INTRODUCTION
The Food and Agriculture Organization of the United Nations predicts that the global population will increase to 9 billion by 2050, with global meat consumption projected to increase as a result [1]. A large amount of feed is required to meet this increase in animal meat consumption [2,3]. Most protein resources added to feed are highly dependent on imports, and the price of protein resources has been steadily rising in recent years [4]. Protein feed resources such as soybean schlegelii and fishmeal are expensive, and most of them are imported from abroad; therefore, resources are needed to replace them [5]. Interest in the use of insect proteins has increased with the increase in localization of protein sources imported from abroad.
Insects have generally been recognized as pests for a long time, but recently, the diversity and utility of insects have been recognized [6]. In the past, the perception of insects was repugnant, but recently, it has brought about a major change in the insect industry in that high-quality protein mass production is possible [7]. The traditional use of insects is limited to only a few areas, such as silkworms and beekeeping; however, they are now recognized as edible and their utility as practical biological resources is developing worldwide [8]. Insects are rich in proteins, unsaturated fatty acids (SFAs), vitamins, minerals, and fiber, and thus have a very high nutritional value [9]. Insect proteins are known to have high digestibility and contain essential amino acids; therefore, only a small amount of protein is required for animal growth [10]. Thus, the high nutritional value and function of insects makes them very promising as feed for livestock [10].
In this respect, research on insect feed has partially progressed, and Choi et al. [11] reported an improvement in broiler productivity when Hermetia illucens powder was added to feed. Jang et al. [12] indicated that the addition of H. illucens to feed improves the productivity and economy of ducks. Jang et al. [7] reported that insect feeding improves profitability by increasing the weight and gain of livestock and decreasing feed intake and feed demand.
Therefore, in this study, we intended to analyze the research trends of insects for feed, evaluate the potential of insects for feed, and suggest opinions for technology development to replace high-protein feed that depends on imports in the future.
APPLICATION OF INSECTS TO MEALS AND THEIR DIGESTIBILITY
The use of edible insects as feed is restricted to the production of fish, poultry, and pork, and does not include beef or eggs. This limitation depends on whether their natural diets are omnivorous [13]. When rearing these animals, feed digestibility is closely related to feed growth performance [14]. When manufacturing feed for animals, poor digestibility and nutritional value of feed could occur when using inaccurate and improper methods [15]. Digestibility can be changed by the species of insects, and the effect of various insect meals on digestibility is given in Table 1. Therefore, understanding the use of insects as feed is important for animal rearing. In this section, the effects of insects on the performance of feed for various animals are reviewed, focusing on the digestibility and nutritional value of insects.
| Targeted animal | Consisted insect species (life stage) | Composition of insect meal | Diets | Estimated ADC (%) | Recommended diets | Reference |
|---|---|---|---|---|---|---|
| Fish | ||||||
| Nile Tilapia Finger lings |
Nauphoeta cinerea (adult) Zophobas morio (larvae) Gromphadorhina portentosa (adult) Gryllus assimilis (adult) Tenebrio molitor (larvae) |
Mixture of 20% insect meal and 80% commercial meal |
Pellet type Three times daily for 15 days |
Dried matter (61.7–95.8) Crude protein (58.3–92.4) Energy (47.0–82.1) Lipid (87.9–98.8) Chitin (59.8–81.3) |
T. molitor meal | [18] |
| Gilthead sea bream | Tenebrio molitor (larvae) | Mixture of 25% and 50% insect meal and commercial meal |
Pellet type Two times daily for 16 days |
Dried matter (78.46 and 87.44) Crude protein (79.19 and 87.26) Ether extract (82.39 and 89.93) |
25% substituted meal | [19] |
| Pacific white shrimp |
Allomyrina dichotoma (larvae) Oxya chinensis (adult) Hermetia illucens (larvae) Protaetia brevitarsis (larvae) Tenebrio molitor (larvae) Gryllus bimaculatus (adult) Bombyx mori (pupae) |
Mixture of 30% insect meal and 70% commercial meal |
Pellet type Two times daily for 6 days |
Protein (82.8–89.0) Lipid (91.2–98.0) Energy (83.6–90.3) Dry matter (78.5–81.0) Chitin (28.0–35.5) |
A. dichotoma and H. illucens meal | [23] |
| Rainbow trout | Tenebrio molitor (larvae) | Insect substituted 0%, 25%, 50% commercial meal |
Pellet type Two times daily for 14 days |
Protein (90.1–92.2) No sigifnicant difference in dry matter, organic matter, and ether extract. |
25% substituted meal | [21] |
| Non-tested |
Hermetia illucens (larvae) Acheta domesticus (adult) |
Substituted 25%, 50%, 75% | Extruded pellet type | Not detected but expected improving digestibility after extruding | 75% substituted meal | [24] |
| Hybrid catfish figerlings |
Zonocerus variegatus (adult) Dung beetle (larvae) |
Substituted 0%, 10%, 20%, 40%, 60% blended (1:1) insect meal |
Pellet type Three times daily for 56 days |
Protein (88.50–90.11) Lipid (85.00–58.70) Fiber (55.26–57.22) |
40% substituted meal | [95] |
| Poultry | ||||||
| Broiler chicken |
Tenebrio molitor (larvae) Zophobas morio (larvae) |
0.2% and 0.3% full-fat meal | Ad libitum 35 days (1–14 days starter diets, 15–35 days grown diets) |
Protein (73–77) Ether extract (93–94) |
All treatments | [26] |
| Hermeita illucens (larvae) | 250 g/kg partially and highly defatted insect meal | Ad libitum 26–32 days |
Dry matter (59–63) Organic matter (64–69) Crude protein (62) Ether extract (93–98) Gross energy (50–61) |
Partially defatted insect meal | [28] | |
|
Tenebrio molitor (larvae) Hermetia illucens (larvae) |
250 g/kg two different insect meal | Ad libitum 26–35 days |
Dry matter (53–60) Organic matter (66) Crude protein (51–60) Ether extract (88–99) Gross energy (64–69) |
T. molitor consisted meal | [30] | |
| Hermetia illucens (larvae) | substituted 10% layer mash meal and 50:50 layer mash: fish offal meal | Ad libitum 16–28 days |
Dry matter Organic matter Ether extract |
10% substituted layer mash meal | [33] | |
| Laying hen | Hermetia illucens (larvae) | Partially defatted insect substituted 25% and 50% meal | Manually distributed 20 weeks |
