INTRODUCTION
Light is a vital indicator for animals to perceive environmental changes and can cause changes in the reproductive cycle according to Webb [1]. Chang et al. [2], Chellappa et al. [3], Chang et al. [4], van der Lely et al. [5], and Rahman et al. [6] showed that the effect of light intensity and duration on the physiology and behaviour of mammals varied with light intensity and duration. According to Mattaraia et al. [7], increasing the duration of light has a positive effect on the reproductive success of female rabbits; the rabbit acceptance rate is greater under a 16 L:8 D photoperiod than under an 8 L:16 D photoperiod. Furthermore, whereas it is usual practice in European rabbit production farms to expose breeding females to artificial light for 15 to 16 hours each day throughout the year, many rabbit farms in China have adopted this lighting scheme of time. However, as studies have progressed, the colour of light has begun to be considered [8]. Colour, according to Bourgin et al. [9], plays a more essential and nuanced function than previously assumed and is a critical element to consider. Despite the relevance of light colour for commercial rabbits maintained in buildings with artificial light, few studies have described the influence of light colour on rabbit reproduction. Because the number of rabbits in China is currently quite high, rabbit farms in China employ the lighting system used in Europe. Due to geographical variations, it is vital to investigate whether there is a better suitable lighting plan for Chinese rabbit farms.
Furthermore, compared to research on the influence of light colour on avian reproductive performance [10,11], there have been comparatively few investigations on the molecular mechanism by which light colour may alter rabbit reproductive performance and follicular growth. Gonadotropin releasing hormone receptor (GNRHR), follicle stimulating hormone receptor (FSHR), and luteinizing hormone receptor (LHR) are three key candidate genes for mammalian reproductive characteristics. GNRHR is expressed in the brain, pituitary gland, and ovaries, as demonstrated by Zerani et al. [12], and rabbit oocytes, granulosa cells (GC), membrane cells, and zona pellucidae express particular GnRH receptors. FSH and LH both play key roles in the growth and maturation of follicles, and both the FSHR and LHR proteins are expressed in rabbit zona. According to the findings, the GNRHR, FSHR, and LHR genes all have a role in rabbit reproduction. The dynamic processes of ovarian follicle growth and atresia in female animals are intimately associated with GC death throughout the whole oestrus cycle [13,14], and GC apoptosis is mediated by the BCL-2 protein family [15,16]. B-cell lymphom (BCL)-2, a crucial apoptosis-related gene, can prevent cells from undergoing additional apoptosis by blocking the ultimate apoptosis process. BAX, a member of the BCL-2 family, has the inverse effect and can accelerate apoptosis. Ovarian follicle formation is the cornerstone of female mammalian reproduction. The proliferation of GCs is an important mechanism essential for optimal follicular development. Adiguzel et al. [17], Li et al. [18] demonstrated that GC cycle arrest at the G0/G1 phase is highly correlated with pathways associated with stress and FOXO signalling and that the increased proportion of GCs at the arrested G0/G1 phase was accompanied by increased FOXO1 expression in vivo and in vitro. In this work, we chose these genes with well-defined roles and investigated the effects of light colour on reproduction and follicular development gene expression in rabbits to see if there is a better lighting system for rabbit breeding facilities than the one now in use. Our work serves as a foundation for future research on the effects of light and colour on female reproductive health.
