INTRODUCTION
The wound is the second most prevalent emergency condition in horses [1]. Additionally, traumatic wounds are common in horses, and a large percentage of these develop into chronic wounds [2]. Horses often display a difficult challenge in distal limb wound healing [3,4]. A large percentage of horses are likely to be impacted by aberrant limb wound healing at some point in their lives [5]. Many horse skin wounds are not healed by the first intention due to difficult suturing owing to substantial soft tissue loss, high skin tension, motion, infection susceptibility, inadequate blood supply, partial or full wound dehiscence, or a long interval after injury [6,7]. Clinically, horses with improperly healed distal limb injuries provide an ongoing challenge due to the prolonged inflammatory phase, decreased contraction, and epithelialization [8,9]. All these factors directly interfere with wound healing; thus, often making healing with a secondary intention the only choice [5,10]. Affected horses lose their economic value and become unsuitable for work over long periods, or their athletic careers are ended [5,11].
Treatment may be challenging owing to the wide range of wound types, locations, and severity, as well as a lack of primary data on optimal treatment techniques. The wound care literature primarily comprises expert opinion or case series, and there are presently no evidence summaries to guide therapeutic decision-making [12].
There are a wide variety of wound care medications for horse practitioners; however, only a few have been clinically studied for their effect on chronic wound healing in horses [13,14]. Various topical wound therapies have been tested in horses’ distal limb models to enhance second-intention wounds, with varying degrees of success [13,15]. Furthermore, several medications had inconclusive outcomes for improving wound healing when compared to the control group [16–21]. Thus, equine veterinarians should be aware that positive findings from these research studies have not been replicated consistently when evaluated in vivo on horse wounds [22].
Polysaccharides are some of the most ancient and popular drugs that promote wound healing [23,24]. Maltodextrins are highly specialized carbohydrates with the intriguing potential to enhance wound healing. Several reports have confirmed the general effectiveness of maltodextrins as a feasible alternative for the treatment of chronic wounds [25–27]. The maltodextrin/ascorbic acid gel combination has exhibited encouraging outcomes, such as softening of necrotic tissues to facilitate debridement and promoting granulation tissue formation and epithelialization in treating chronic wounds. Moreover, it showed efficacy in both infected and non-infected wounds, without being toxic or absorbed systemically [25,28]. Furthermore, it has a quick ability to penetrate wound irregularities to fill tunneling and undermining tissues [29,30]. Several studies have shown that maltodextrin/ascorbic acid gel appears to improve chronic wound healing via increased transforming growth factor β1 protein expression, collagen turnover, and cytokine attraction [25,31]. In addition, maltodextrins have the potential to deliver nutrients to the wound bed by delivering glucose for cell metabolism via hydrolysis [32,33]. Furthermore, maltodextrin not only has chemotactic properties but also has antibacterial and bacteriostatic properties [31,32].
To the best of our knowledge, this is the first clinical study to use maltodextrin/ascorbic acid gel to treat chronic cutaneous wounds on horses’ distal limbs that heal by second intention. This investigation aimed to test the effectiveness of this wound care product in field settings to assist horse practitioners in treating these types of delayed wounds.
MATERIALS AND METHODS
A randomized, multi-center clinical study including horses with distal limb old wounds was performed from January to September 2021. Horses were admitted to the Department of Surgery, Anesthesiology, and Radiology, Faculty of Veterinary Medicine, Suez Canal University, Ismailia, Egypt, and the Equestrian Center Clinic, King Abdulaziz University, Jeddah, Kingdom of Saudi Arabia. The study included 18 adult horses of both sexes ranging in age from 3 to 15 years. The horse breeds were as follows: six Baladi, eight Thoroughbred, and four Arabian. Based on the therapeutic protocol used, the horses were randomly allocated to different groups based on the priority of their attendance at the clinic using regular sampling. Horses were divided into one of three groups following wound preparation, with a similar mean wound area in each group. Group 1 (n = 6): As a control group, wounds were irrigated using a sterile 0.9% physiological saline solution before applying a wet dressing moistened with 1% povidone-iodine solution (BETADINE®, Nile Pharma, Cairo, Egypt). Group 2 (n = 6) wounds were irrigated with a sterile 0.9% physiological saline solution before being dressed with maltodextrin/ascorbic acid gel (Multidex®, DeRoyal, Powell, TN, USA). Wounds in Group 3 (n = 6) were irrigated with a sterile 0.9% physiological saline solution followed by a 1% povidone-iodine solution before being dressed with Multidex®.
Before treatment, horse owners were informed of their animals’ participation in the clinical study. The Institutional Animal Use and Care Committee of the Faculty of Veterinary Medicine at Suez Canal University in Egypt evaluated and approved the research protocol (2021022). All treatments were performed with the utmost consideration for animal welfare.
Horses were selected based on predetermined selection criteria as follows: Mature (non-geriatric) healthy horses without signs of infection or other systemic diseases. Cutaneous chronic wounds were included when they were at the distal limbs under the level of the carpus or tarsus, with an incomplete granulating bed (approximately 0.5–1.0 cm depth when measured with a probe). Wounds had nearly the same mean surface area in the different groups (12.67 ± 1.2, 12.67 ± 3.71, and 12.67 ± 2.73 cm2 for groups 1, 2, and 3, respectively).