Dry matter (64.29–70.29) Organic matter (67.23–73.66) Crude protein (76.06–81.12) Ether extract (89.33–90.83) |
25% substituted meal more suitable | [34] |
| Laying quail | Tenebrio molitor (larvae) | Substituted 5%, 10%, and 20% of protein of commercial meal | 54 days |
Dry matter (75.4–77.6) Organic matter (77.5–80.1) Crude protein (72.3–77.5) Ether extract (89.9–90.5) Calcium (79.1–79.7) |
5% substituted meal | [35] |
| Turkey | Hermetia illucens (larvae) | 50% and 100% insect fat extract substituted soy bean oil | Ad libitum 7–35 days |
Crude protein (83.10–84.88) Ether extract (96.38–07.13) |
All treatments | [36] |
| Duck |
Periplaneta americana (nymph) Hydrous cavistanum (adult) Tenebrio molitor (larvae) Zophobas morio (larvae) Bactrocera dorsalis (larvae) Hermetia illucens (prepupae) Musca domestica (larvae) Achroia grisella (larvae) Bombyx mori (larvae and pupae) Philosamina ricini (pupae) Allomyrina domesticus (adult) Gryllotalpa Africana (adult) Gryllus bimaculatus (adult) Gryllus testaceus (adult) Locusta migratoria (adult) Patanga succincta (adult) |
Dried ground insect | In vitro digestibility (stomach and small intestine) |
Organic matter (over 50%) Crude protein (over 20%) |
T. molitor
Z. morio A. grisella M. domestica B. mori P. Americana |
[37] |
| Pig | ||||||
| Piglets | Hermetia illucens (larvae) | Substituted 5%, 10%, and 20% commercial meal | Pellet type 4 weeks |
Dry matter (82.7–83.0) Crude protein (77.3–78.4) Starch (99.7–99.8) Crude fat (78.0–80.0) Acid detergent fiber (27.9–29.2) Amylase-treated neutral detergent fiber (36.9–39.4) Phosphorus (51.5–56.4) Ash (57.4–61.2) Energy (81.8–82.3) |
20% substituted meal | [39] |
| Hermetia illucens (larvae) | Defatted insect substituted 0, 5, and 10% | Ad libitum 61 days |
Dry matter (95.4–95.9) Organic matter (96.0–96.4) Crude protein (77.7–82.8) Ether extract (82.8–85.7) |
All treatments | [40] | |
| Ptecticus tenebrifer (larvae) | Substituted 0, 50, and 100% fishmeal |
Ad libitum Pellet type 35 days |
Dry matter (78.81–80.41) Nitrogen (78.57–80.27) Gross energy (79.28–79.46) |
100% substituted meal | [41] | |
| Growing pig | Tenebrio molitor (larvae) | Compared with fish, poultry, and meat meal | 2 weeks |
Dry matter (89.44) Gross energy (89.53) Crude protein (89.58) Total amino acid (89.60) |
Insect meal | [42] |
| Tenebrio molitor (larvae) | Compared with defatted and hydrolyzed T. molitor, fermented poultry offal, and hydrolyzed fishmeal | 2 weeks |
Dry matter (87.45, 89.47) Crude protein (86.37, 89.31) Crude fat (82.12, 89.80) Total amino acid (78.09, 79.52) |
Hydrolyzed T. molitor | [43] |
Fishmeal and oil have been consumed as aquafeed, but the high demand for seafood and rapid growth could be problems of conventional feeds, such as forage fish stock, environmental issues, and waste disposal. To address these problems, plant-based substitution feeds have been developed [16]. However, greater water and land efforts to reduce waste are needed to produce plant- and fish-derived meals. As an alternative to fish- and plant-derived meals, insect-derived meals have emerged with great potential as good protein suppliers [17]. When insects are used as fish feed, the species and conditions of the insects should be considered first. Fontes et al. [18] reared Nile tilapia fingerlings and used five different insects as feed alternatives. Among adults of Nauphoeta cinerea, Gromphadorhina portentosa, and Gryllus assimilis and larvae of Zophobas morio and Tenebrio molitor showed the highest apparent digestibility coefficient (ADC), while G. assimilis had the lowest ADC for Nile tilapia fingerlings [18]. Piccolo et al. [19] reared gilthead sea bream and used T. molitor as a substitute for commercial meals. When the meal was replaced with up to 25% T. molitor, the ADC of the replaced meal was similar to that of the control meal. However, there was a significant difference in the insect ratio and ADC of dry matter, crude protein, and ester extract when there was a high replacement ratio (50%) and ADC was lowest. Piccolo et al. [19] expected that the presence of chitin would inhibit digestion, and a reduction in nutrient digestibility was also shown in other studies [18,20,21]. Belforti et al. [21] estimated the effect of mealworms (T. molitor) as rainbow trout feed. Although a significant difference in ADC of dry matter, organic matter, and ether extract was not observed, a higher protein ADC was observed in control diets, and this value decreased when the substitution ratio was increased. However, low concentrations of chitin can improve growth performance because some fish have chitinase genes, and chitin degradation can improve chitin digestibility [22]. The high capacities of chitin to bind proteins and water, form ionic bonds with lipids, increase intestinal length, and react as a prebiotic could enhance the growth performance of gilthead sea bream [19,22]. Shin and Lee [23] tested the effects of mealworms, silkworms, rice grasshoppers, two-spotted crickets, dynastid beetles, and white-spotted flower chafers as fish feeds. The ADCs of the insect meals were 83%–89% protein, 91%–98% lipid, 84%–90% energy, 77%–81% dry matter, 28%–36% chitin, 76%–96% amino acids, and 89%–93% fatty acids. The growth performance of shrimp could be improved when black soldier flies or dynastid beetles were included as meals. These previous studies suggested that the selection of insects as fish feed should be considered carefully because apparent digestibility was not significantly different, but growth performance could be changed.
The processing conditions should also be considered to improve digestibility. Some processing methods have been developed to enhance the quality of protein sources [20]. Extrusion processing has been used to improve the digestibility and bioavailability of fish feed [24]. Irungu et al. [24] reported that freshwater shrimp in good quality extruded pellets can be substituted by 75% black soldier fly larvae or adult crickets. Although they did not estimate digestibility directly, they expected extrusion processing to enhance the digestibility of insect meal. Because of the increased water solubility and expansion ratio, which are closely related to feed digestibility, substitution of shrimp with insects might have the potential to improve the nutritional value of insect meal [24]. Likewise, the processing method is also considered to enhance the digestibility of insect meal.
