supplementation, while in other studies, dietary supplementation with CTL has been proven to increase weight gain in lambs [18,40,41]. This lack of effect on the weight gain of the lambs might be due to the limited sample size used in our study. On the other hand, Pond et al. [42] mentioned that a CTL advantage on lamb growth performance appeared when diets contained an intact protein source and a high protein level. According to the previous study, it is suggested that high protein intake elevates plasma urea-nitrogen and ruminal ammonium (NH4+) concentrations [42]. Since CTL has a high NH4+ binding capacity, NH4+ ions are immediately exchanged for the compensating cation of the zeolite and held there until they are released when saliva enters the rumen during rumination. It is possible that the dosed release of NH4+ ions enhanced ruminal bacteria growth, which in turn could improve ruminal fermentation patterns and microbial protein production. Moreover, Abdelrahman et al. [41] observed that lambs treated with 1% zeolite presented a similar performance to the lambs of the control group, while lambs fed with 2% zeolite showed a better growth performance. In the present work, the failure of CTL to affect weight gain in hair-breed lambs might be due to either an inappropriately low dose of CTL (chosen in this study) or balanced protein intake.
FA supplementation did not affect either the carcass characteristics or the wholesale cuts. The only change shown in these traits was a reduction in the conformation of the FA carcasses. The lack of effects of FA in our results is in accordance with [9,10,30] findings. In contrast, Peña-Torres et al. [6] found that by including 250 ppm FA in the diet of heifers, dressing weight and loin area were improved. In addition, previous studies have shown that phytochemicals and plant extracts did not alter carcass characteristics in fattening lambs [39]. Although our values were within the reference ranges, the lack of an effect on the carcass traits and wholesale cuts suggests that FA supplementation does not have anabolic stimulation in hair-breed lambs, as shown in younger lambs and other species [7,9,29,30]. Since the results of carcass characteristics and wholesale cuts have not been consistent throughout studies, these inconsistencies may be due to the growth effect shown by FA is associated with the breed, sex, species, environmental conditions, or the dose used [43].
Regarding CTL addition, there was no evidence of remarkable effects on carcass characteristics and wholesale cuts. Previous reports indicate that CTL did not affect any carcass characteristics of hair-breed lambs [15,40,43]. Since the CTL supplementation did not improve lambs’ muscular development in our study, consequently, no changes in carcass characteristics were expected. Deligiannis et al. [40] reported that lambs supplemented with 3% of CTL showed a better weight gain with no change on carcass traits. Similarly, other studies have reported that CTL supplementation enhances weight gain (carcass characteristics were not evaluated) [17,41,42]. The effects of CTL supplementation in lambs in other studies, could be related to the ability of CTL to modify ruminal fermentation due to its cation exchange property, resulting in better energy efficiency by improving the acetate:propionate ratio or by stimulating some populations of ruminal bacteria [18,44,45].
In the present study, FA supplementation did not affect the meat quality of hair lambs. These parameters (pH, WHC, cooking loss, color, and WBSF) were maintained within the reported values for lambs [9,46-48]. The lack of effects on color parameters between groups is consistent with previous reports with FA supplementation [6-9,48]. It has been reported that phytochemicals that exhibited antioxidant properties could delay metmyoglobin formation by reducing myoglobin and lipid oxidation, improving meat color stability [5,49]; however, FA did not show any antioxidant properties in our study. Phytochemicals (such as FA) are biotransformed by methylation, glucuronidation, and sulphation into conjugated metabolites because the body recognizes phytochemicals as xenobiotics. Therefore, after intake, concentrations of phytochemicals remain relatively low. Thus, these metabolites can partially reach the target tissues. Besides, they are rapidly excreted via urine and bile [28], limiting their tissue accumulation.
To our knowledge, this is the first report to evaluate meat quality in hair lambs supplemented with CTL. Meat quality characteristics (WHC, pH, WBSF, and cooking loss) were not influenced by CTL supplementation. The only change shown in these traits was an increase in some color variables. Information about the influence of CTL on meat color parameters is limited. In the current study, CTL supplementation improved L*, a*, and C* in the meat of lambs. In contrast, Hcini et al. [13] reported no change in the color of breast meat of turkeys supplemented with 1% and 2% CTL. No antioxidant status of lambs was measured in this work; however, the improvement observed in color parameters of lamb meat could be attributed to the antioxidant activity of CTL [49]. The antioxidant capacity of CTL is associated with the scavenging of reactive oxygen species (ROS) and transition metals or to the increased activity of endogenous antioxidant enzymes (glutathione peroxidase, superoxide dismutase, catalase, and nitric oxide synthase) by offering cofactors such as trace elements selenium, copper, zinc, and manganese within the CTL structure [13,50]. As mentioned above, we hypothesize that the microelements contained in CTL could reach muscle tissue and perform its antioxidant activity. However, future research is needed to demonstrate the fate and effect of CTL on meat quality of lambs.