MATERIALS AND METHODS
Female New Zealand rabbits were obtained from the Liu He Animal Science Base (118°36′E, 32°29′N), Jiangsu Academy of Agricultural Sciences, China (experimental rabbit production licence number: SCXK (SU)2017-0008). The Care and Use of Laboratory Animals (Ministry of Science and Technology of the People’s Republic of China) and the Animal Care and Use Committee of Yangzhou University, Yangzhou, China (licence number: SYXK(SU)2017-0044) authorized all procedures. The 5-month-old nulliparous New Zealand rabbits (total of 1,068) weighed 3.6–4.4 kg live body weight. All rabbits were randomly assigned to one of four groups and confined to wire mesh cages (600 × 500 × 400 mm) with red light-emitting diode (LED) light (660–700 nm, Group R), green LED light (500–560 nm, Group G), blue LED light (440–480 nm, Group B), or white LED light (400–700 nm, Control Group W). The illumination was given by various coloured LED strip lights placed in the centre of the cage ceiling. The light intensity was evaluated in the cage’s centre using a Spectronics (SP) XRP-3000 photometer (Spectronics Corporation, Melville, NY, USA), and the measured values were 150 ± 3.0 lx, 150 ± 2.8 lx, 150 ± 3.1 lx, and 150 3.2 lx, indicating that the intensity was similar for each group. The lighting schedule was 16 L:8 D-15 d/6:00 am–22:00 pm (3 days before artificial insemination (AI) to 12 days after AI) (Fig. 1).
During the studies, the rabbits were kept in a closed and ventilated facility with a maximum temperature of 26°C, a minimum temperature of 22°C, and a relative humidity of 50% to 60%. All cages included food and drinking faucets. Water from the nipple drinkers and a commercial feed (digestible energy: 11.2 MJ/kg, crude protein: 186 g/kg, crude fibre: 155 g/kg) were freely accessible.
On day 7, AI was conducted, and ovulation was stimulated with an intramuscular injection of LHR-A3 (1 g, Ningbo Second Hormone Factory, Zhejiang, China). Palpation was used to diagnose pregnancy on day 19 of the trial (12 d postinsemination). Then, on day 76 of the trial, AI was conducted, and ovulation was induced by intramuscular injection of LHR-A3 (1 g, Ningbo Second Hormone Factory). On day 88 of the trial, pregnancy was determined by palpation (12-d postinsemination). The experiment was Kindled on day 107 and weaned on day 142. (The kits were weaned at 35 d of age). The lighting schedule was as follows: 16 L:8 D-15 d/6:00 am–22:00 pm (3 d before AI to 12 d post-AI). On day 145 of the trial, slaughter was carried out (Fig. 2). The conception rate was estimated by dividing the total number of inseminated does by 100. The total litter size, live litter size, and litter weight were measured on the day of kindling, and the kindling rate (the number of kindled does multiplied by the number of inseminated does multiplied by 100) was computed. Following delivery, the litters were divided into groups based on the average number of living kits (maximum 8 kits). Preweaning mortality was estimated as the number of kits deceased at weaning divided by the total number of kits born alive multiplied by 100 on the day of weaning (35 d postparturition). At 35 days old, the kittens were weaned.
On the 145th day following the LED light treatment, 15 female rabbits with similar body weights and at the same physiological stage (marked by a vulva colour of dark red or purple) were chosen for slaughter in each group of experimental rabbits. The right ovarian tissues were immediately snap-frozen in liquid nitrogen and kept at −80°C until the relative quantity of mRNA was determined. The ovarian tissues were dissected free of fat and mesentery and placed in 4% paraformaldehyde.
The ovarian samples were preserved in 4% formalin solution (Sigma-Aldrich, St. Louis, MO, USA) for 24 hours immediately after the rabbits were killed. They were subsequently treated using normal histological procedures before being embedded in paraffin blocks with a Leica EG 1150H paraffin embedding station (Leica Microsystems, Wetzlar, Germany) [19,20]. Each sample was sliced into three- to five-mm-thick slices with a microtome (Leica RM2255, Leica Microsystems) and placed on standard glass slides. Haematoxylin and eosin were used to stain the slices. Primary and secondary ovarian follicles, as well as large nonovulated haemorrhagic follicles, were identified, classified, and counted. All follicles were measured twice, and only those with clearly visible oocytes were counted.
Total RNA was isolated from ovarian samples using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s procedure. The optical density at 260 and 280 nm was used to calculate the quantity and purity of total RNA, and 1.8% agarose gel electrophoresis was used to determine RNA integrity. After that, 1.5 g of RNA was processed with DNase before being reverse-transcribed into cDNA with a PrimeScript® RT Reagent Kit (Takara, Dalian, China). The cDNA samples were kept at −80°C.