The apparent digestibility of insect feeds for poultry differed according to the condition of the insect feed. The size and weight of mealworms and substrates have significant effects on the nutrient and amino acid values of broilers [25]. Various bioactive components in insects, such as chitin, melanin, and peptides, can enhance the health of poultry [26]. Addition of small amounts of insects can enhance poultry health. Small amounts of 0.2%–0.3% supplementation with mealworm full-fat meal did not have any negative effect on ileal digestibility and reduced the potential pathogenic bacteria in poultry [26]. In poultry production, broilers and laying hens are the major sources of meat and eggs, and most studies have focused on rearing broilers [27]. When feeding H. illucens (black soldier fly) to broiler chickens, defatted black soldier flies could be used for more efficient nutrient digestion [28,29]. Defatting had a significant effect on ADC, and highly defatted insects had lower ADC values than partially defatted insects [28]. Therefore, fully defatted insects may not be helpful in increasing the digestibility of poultry. De Marco et al. [30] compared the digestibility of two different insect species (T. molitor and H. illucens). There was a significant difference in the ADC of the ether extract only; H. illucens had a higher ADC value [30]. Significant differences in the ADC of dry matter, organic matter, crude protein, gross energy, and in amino acid apparent ileal digestibility between T. molitor and H. illucens were not observed in broilers [30]. However, insect feed should be carefully considered. Chitinase has been found in the proventriculus and hepatocytes of poultry [31]. However, chitin supplementation inhibits nutrient absorption from the intestinal tract, and increased chitin content negatively affects protein digestibility [32]. In addition, the type of supplement should be carefully chosen. When diet included 10% H. illucens in layer mash meal (IM1), there was no significant effect on the apparent digestibility of nutrients, mortality, or carcass yield compared with commercial meals. Although digestibility was higher for dry matter and organic matter than in the IM1 group when the 10% H. illucens diet included a 50:50 layer mash: fish offal meal, the growth rate and carcass weight of broilers were affected negatively. This result may be due to the reduced ADC of the ether extract and different metabolizable energies of starch and offal [33]. The apparent digestibility of laying hens is also affected by supplementation with insects [34]. Significant differences in apparent ileal digestibility between commercial meal and 25% H. illucens substituted meal were not observed, and the ADC of crude protein was reduced after substitution. However, meals substituted by over 25% reduced the ADCs of dry matter and organic matter [34]. They suspected chitin content, which has a high protein binding capacity, as a reason for the reduced ADC values of laying hens. However, chitin can also reduce serum cholesterol and triglyceride levels. Secci et al. [35] described T. molitor as a novel protein source for laying quails. The ADC of dry matter, organic matter, and crude protein was reduced with increasing amounts of substituted insects, but albumin and yolk weight increased. Thus, H. illucens might be a more suitable source than T. molitor for laying hen feed.
Insects have been used and studied as novel nutritional feed for poultry. Sypniewski et al. [36] used H. illucens fat extract as a soybean oil replacement and found no significant effect of substituted fat on the ADC of crude protein and ether extract. Kovitvadhi et al. [37] estimated the in vitro digestibility of ducks from 17 different insects and compared the digestibility with commercial meals (fishmeal, chicken/pork offal, and soybean). Among the 17 species in the study, T. molitor, Z. morio, Achroia grisella, Musca domestica, Bombyx mori, and Periplaneta americana were recommended as substitutes for commercial meals. When the correlation coefficients between chemical composition and digestibility were estimated, a significant negative coefficient was observed between fiber components and digestibility of organic matter and crude protein. Indigestible components, such as chitin, can inhibit the digestibility of insects. However, chicken has an mRNA gene cord of chitinase within the glandular stomach, which can act as a health promoter for poultry [27]. In conclusion, although apparent digestibility can be slightly reduced, the use of insects as poultry feed can improve the health of poultry.
The huge feed costs (60%–70% of total costs), increased grain prices, environmental problems, increased consumption, and insufficient supply can be reasons for shifting to the use of insects as novel feed when producing pork [38]. Owing to these problems, insects have been used as high-quality protein resources to produce pork. Håkenåsen et al. [39] studied pellet-type feed and full-fat H. illicens as a an alternative. They replaced 5%, 10%, and 20% of commercial meals with insects, and observed that the ADC of crude protein was reduced slightly when insect ratio increased. They are related to the reduced ADC of crude protein and chitin contents. In addition, an overestimation of protein content might be another reason for the decline in ADC [39]. However, the ADC of crude fat, phosphorus, and ash increased gradually, which might be due to the increased amounts of fat, phosphorus, and ash [39]. Biasato et al. [40] defatted H. illucens and used it as a replacement. In full-fat H. illucens, there was no significant effect on ADC regardless of the type of nutrient when fed partially defatted H. illucens [40]. When supplying Ptecticus tenebrifer as a replacement of fishmeal in piglets, the ADC of dry matter and nitrogen was slightly reduced [41]. Insect species, life stage, dietary inclusion, and processing methods can affect the ADC of nutrients, and similar results have been reported in piglets [6, 38–41]. The ADC of pigs are changed when they grow from piglets to pigs, and the differences may be due to the changes in digestibility between piglets and pigs [40–42]. Yoo et al. [42] reported a non-significant effect of T. molitor on ADC when used as a replacement supplement for fish, poultry, and meat meal. Therefore, T. molitor has good potential as a replacement for conventional feed, such as fish, poultry, and meat for growing pigs [42]. Cho et al. [43] compared the effects of defatted and hydrolyzed T. molitor on the ADC. Hydrolyzed T. molitor had higher ADC values for dry matter, crude protein, crude fat, and total amino acids than hydrolyzed fishmeal and fermented poultry offal [43]. Insect supplementation can affect digestibility, feed efficiency, and quality characteristics of finishing pigs (slaughtered pigs). According to Chia et al. [44], meal-fed insects had a higher carcass weight, feed conversion ratio, and crude protein content than conventional fishmeal-fed pigs. In conclusion, insect species, life stage, and processing methods can be carefully selected to enhance the digestibility of livestock, and these factors can affect the final quality of livestock production, such as carcass weight, quality of egg, and nutritional composition of production.
BIO-FUNCTIONAL PROPERTIES EXPECTED FOLLOWING INSECT MEAL APPLICATION
Many studies have reported the excellent biofunctional activity of various components of edible insects, such as lipids, proteins, and chitin. Representative biofunctional properties include antioxidant, anti-hypertensive, anti-cancer, anti-inflammatory, anti-obesity, anti-diabetic, and anti-microbial activities [10]. Many insect species with biofunctional properties have been reported, including H. illucens [45], B. mori [46], M. domestica [47], T. molitor [48], Acheta domesticus [49], and Spodoptera littoralis [50]. The reported functional properties of these insects vary according to the growth status of the insect (larvae, pupae, and adults), insect components (protein, fat, and chitin), and extraction method (water extracts, solvent extracts, and enzymatic hydrolysates) [10]. Accordingly, insects can be selectively used in animal diets, based on the functionality required for rearing animals. In this section, the biofunctional properties that can be obtained by replacing the protein or lipid components of animal feed with various insect components are reviewed and summarized (Table 2).