The addition of FA or CTL to the lamb diet did not negatively affect the chemical composition of lamb meat. Moisture, ash, fat, and protein were maintained within the range reported by other authors [46,47] for hair lambs. Since the fat content was slightly higher than that reported in other studies, we believe that this result may be attributed to the older lambs used in this study. Mature animals deposit around 85% of their energy as fat [51].
In the current study, FA supplementation did not change the fatty acid profile, the overall fatty acids, or the nutritional indices of hair lambs, whereas CTL and FA × CTL positively modified the fatty acid profile. In agreement with our findings, Mallek et al. [52] reported a decrease in myristic (C14:0), palmitic (C16:0), and stearic (C18:0) acids in broilers’ fatty acid profile by zeolite supplementation as well as an increase in linolenic acid (C18:3ɷ3). Additionally, a study reported a reduction in DHA (C22:6 n-3) and eicosenoic acid (C20:1 n-9) in lambs’ fatty acid profile by monensin sodium supplementation (33 mg/kg of Rumensin 200® for 70 d) [53]. The potential pathways by which CTL or FA × CTL modify lambs’ fatty acid profile is unclear, but an enhancement of the fatty acid desaturase activity [13,52] or changes in the rumen microbial population [53,54], could be involved.
Fatty acids desaturases such as Δ9-desaturase introduce a double bond across carbons of a SFAs carbon chain. A study reported that a higher desaturase product in the muscle of supplemented kids (Terminalia chebula extract 2 and 6 mg/mL of rumen volume) is due to the action of the Δ9-desaturase enzyme, suggesting that the desaturase activity was influenced by the presence of phenolic compounds [55]. Also, Δ9-desaturase activity could be regulated by some elements in CTL [13-50,52]. Concerning the second pathway proposed, CTL ion-exchange activity may modify the ruminal environment, resulting in changes in the rumen microbial population, especially in cellulolytic bacteria [44]. Cellulolytic bacteria such as Ruminococcus albus y Butyrivibrio sp. are placed in the first group of rumen bacteria which hydrogenate unsaturated fatty acids. Butyrivibrio fibrisolvens have been used to explore the multiple biohydrogenation (BH) pathways. These bacteria can hydrogenate linoleic and alfa-linolenic acids to a conjugated form as the final product (without forming stearic acid as the end products) [54]. Based on the aforementioned, our results could be caused by a regulation of Δ9-desaturase activity or by a slight inhibition of the BH of unsaturated fatty acids [53,54], which may increase the total PUFA, n-6, and n-3 contents and enhance their nutritional ratios. Nevertheless, further studies are needed to show whether changes in fatty acid profile were modified via regulation of Δ9-desaturase activity or by changes in the ruminal microbial population.
In addition, although the ∑ PUFA, ∑ n-6, and PUFA/SFA ratios in our results did not reach significance, these values tended to improve, which may lead to healthier meat from a nutritional viewpoint [56]. Recently, there has been an interest in improving the nutritional indices of meat, as high values of PUFA/SFA and n-6/n-3 ratios represent a risk factor for cancer and coronary heart disease. The recommended ratio of n-6/n-3 is less than 4, and PUFA/SFA should be above 0.4 [56]. In general, in this study, the n-6/n-3 ratio was high for all treatments, which may have occurred due to the low content of omega-3 fatty acids found in our results. In the current work, DHA (C22:6 n-3) was not detectable, and eicosapentaenoic acid (EPA; C20:5 n-3) was found at a low concentration in lamb meat [47-53,57] in all treatments, which could explain the undesirably high n-6/n-3 ratio found in this work.
CONCLUSION
Feeding FA and CTL did not have any combined effect on the growth performance, carcass characteristics, wholesale cut yields, or meat quality of hair lambs. This study provides information about how adding FA reduces daily feed intake without compromising weight gain, which could represent an advantage for lowering feeding costs.
Additionally, this experiment provides new evidence that CTL supplementation improves some meat quality traits and the fatty acid profile of hair lambs. Nevertheless, further studies in ruminants, including a high CTL dose, are needed in order to understand the conditions of CTL beneficial effects on feedlot performance traits.
Factors such as dosage, age, diet composition, and environmental conditions need more investigation to elucidate the beneficial effects of FA and CTL supplementation on hair lambs. Besides, further research is necessary to investigate the effects of FA and CTL supplementation on the ruminal fermentation parameters and microbial populations of the rumen of hair-breed lambs to complement the findings in this work.