RT–PCR examination of the relative abundance of GNRHR, SHR, LHR, Bcl, BAX, FOXO1 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) in ovarian samples was performed using an Applied Biosystems 7500 Real Time PCR System and SYBR Premix Ex Taq Kit (Applied Biosystems, Carlsbad, CA, USA) (Takara, Dalian, China). Each PCR mixture contained 20 L of cDNA, 10 L of SYBR Premix Ex Taq, 0.5 L of forward PCR primer, 0.5 L of reverse PCR primer, 0.5 L of ROX Reference Dye II, and 6.5 L of ddH2O.
Rabbit mRNA sequences for GAPDH, GNRHR, FSHR, LHR, BCL-2, BAX, and FOXO1 were acquired from NCBI GenBank. Primer Premier 5.0 software was used to create the primers. Table 1 shows the sequence, GenBank number, and length of each primer set.
RT–PCR was carried out with an initial incubation at 95°C for 45 seconds, followed by 40 cycles of 95°C for 10 seconds and annealing for 40 seconds; real-time fluorescence data were obtained throughout this time. A melting-curve methodology was used to heat the reactions from 60°C to 95°C in 0.5°C 15 s increments while collecting fluorescence data to evaluate the specific amplification. The relative abundances of the distinct mRNA molecules were estimated using the 2−ΔΔCt technique [21] after all data were standardized to the internal reference GAPDH. Each PCR result was tested for specificity using 1.8% agarose gel electrophoresis followed by sequencing.
The results for litter size (total and alive), litter weight, and individual weight are presented as the means and standard errors. Data were compared using one-way repeated analyses of variance (ANOVAs, SPSS software version 22.0) with Bonferroni post hoc tests. Simultaneously, nonparametric chi-square testing was used to analyse conception and kindling rates, as well as preweaning mortality [22]. A statistically significant p value of 0.05 was considered.
In the serial sampling experiment, relative mRNA abundances were calculated using one-way ANOVA testing for the four monochromatic light hues’ major treatments. The mean differences for each therapy were compared using the mean standard error of the mean, and P 0.05 was judged significant. The means were ranked using Duncan’s multiple range test. IBM SPSS software was used for statistical analysis (ver. 13.0, IBM SPSS, Armonk, NY, USA).
RESULTS
As shown in Table 2, red light and white light affected the conception rate and kindling rate and increased the total litter size at birth (p < 0.05). The effects of red light on litter size at weaning, litter weight at weaning, and individual weight at weaning increased compared with the green and blue groups. The effects of red light on live litter size at birth were increased compared with those in the blue group (p < 0.05).
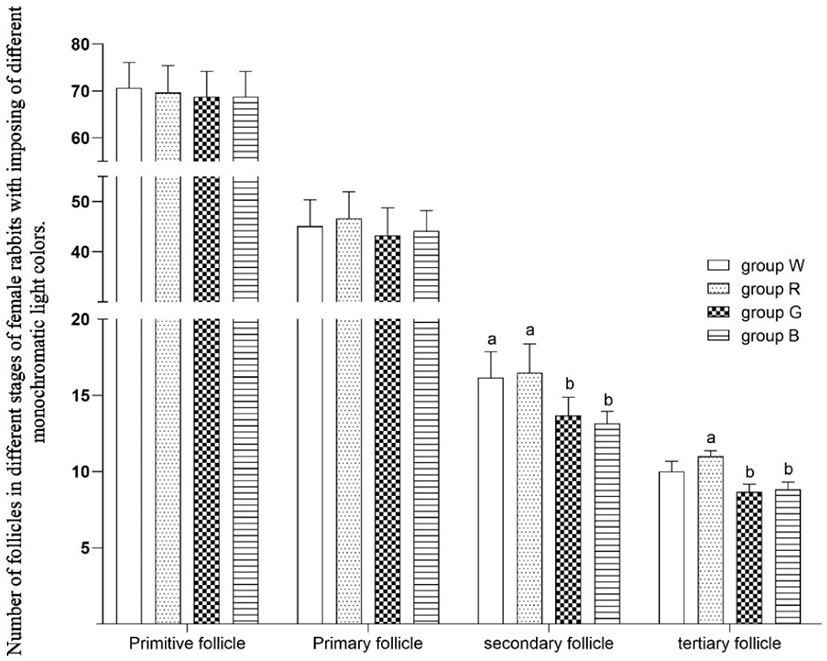
Primitive follicles are located outside the cortex in great numbers. As shown in Fig. 3, compared to white light, green and blue light reduced the number of secondary follicles (p < 0.05). Compared to red light, green and blue light reduced the number of tertiary follicles (p < 0.05).