| Targeted animal | Consisted insect species | Composition of insect meal | Bio-functional properties | Effect mechanisms | Reference |
|---|---|---|---|---|---|
| Poultry - Laying hens | Hermetia illucens larvae meal (defatted) | 7.3%, 14.6% | Gut health and microbiota |
- Positive effect on the morphology of the small intestine and the activity of brush border enzymes and cecal microbiota - Increases the production of VFAs |
[51] |
| Poultry - Laying hens | Hermetia illucens larvae meal (defatted) | 17% | Gut health and microbiota |
- Increases relative abundance of Elusimicrobia, Lentisphaerae and Cyanobacteria in the gut - Decreases relative abundance of Fusobacteria in the gut - Increases production of SCFAs (acetate, propionate, and butyrate) |
[52] |
| Poultry - Broiler chickens | Hermetia illucens larvae meal (defatted) | 5%, 10%, and 15% | Gut health and microbiota |
- Positive effect on the cecal microbiota and gut mucin dynamics - Increases the ratio of L-Ruminococcus, Faecalibacterium, and Blautia in cecal - Increases in villi mucins |
[53] |
| Poultry - Broiler chickens | Tenebrio molitor larvae meal (full-fat) | 7.5% | Gut health and microbiota |
- Increases relative abundance of Clostridium, Oscillospira, Ruminococcus, Coprococcus, and Sutterella - Decreases relative abundance of Bacteroides |
[56] |
| Poultry - Broiler chickens | Shelfordella lateralis imago meal (full-fat) | 0.05%, 0.1%, and 0.2% | Gut health and microbiota |
- Increases the number of total microbiota counts, Clostridium leptum subgroup, and Clostridiumcoccoides-Eubacterium rectale in the crop - Increases the number of Lactobacillus spp./Enterococcus in the ileum |
[57] |
| Poultry - Broiler chickens | Tenebrio molitor larvae meal, Zophobas morio larvae meal | 0.2%, 0.3% | Gut health and microbiota |
- Stimulates the colonization of cecal probiotics - Increases relative abundance of Actinobacteria by Z. morio larvae meal - Increases relative abundance of family Ruminococcaceae by T. molitor larvae meal |
[58] |
| Poultry - Turkey | Hermetia illucens fat | 2.5%, 5% | Gut health and microbiota |
- Decreases the activity of trypsin - Reduces the proliferation of potentially pathogenic bacteria |
[36] |
| Weaning piglets | Hermetia illucens larvae meal (full-fat) | 1%, 2%, and 4% | Gut health and microbiota |
- Increases the number of probiotic bacteria (Lactobacillus and Bifidobacterium) and the concentrations of lactate and SCFAs - Decreases the number of Escherichia coli - Down-regulates the expression of TLR4, NF-κB, MyD88, and TNF-α - Up regulates the expression of anti-inflammatory IL-10 |
[60] |
| Weaning piglets | Hermetia illucens larvae meal | 5%, 10% | Gut health and microbiota |
- Increases the β-diversity - Increases relative abundance of Blautia, Coprococcus, Eubacterium, Prevotella, and Roseburia |
[61] |
| Poultry - Laying hens | Hermetia illucens larvae meal (defatted) | 7.3%, 14.6% | Immune activity |
- Shows low albumin/globulin ratio - Decreases the contents of serum cholesterol and triglyceride |
[34] |
| Poultry - Broiler chickens | Hermetia illucens larvae meal | 1%, 2%, and 3% | Immune activity |
- Increases the percentage of CD3+CD4+ T lymphocytes in the spleen - Increases the proliferation of spleen cells and serum lysozyme activity |
[65] |
| Poultry - Broiler chickens | Tenebrio molitor larvae meal | 30% | Immune activity |
- Shows low albumin/globulin ratio - Increases the concentration of aspartate aminotransferase and alanine aminotransferase |
[66] |
| Poultry - Broiler chickens | Tenebrio molitor larvae meal (full-fat) | 5%, 10%, and 15% | Immune activity |
- Increases the number of erythrocytes - Shows low albumin/globulin ratio - Increases the concentration of gamma glutamyl transferase |
[67] |
| Poultry - Turkey | Hermetia illucens fat | 2.5%, 5% | Immune activity | - Decreases IL-6 and TNF-α concentrations in serum | [36] |
| Weaning piglets | Hermetia illucens larvae meal (full-fat) | 1%, 2%, and 4% | Immune activity |
- Decreases IFN-γ concentrations in serum - Increases IL-10 and IgA concentrations in serum |
[68] |
| Growing pigs | Hermetia illucens larvae meal (full-fat) | 9%, 12%, 14.5%, and 18.5% | Immune activity |
- Increases the number of neutrophils - Shows the number of lymphocytes outside the normal physiological range |
[71] |
| Poultry - Broiler chickens | Hermetia illucens larvae meal | 1%, 2%, and 3% | Antimicrobial activity |
- Decreases the number of S. Gallinarum in infected tissues (liver, spleen, bursa, and cecum) - Increases the survivability of infected chickens |
[65] |
| Poultry - Broiler chickens | Tenebrio molitor meal, Zophoba morio meal | 0.4% | Antimicrobial activity |
- Increases the concentration of IgG and IgA - Decreases the content of pathogenic bacteria E. coli and Salmonella spp. in the cecal - Increases the survivability of infected chickens |
[73] |
| Weaning piglets | Tenebrio molitor meal, Musca domestica larvae meal, Zophoba morio meal | 5% | Antimicrobial activity | - Reduces incidence of diarrhea between 15 and 28 day | [76] |
| Weaning piglets | Hermetia illucens larvae/prepupae meal (full-fat) | 4%, 8% | Antimicrobial activity |
- Inhibits the growth of D-Streptococci in vitro - Reduces D-Streptococci 0.5 log fold in the gut of piglets |
[78] |
| Rabbits | Hermetia illucens larvae fat, Tenebrio molitor larvae fat | 1.5% | Antimicrobial activity |
- Increases the production of VFAs in the cecum - Increases the microbial diversity of cecal of rabbits |
[80] |
Many studies have been conducted on the use of defatted insects to replace soybean, which is generally used as a protein source, in animal feed. Moniello et al. [51] reported that the replacement of soy protein with an H. illucens larvae meal had an effect on small intestine morphology and brush border enzymatic activity, as well as cecal microbial activity. They explained that chitin present in the H. illucens larvae meal induced an increase in volatile fatty acids (VFAs), such as acetate and butyrate, which may have a positive effect on the gut health of laying hens.
Borrelli et al. [52] also reported that a diet of H. illucens larvae affects the production of cecal microbiota and short-chain fatty acids (SCFAs) in laying hens. They confirmed that Elusimicrobiota, Lentisphaerae, and Cyanobacteria were increased in the insect diet group through 16S rDNA sequencing analysis, whereas Fusobacteria were decreased compared to the soybean diet group used as a control group. Furthermore, the insect diet groups showed increased production of SCFAs, such as acetate, propionate, and butyrate. It has been reported that an increase in SCFAs is because chitin contained in insect meal acts as a prebiotic and produces SCFAs molecules together with intestinal bacteria. Consequently, it has been reported that SCFAs may help to promote gut and overall health of laying hens.
Biasato et al. [53] conducted a study that confirmed the positive effect of H. illucens larvae meal (5%) intake on the cecal microbiota and gut mucin dynamics in an experiment with broiler chickens. In particular, they reported that the ratio of Ruminococcus, Faecalibacterium, and Blautia in the ratio of the cecal microbiota was higher in the group fed 5% H. illucens larvae meal compared to the control group. This microbial group is well known to produce butyric acid and SCFAs, which are known to play a major role in optimal intestinal health and have the ability to inhibit enteric pathogens [54,55].
Many studies have confirmed the expression of bio-functional properties using insect diets that include fats without the defatting process. Biasato et al. [56] also reported that full-fat T. molitor larvae meal significantly affected the microbiota composition of chickens. The relative abundances of Clostridium, Oscillospira, Ruminococcus, Coprococcus, and Sutterella genera were higher in the T. molitor diet group than in the control group, whereas the relative abundance of the Bacteroides genus was lower than that in the control group. These changes in the gut microbiota were made without any specific effect on intestinal morphology and mucin composition, and it was reported that the use of the T. molitor diet would not have a negative effect on the gut health of birds.
Józefiak et al. [57] reported that supplementation with 0.2% of Shelfordella lateralis improved the body weight gain, feed intake, and feed conversion ratio in broiler chicken experiments. In addition, analysis of the gastrointestinal tract (GIT) microbiota showed that the total microbiota counts, Clostridium leptum subgroup, and Clostridium coccoides–Eubacteriumrectale of the S. lateralis diet group increased in the crop. The ileum analysis confirmed an increase in Lactobacillus spp./Enterococcus spp. in the S. lateralis diet group.
Józefiak et al. [58] studied the effects of insect addition (Z. morio and T. molitor) on the cecal commensal microbiome of chickens. The two insect diets had different effects on the chicken cecal microbiome. The Z. morio diet increased the relative abundance of Actinobacteria (including Bifidobacteriaceae), while the T. molitor diet significantly increased the relative abundance of Ruminococcaceae. The authors explained that this microbial community change could help prevent pathogenic bacterial infections by stimulating the colonization of cecal probiotics.
On the other hand, there are studies that extract only lipids from insects to replace the lipid components in feed and then confirm the expression of physiological activity. Sypniewski et al. [36] reported a positive effect of replacing soybean oil with H. illucens fat on turkey nutrition. In their study, it was confirmed that when soybean oil was replaced with H. illucens fat, trypsin activity decreased without any negative effect on growth performance, nutrients, or energy utilization. It is known that excessively increased trypsin activity can cause stunting syndrome in birds [59]. It was also reported that a H. illucens fat diet reduced the proliferation of potentially pathogenic bacteria. It was explained that this was due to the lauric acid and medium-chain fatty acids (MCFAs), which were effective against the pathogenic bacteria of poultry, in H. illucens fat through a fat profile analysis.
A study on the effect of an insect diet on changes in the intestinal microbiome of pigs and poultry was conducted. Yu et al. [60] conducted a study on the changes in the gut microbiota that appeared when fishmeal was replaced with H. illucens larvae in the diet of weaning piglets. The authors reported that a 2% H. illucens diet affected specific ileal and cecal bacterial populations, metabolic profiles, and ileal immune status in weaning piglets. When the 2% H. illucens diet was applied, the number of probiotic bacteria (such as Lactobacillus and Bifidobacterium), and the concentrations of lactate and SCFAs increased, whereas the number of Escherichia coli decreased. This results in the down-regulation of the expression of Toll-like receptor 4 (TLR4), uclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), myeloid differentiation factor 88 (MyD88), and tumor necrosis factor (TNF)-α, which are negatively correlated with SCFA- and lactate-producing bacteria; and on the other hand, increase the expression of anti-inflammatory interleukin (IL)-10. In addition, it was reported that the H. illucens diet maintained the health and immune status of the ileum by increasing the expression of mucin 1 (MUC1), zonula occludens (ZO)-1, occludin, and Claudin-2, which are genes related to the barrier function of the ileum.
Biasato et al. [61] reported that the β-diversity, indicating the diversity of the microbial community, was increased in the weaned piglet experimental group fed the H. illucens diet compared to the control group. As a result of the analysis of the cecal microbiota of the H. illucens diet group, it was confirmed that Blautia, Coprococcus, Eubacterium, Prevotella, and Roseburia were predominant. These microbial taxa are known to be involved in polysaccharide degradation and fermentation, and promote the production of SCFAs (mainly butyrate).
It is important to properly control the intestinal microbiota during livestock breeding. This is evidenced by the growing importance of the gut microbiome in a variety of health-related studies on humans and livestock [62,63]. It is thought that it will be possible to manage the health of livestock more easily by increasing the ratio of beneficial bacteria and lowering the ratio of harmful bacteria in the intestinal microbiome of livestock. Combining previous studies, it was confirmed that various factors, such as the type of insect, whether or not the insect was degreased, and the type of ingredients affected the intestinal environment of livestock. Furthermore, it was confirmed that there was a difference in the effect of the insect diet on changes in the intestinal environment according to the type, sex, and age of the livestock.
Studies have confirmed an improvement in immunity in livestock when an insect diet is used. Bovera et al. [34] reported that the level of serum globulin increases when laying hens are bred by replacing 25% or 50% of soybean meal with H. illucens larvae meal. This results in a lower albumin-to-globulin ratio, which results in better resistance to disease and better immune response. In addition, it was reported that the levels of serum cholesterol and triglycerides were significantly lower in both H. illucens larvae meal groups than in the control group (soybean meal). This was attributed to the effect of chitin in the H. illucens larvae meal. According to Hossain and Blair [64], the presence of positively charged chitin lowers cholesterol and triglyceride contents by attracting negatively charged bile acids and free fatty acids.
Lee et al. [65] studied the immunoprophylactic effects of H. illucens larvae against Salmonella enterica serovar Gallinarum as a feed additive for breeding broiler chicks. In the 2% and 3% H. illucens larvae meal diet groups, it was confirmed that the percentage of CD3+CD4+ T lymphocytes in the spleen increased. In addition, the proliferation of spleen cells and serum lysozyme activity increased. These results suggest that H. illucens larvae meal exhibits prophylactic properties by stimulating the non-specific immune response in chicks.
Bovera et al. [66] studied the potential of T. molitor larvae as a substitute for soybean meal in broiler diets. These authors also confirmed that the lowest albumin/globulin ratio was observed in the T. molitor feed group. They explained that this result might have been due to the prebiotic effect of chitin present in the T. molitor meal.