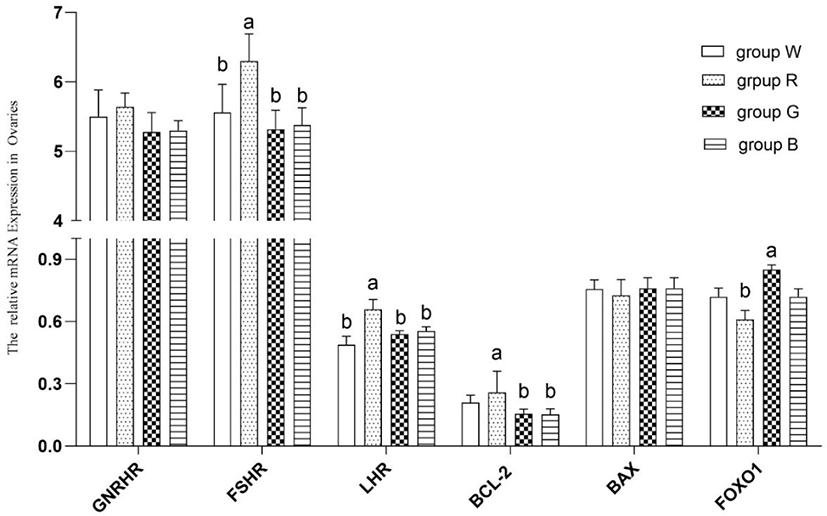
As shown in Fig. 4, compared with white light, red LED light resulted in greater ovarian FSHR and LHR mRNA expression (p < 0.05). Compared with green and blue LED light, red LED light resulted in greater BCL-2 mRNA expression (p < 0.05). Compared with green LED light, red LED light inhibited FOXO1 mRNA expression in rabbit ovaries (p < 0.05).
DISCUSSION
In comparison to studies on the effects of light colour on avian reproductive performance, there have been comparatively few publications on the effects of LED light colour on rabbit reproductive performance. Existing research has tended to focus on the effect of photoperiod on rabbit reproductive success. Sendrő et al. [23] discovered that switching from an 8 h light period to a 16 h light period 8 days before insemination on a large-scale rabbit farm effectively boosted the oestrus rate of female rabbits. According to Sendrő Z et al. [23], compared to normal white light, blue light has a positive influence on litter weight at 23 days of age. The influence of light colours is mostly due to differences in light wavelengths. Wu et al. [22] found that light colour had no discernible effect on the conception and kindling rates of female rabbits, but in this large sample study, we discovered that red and white light affected the conception and kindling rates and increased the number of kits at birth (total litter size and live litter size) (p < 0.05, Table 2). The effects of red light on litter size and litter weight at weaning were superior to those of other light colours (p < 0.05, Table 2). Because of its long wavelength, red light has the greatest biological permeability [14]. It has been utilized in photobiomodulation treatment to stimulate brain function [24]. It is thought that red light may infiltrate the cerebral brain of rabbits and produce biological responses, ultimately improving rabbit health. Based on our findings, we advocate replacing other colours of light with red light in rabbit farms.