Biasato et al. [67] studied the effects of T. molitor larvae meal on the health of female broiler chickens. In this study, feed supplementation with T. molitor larvae was shown to improve body weight and feed intake, but partially decreased feed efficiency. In addition, a positive effect was observed in the analysis of the hematochemical parameters, which resulted in an increase in erythrocytes and a decrease in albumin and gamma glutamyl transferase (GGT). A lowered albumin concentration induces a decrease in the albumin/globulin ratio, which increases resistance to disease. Additionally, high GGT concentrations are used as indicators of liver disease and impaired bile flow. Therefore, it can be seen that the reduction of GGT according to T. molitor larvae meal has a positive effect on immunity.
Meanwhile, Sypniewski et al. [36] reported that the replacement of soybean oil with H. illucens fat in turkey nutrition had a supportive effect on the immune response. The H. illucens fat-diet group showed a decrease in serum IL-6 and TNF-α concentrations. These are well-known factors related to GIT inflammation, confirming that a H. illucens fat diet can help relieve GIT inflammation.
Studies have also reported improved immunity in pigs fed an insect diet. Yu et al. [68] studied the effects of replacing fishmeal with full-fat H. illucens larvae in the diets of weaning piglets. Dietary supplementation with H. illucens larvae meal decreased the levels of the pro-inflammatory cytokine interferon (IFN)-γ while increasing the serum levels of the anti-inflammatory cytokines IL-10 and immunoglobulin A (IgA). It has been reported that H. illucens larvae meal has a positive effect on systemic immunity. Cytokines play a major role in immune and inflammatory responses, and a proper balance between pro-inflammatory and anti-inflammatory cytokines is important in preventing infection [69]. Therefore, proper regulation of pro- and anti-inflammatory cytokines (IFN-γ and IL-10) by H. illucens meal can have a positive effect on animal immunity. In addition, serum IgA, a major component of humoral immunity in mammals, plays a major role in protecting the extravascular compartment against microbes [70].
Chia et al. [71] reported that supplementation with H. illucens larvae meal to replace fishmeal significantly improved immunity in growing pigs. In this study, it was reported that, as the proportion of H. illucens replacing fishmeal increased, the number of neutrophils significantly increased to the normal physiological range. These neutrophils are known to play a major role in wound healing through microbial sterilization and macrophage attraction [72]. In addition, it was confirmed that the number of lymphocytes in the group fed H. illucens larvae meal was outside the normal physiological range, which may be a result of stimulation of the cellular and humoral immune response systems of pigs.
In summary, many studies have reported that an insect diet improves the immune activity of animals. It has been shown that insect diets lower the albumin/globulin ratio to increase the prevention of diseases or enhance immunity by increasing the activity of immune-related enzymes (such as aspartate aminotransferase, alanine aminotransferase, and GGT). In addition, it has been confirmed that insect diets improve immune activity by increasing the activity and number of immune cells, such as lymphocytes, splenocytes, red blood cells, and neutrophils; and helps in immune regulation by regulating the expression of cytokines related to inflammation and immunity.
The antibacterial activity of insect components is utilized as a good biofunctional property in breeding animals. Lee et al. [65] reported that H. illucens larvae diet showed excellent antibacterial effects against Salmonella Gallinarum in broiler chicks. The H. illucens diet enhanced bacterial clearance and increased the survivability of broiler chicks to S. Gallinarum. The number of viable bacterial cells in the tissues of the H. illucens diet group tended to decrease during the entire experimental infection period compared to that in the control group. The number of S. Gallinarum decreased in all tissues, including the liver, spleen, bursa, and cecum. The authors also reported that the survivability of chicks infected with S. Gallinarum increased with increasing H. illucens dietary feed concentrations.
Studies on the application of fermented insects in animal diets have also been conducted. Islam and Yang [73] studied the antimicrobial effect of a diet containing fermented T. molitor and Z. morio larvae using probiotics (Lactobacillus plantarum and Saccharomyces cerevisiae mixture) in chicks. The effects of a fermented T. molitor and Z. morio diet on mortality, immunity, and cecal and fecal microbiota in chicks infected with Salmonella Enteritidis and E. coli were studied. The authors reported that the increased mortality due to pathogenic infection was significantly reduced by a fermented insect diet. In addition, it has been reported that immunoglobulin G (IgG) and IgA levels are significantly increased when feeding with a fermented insect diet. Analysis of the microbiota of cecal and fecal samples revealed that the content of pathogenic bacteria E. coli and Salmonella spp. in the cecum was reduced. The authors explained that this was the combined effect of chitin contained in the insect diet and probiotics used during fermentation. Various studies have reported that chitin in exoskeletons has antioxidant and antibacterial effects against bacteria, mold, and yeast [74]; and metabolites produced during the growth of probiotics have been reported to have excellent antibacterial activity against pathogenic microorganisms [75]. Therefore, it has been demonstrated that fermented insect diets can replace the use of antibiotics in chick rearing.
Studies on the antibacterial activity of insects in animal diets have been conducted not only in poultry, but also in pigs and rabbits. Ji et al. [76] conducted a study to confirm the feasibility of using T. molitor, M. domestica larvae, and Z. morio powder as protein sources for piglets. It has been reported that early weaning in pig breeding can often increase the incidence of diarrhea and mortality, and cause stress and growth retardation in piglets [77]. Therefore, special attention is required when early weaning is performed. The authors conducted an experiment in which 5% of insect feed was added at the beginning of weaning, and as a result, it was confirmed that the incidence of diarrhea significantly decreased between 15 and 28 days. The antibacterial peptides present in the insect diet are thought to help prevent intestinal inflammation and mucosal damage.
Spranghers et al. [78] conducted a study to confirm whether a H. illucens larvae/prepupae diet had gut antimicrobial activity in piglets through in vitro and animal trials. First, it was confirmed that treatment with full-fat H. illucens larvae/prepupae meal significantly inhibited the growth of Group D-streptococci in vitro. Second, when animal trials were conducted, only a 0.5 log fold reduction was observed for Group D-streptococci in the gut of piglets fed H. illucens larvae/prepupae-containing diets. The authors explained that H. illucens larvae/prepupae have a high fat content, especially lauric acid, which is known to be the highest among them. This lauric acid is known to have a strong growth inhibitory activity against gram-positive bacteria [79], which is a major factor in the antibacterial activity of the H. illucens larvae/prepupae diet.
Dabbou et al. [80] also reported a study result showing that when soybean oil was replaced with insect (H. illucens larvae and T. morio larvae) oil, the antibacterial effect was superior to that of soybean oil due to the lauric acid present. In vitro tests showed that H. illucens oil had superior antibacterial activity compared to T. morio oil. This was explained by the high content of SFAs in H. illucens, and among them, the content of lauric acid is high. This effect was also confirmed in animal experiments using rabbits. It has been reported that when a diet containing H. illucens and T. morio oil was provided, the production of VFAs in the cecum was increased and the microbial diversity of the cecum was increased. High diversity is generally known to increase resistance to invading pathogens and help regulate animal responses to stress [81].