GNRHR, FSHR, and LHR are significant genes associated with mammalian reproductive success and play key roles in follicle growth and maturation, as demonstrated by Zerani et al. [12] and Ramakrishnappa et al. [25]. Few studies have been conducted on the effects of different light colours on rabbit reproductive performance, and current studies have tended to focus on the effects of photoperiod and light intensity on female rabbit reproduction and associated genes. Sun et al. [26] investigated the influence of light intensity on ovary gene expression, reproductive success, and body weight in rabbit does with three different light intensities: 60 lx, 80 lx, and 100 lx. The relative abundance of growth hormone receptor (GHR) mRNA expression was more abundant in 60 lx than in 80 lx or 100 lx (p < 0.05); at first insemination, second insemination, and the second postpartum period (p < 0.05), the bodyweight of the dose in Group 60 lx was higher than that in the other two groups. For the first time, the findings of this study show that different LED light colours can influence the expression of FSHR and LHR mRNA in female rabbits (as shown in Table 2). Although rabbits are nocturnal creatures and hence less sensitive to light colour than animals that are active during the day, substantial differences in several features were discovered between groups lit by white, red, green, and blue light. According to our findings, red light can influence the reproductive success of inulliparous doesrabbits as well as the expression of essential genes for follicular development. When compared to white light, red LED light led to higher ovarian FSHR and LHR mRNA expression (p < 0.05). Red LED light increased BCL-2 mRNA expression (p < 0.05) compared to green and blue LED light. Red LED light decreased FOXO1 mRNA expression in female rabbit ovaries (p < 0.05) when compared to green LED light. As a result, the prospective influence of light colour merits further investigation.
Numerous studies on the influence of environmental variables on the development of female mammalian follicles have been performed, and some of them have achieved significant progress [17]. For example, Shen et al. [27] discovered that oxidative stress generated by environmental variables affects follicle growth. FOXO transcription factors are recognized as important mediators of oxidative stress and apoptotic regulation [28]. FOXO1 was found to be involved in oxidative stress-induced apoptosis of mouse follicular GCs both in vivo and in vitro in mice. When mice were treated with the oxidant, it was shown that higher apoptotic signals were associated with increased expression of FoxO1 in GCs [29]. FOXO1 expression was also upregulated in proapoptotic and antioxidative genes [27,28,30]. Red light substantially decreased the expression of FOXO1 mRNA in this research. This finding is consistent with the data on tertiary follicle number given in Fig. 2. Red light has a longer wavelength than other visible light colours, and it contains less energy than other colours of light. The oxidative stress imparted to the retina may have been milder in the red light group than in the other groups, resulting in considerably lower FOXO1 mRNA transcription levels in the red light group than in the other groups. It is hypothesized that the oxidative stress response in female rabbits differs following different LED light treatments. These variations may be due to the varied photon energy conveyed by different wavelengths of LED light. When varied energy levels of LED light contact the retina, the biological efficiency changes, resulting in variances in FOXO1 mRNA expression levels in female rabbit ovaries.
Throughout the oestrus cycle, follicular development is linked to granulosa cell death, as explained in the introduction. The BCL-2 gene family is primarily involved in granulosa cell apoptosis, and BCL-2 and BAX are important apoptotic genes. BCL-2 can prevent additional apoptosis by blocking the final route of apoptosis, whereas BAX has the opposite effect and can accelerate apoptosis [14]. In this work, we chose two genes with distinct roles and examined their expression patterns in female rabbits exposed to various LED light colours. The results revealed that red LED light resulted in greater BCL-2 mRNA expression (p < 0.05), we did not clarify the exact process, and will investigate it more in the future work.
CONCLUSION
Although rabbits are nocturnal creatures and hence not as sensitive to light colour as animals that are active during the day, there were substantial differences in several features between groups lighted by white, red, green, and blue light. Red light has been shown to have a positively effect on the reproductive success of rabbits as well as the expression of critical genes for follicular development.
Furthermore, according to common sense, LED lamps are more energy efficient than incandescent lamps, it is recommended to promote the use of LED lights in the production of domestic rabbits to reduce energy consumption.