In summary, it was confirmed that various components with antibacterial activity had a positive effect when an insect diet was carried out in animal breeding. Materials present in insect diets (chitin, saturated fatty acids [especially lauric acid], antibacterial peptides, etc.), metabolites produced when insect diets are processed (VFAs), and probiotics present in fermented insect diets have been confirmed to show antibacterial activity. These ingredients with antibacterial activity reduced the incidence of diarrhea and mortality caused by pathogenic bacterial infection in animals, and were confirmed to be effective in relieving intestinal inflammation and preventing stress caused by pathogenic bacterial infection.
SAFETY OF INSECTS AS FEED MATERIALS
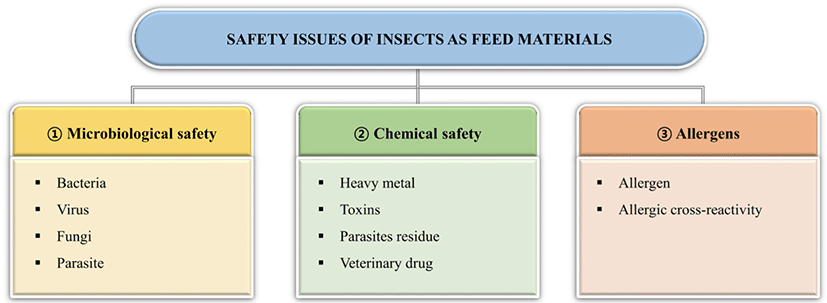
With growing interest in and efforts to develop insect-based feed, concerns about the safety of insect-fed animals are increasing. The challenges of using insects as feed include microbiological and chemical hazards and allergens [82]. Although more long-term studies are required to evaluate the safety and adequacy of insect-based feed, the current questions on safety is presented in this section. The potential safety risks of insect-based feed to animals are summarized in Fig. 1.
Because insects are reared in crowded production units, microorganisms, including bacteria, fungi, and viruses, can easily spread. In recent years, an increasing number of studies have proposed microbiological hazards of insects as animal feed. When Netherlands Food and Consumer Product Safety Authority [83] investigated the microbiological status of 55 freeze-dried insects, including mealworms and locusts, more than half of the insects had a total amount ofaerobic bacteria exceeding 6 log CFU/g, and Enterobacteriaceae had more than 3 log CFU/g. According to Vandeweyer et al. [84], Clostridium spp., Staphylococcus spp., and Bacillus (cereus group), as well as the fungi Aspergillus and Penicillium are regularly found in insect species. In this study, Clostridium spp. and B. cereus were spore-forming bacteria, and their endospores were more difficult to eliminate than their vegetative cells. Viruses are also an important issue in the use of insects as feed. In general, human viruses taxonomically related to insects are unable to replicate in insects and are safe for human health. However, some viruses (norovirus, hepatitis A, hepatitis E, and rotavirus) can be introduced with substrates into insect production units and transferred to humans [82]. Maciel-Vergara and Ros [85] reviewed the viruses of insects commonly reared as feed and provided a few strategies for the prevention and management of insect viral diseases.
Another microbiological hazard to insects is their potential to harbor and transmit parasites through the oral route. Some species, including P. americana and Blatella germanica, have been demonstrated to harbor pathogenic protozoa such as Giardia lamblia, Gongylonema pulchrum, Entamoeba histolytica, Sarcocystis spp., and Toxoplasma spp. [82]. When Müller et al. [86] investigated the transmission of parasites via black soldier fly larvae feed, less than 1% of the parasital oocysts of Eimeria tenella, Eimeria nieschulzi, or Ascaris suum eggs where present in the larval gut. This indicates a low possibility of contamination, but it is difficult to neglect the potential risk of parasite transmission when using black soldier fly larvae as animal feed [84].
Chemical contamination can also occur in insects. Chemical contaminants include heavy metals, mycotoxins, pesticides, and veterinary drugs [82]. Among these chemicals, heavy metals such as lead (Pb), arsenic (As), mercury (Hg), and cadmium (Cd) can accumulate in insect bodies and affect the health of animals fed with insects. Accumulation in insects depends on the type of heavy metal, substrate, and insect species [87]. Mycotoxins are toxic secondary metabolites produced by certain fungi, including Aspergillus spp., Fusarium spp., and Penicillium spp., and are capable of causing diseases in animals. However, there was no distinct evidence of mycotoxin accumulation in insects when feeding trials were conducted. Further analysis is required regarding the accumulation of mycotoxins in insects [88]. To date, limited studies have been conducted on the accumulation of pesticide residues, veterinary drugs, and hormones in insects, but most have reported that these chemicals can be degraded during insect growth [82].
An allergy is a hypersensitivity reaction initiated by specific immunological mechanisms [89]. According to van der Fels‐Klerx et al. [82], the insects which can cause anaphylactic shock are grasshoppers, locusts, silkworm pupae, cicada, and bee larva and pupa. To use insects widely as animal feed, their allergenicity should be investigated. Chitin is an allergenic material found in insect cuticles. Fortunately, smaller chitin particles can reduce the inflammatory response [38].
Allergy by the intake of insects can be due to cross-reactivity with another allergen. Cross-reactivity in allergic reactions occurs when allergies to related proteins induce a similar allergic response [89]. Previous studies have reported that tropomyosin and arginine kinase are possible proteins responsible for the cross-reactivity of allergens in edible insects [82]. Novel processing techniques are required to reduce the allergic potential of insect proteins and make them a more sustainable animal feed.
REGULATIONS FOR UTILIZATION OF INSECTS AS FEED
Guidelines or regulations are needed for the use of insects as animal feed because of various safety issues. This regulatory system using insects as feed differs in different countries because each country has its own laws and background history [15]. This section discusses feed regulations in different countries, as it is the most critical issue for insect businesses. The regulations on the use of insects as feed are summarized in Table 3.
| Country | Authority | Regulation and content | Reference |
|---|---|---|---|
| European Union (EU) | European Food Safety Authority (EFSA) |
▪ Regulation: EU Decisions/regulations ▪ New feed materials needs authorization. ▪ Seven insect species (black soldier fly, house fly, yellow mealworm, lesser mealworm, house cricket, banded cricket, and field cricket) reared with feed materials which are approved in the EU regulation were permitted for use in feed for aquaculture. |
[90] |
| United States | Federal Food and Drug Administration (FDA) & Association of American Feed Control Officials (AAFCO) |
▪ Regulation: Federal Food, Drug, and Cosmetic Act (FFDCA) ▪ New feed materials needs authorization, but normal feed rules were applied to insects (additive approval or GRAS needed for insects). ▪ Black soldier fly is permitted for use in feed for aquaculture. . |
[90] |
| Canada | Canadian Food Inspection Agency (CFIA) |
▪ Regulation: Feeds Act and the Feeds Regulations (FAFR) ▪ New feed materials needs authorization. ▪ Black soldier fly is permitted for use in feed for aquaculture and all poultry. |
[15] |
| Korea | The Ministry of Agriculture, Food, and Rural Affairs (MAFRA) |
▪ Regulation: Control of Livestock and Fish Feed Act ▪ New feed materials needs authorization. |
[92,93] |
| China | The Ministry of Agriculture and Rural Affairs |
▪ Regulation: Administrative Measures for Feed and Feed Additives ▪ New feed materials require authorization. |
[15] |
| Japan | The Ministry of Agriculture, Forestry and Fisheries |
▪ Regulation: Act on Safety Assurance and Quality Improvement of Feeds ▪ New feed materials require authorization. |
[90] |
| Australia | Australian Pesticides and Veterinary Medicine Authority (APVMA). |
▪ Regulation: APVMA Good Manufacturing Practice, Australian animal feed industry codes of practice, and an Australian Standard for animal feed manufacture. ▪ New feed materials do not require authorization if it meets specific requirements. |
[38] |
| Nigeria | National Agency for Food and Drug Administration and Control (NAFDAC) |
▪ Regulation: NAFDAC Act ▪ There are not yet specific regulation for insect feed. |
[94] |
EU regulations regarding the use of insects as feed are greatly affected by bovine spongiform encephalopathy (BSE), a progressive neurological disorder in cattle that can be transmitted to humans by eating beef [15]. Because BSE possibly originated as a result of feeding cattle proteins, the use of processed animal proteins as feed material was banned in 2001. A few years later, in 2013, the ban was modified so that processed animal protein, except for ruminants, can be used as feed in aquaculture. This change is essential because most farmed fish are carnivorous [90]. In the 2017 newly amended catalogue of feed materials, processed animal proteins and fats from invertebrates were permitted as feed materials. Accordingly, seven insect species can be used for inclusion in aquaculture diets: house crickets (A. domesticus), field crickets (G. assimilis), banded crickets (Gryllodes sigillatus), yellow mealworms (T. molitor), lesser mealworms (Alphitobius diaperinus), black soldier flies (H. illucens), and house flies (M. domestica). The regulation also specifies the substrates allowed as feed for insects; thus, animal-origin biowaste cannot be used to feed insects. In contrast, the use of fat from insects is permitted for every animal, unlike processed insect proteins [15]. According to Lähteenmäki-Uutela et al. [90], the possibility of extending the authorization of their use to poultry and swine feed is under discussion, but is still being delayed.
In the United States, the Federal Food and Drug Administration (FDA) is the authority responsible for monitoring, inspecting, and ensuring the safety of animal feed. Several states have regulations based on the official publication of the Association of American Feed Control Officials (AAFCO) [15]. Since 2016, AAFCO has permitted only one insect species (black soldier fly, H. illucens) as feed material for salmonids such as salmon, char, and trout. As feed for animals, black soldier fly larvae can be reared on approved feed-grade materials including food manufacturing by-products and pre-consumer food waste [90]. According to the FDA, if new feed materials or additives are not generally recognized as safe (GRAS) for that use, it must be in accordance with the Food Additives of the Act [15]. However, several states allow insect-based pet foods, while other states wait for the FDA and AAFCO’s decisions. Pet foods do not have to comply with all the AAFCO regulations [90].
In Canada, the Animal Feed Division, Animal Health Directorate of the Canadian Food Inspection Agency (CFIA), is responsible for animal feed regulations. In Canada, insects are considered a novel feed source if they do not have a history of safe use. As novel feed falls under federal jurisdiction and requires authorization, own safety tests must be conducted on insect-based feed [91]. In 2016, the CFIA authorized the use of black soldier fly larvae in broiler chicken feed. Authorization was extended to aquaculture in 2017 and to all poultry such as ducks, geese, and turkeys in 2018. However, Canada has no restriction on using insects as pet food, so insect-based pet foods are already available in Canadian markets [90].
In the Korea, the Ministry of Agriculture, Food, and Rural Affairs (MAFRA) is responsible for animal feed regulations. In December 2012, MARFA published a regulation on hazard analysis and critical control points (HACCP), which prohibits the use of animal-based protein in animal feed. Because insects are considered animal-based proteins, they were not allowed to be used in animal feed at that time [92]. However, since 2018, the feed-related laws have been amended to expand the insect industry. According to the animal feed regulations, including the “Control of Livestock and Fish Feed Act” and “Insect Industry Promotion and Support Act,” specific insects can now be used as animal feed. For example, mealworm larvae, crickets, grasshoppers, black soldier fly larvae, and mosquito larvae can be used when reared under specific conditions. If an insect is not registered as a feed material, it cannot be used legally [93].
China has a regulatory framework for insect production; therefore, it is expected to upscale insect production. In China, the major regulations for animal feed are the “Administrative Measures for Feed and Feed Additives”. Unauthorized insects cannot be used for animal feed, and new feed materials must be approved and added to the “Feed Materials Catalogue’ [38].
In Japan, new additives require pre-market authorization. The major regulation about animal feed is responsible for Ministry of Agriculture, Forestry and Fisheries which has given the Act on Safety Assurance and Quality Improvement of Feeds. Feed manufacturers, importers, and dealers must submit notification prior to using new feed and starting a business [90].
In Australia, animal feed materials are regulated by the Australian Pesticide and Veterinary Medicine Authority (AVPMA). Animal feed materials generally do not require registration if they meet the following conditions: (i) they are intended solely for nutritional purposes; (ii) they are only represented as being suitable and used to help maintain normal health or performance; (iii) they are fed as part of a normal diet; and (iv) they do not contain medications or other active ingredients (do not make any health or production claims). Additionally, insects are prohibited from being fed catering waste, manure, or unprocessed meat products [38].
According to Usman and Yusuf [94], there is a lack of clear legislation guiding the rearing and consumption of insects in Nigeria. Nigeria needs an amendment to the National Agency for Food and Drug Administration and Control (NAFDAC) Act to include regulations for using insects as feed [90].
CONCLUSION
As mentioned above, insects have been used as novel nutritional meals when feeding livestock. The effect of insects on digestibility differed according to insect species, stage, and processing methods; and the use of insects as feed had good potential despite lower digestibility. Chitin content could decrease digestibility but had a positive effect on livestock health. When insects were adapted to animal feed, various insect components showed biofunctional activities. Due to the substances having antibacterial activity, positive changes in the intestinal microbiota of livestock were induced, such as improvement of immunity and prevention of infection by pathogenic bacteria. In addition, the safety of insects has recently been studied and regulations on insect feed have been established. Therefore, the use of insects as feed has good potential.