Horses were restrained if necessary, either by application of a nose twitch or by intravenous doses of 1.1 mg/kg Xylazine HCL (Thiazine 100, Ceva Animal Health, Glenorie, NSW, Australia) [34]. The wounded areas were routinely clipped and aseptically prepared. Chronic incomplete granulating wounds were debrided, all necrotic tissues were removed, cleansed with isotonic saline, and the treatment regimen for the assigned group was applied daily. After application of the prescribed treatment, the wound surface was covered with a non-adherent, non-occlusive dressing (MultiPad™, DeRoyal) which was secured in place by cohesive elastic bandages (STRAP BAND, Génia, Saint-Hilaire-de-Chaléons, France). Following this, the second layer of cotton padding was applied and covered using cohesive elastic bandages. Bandages were changed once a day as prescribed by Howard et al. [26] until healthy granulation tissue had filled the wound depth, and treatment was subsequently provided without the need for a bandage cover. The bandage was changed with caution to retain the healthy granulation tissue that had grown. If the dressing stuck to the wound, it was soaked in saline for several minutes before being removed to prevent disrupting the delicate granulation tissue.
Wound healing quality was evaluated at week intervals by clinical examination of the appearance, color, contraction, regularity of granulation tissue, epithelization, and wound exudation.
Wounds were assessed weekly by a blinded investigator for signs of complications and, later complete epithelialization, which was then recorded. Digital photographs and subjective wound data were collected all over the study period (35 days). The wounds were gently wiped clean using sterile saline before closer evaluation to facilitate data acquisition. Before photographing, the hair at the wound borders was clipped, and a tape measure was used as a calibration reference. These images were used for the evaluation and scoring of the measured parameters. Each parameter was measured thrice and the mean was used for calculation. Wounds were measured using Fiji in Image J software, version 1 (National Institutes of Health, Bethesda, MD, USA) [35]. The surface area was calculated by multiplying the greatest length by the perpendicular greatest width [36]. For each wound, the percentage of wound contraction, epithelialization, and overall wound healing was determined using the formulae provided by Bohling et al. [37]. The wound contraction % was calculated as follows: the total wound area on day n as a % of the original wound = (total wound area at day n × 100) / original wound area at day zero, then the % of wound contraction at day n = 100 – total wound area at day n as a % of the original wound size. The epithelization % at day n = (area of epithelization day n × 100) / total wound area day n. The total wound healing % was calculated as follows: the open wound day n as a % of the original wound size = (open wound area at day n × 100) / original wound area at day zero, then the % of total wound healing at day n = 100 – open wound day n as a % of the original wound size.
All quantitative data are expressed as the mean ± standard deviation. SPSS software version 21 (IBM, Armonk, NY, USA) was used for statistical analysis. Data were analyzed using a two-way analysis of variance followed by Tukey’s post hoc test. The p-value was estimated at p < 0.05. GraphPad Prism software version 6 was used to create the graphs (GraphPad Software, La Jolla, CA, USA).
RESULTS
During the study period, the horses were clinically healthy without the use of any antibacterial or anti-inflammatory drugs. Additionally, there were no exclusions of any horses due to the topical treatment applied. Wound debridement was extremely significant for the elimination of dead and necrotized tissues providing a suitable environment for the treatment’s action for efficient and clean healing till the wound surface became bright red and moist. Packing was used to halt and control any bleeding that may have occurred as a result of debridement.
The wounds in all study groups were filled with granulation tissue (Fig. 1) with varying periods. Bandages were removed from all horses when healthy granulation tissue had filled the wound depth between 9–16 days (mean 13.83 ± 2.48), 7–12 days (mean 9.67 ± 1.97) and 7-10 days (mean 8.83 ± 1.17) for groups 1, 2 and 3 respectively. No exuberant granulation tissue was reported in any of the wounds, and there were no adverse side effects related to the Multidex® application.
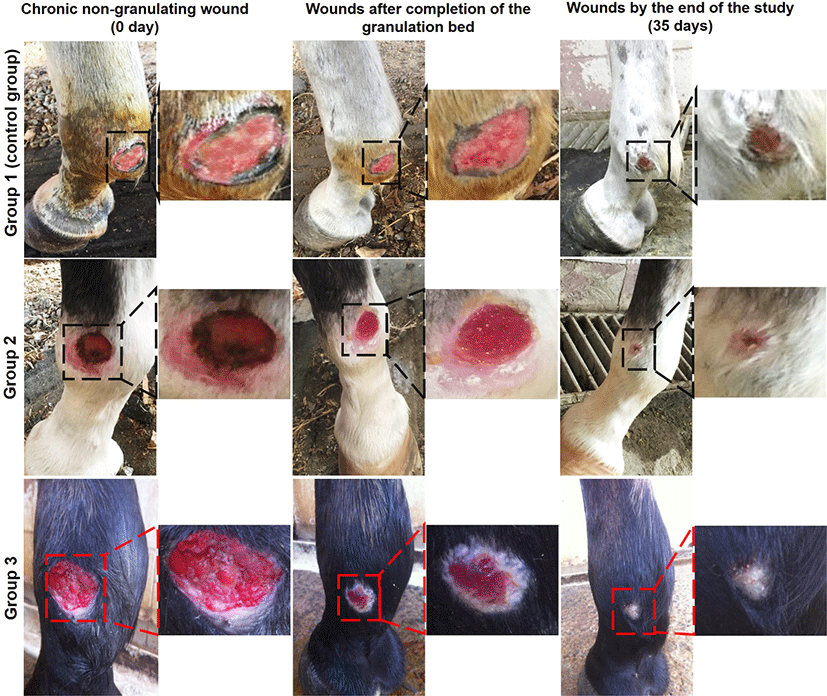
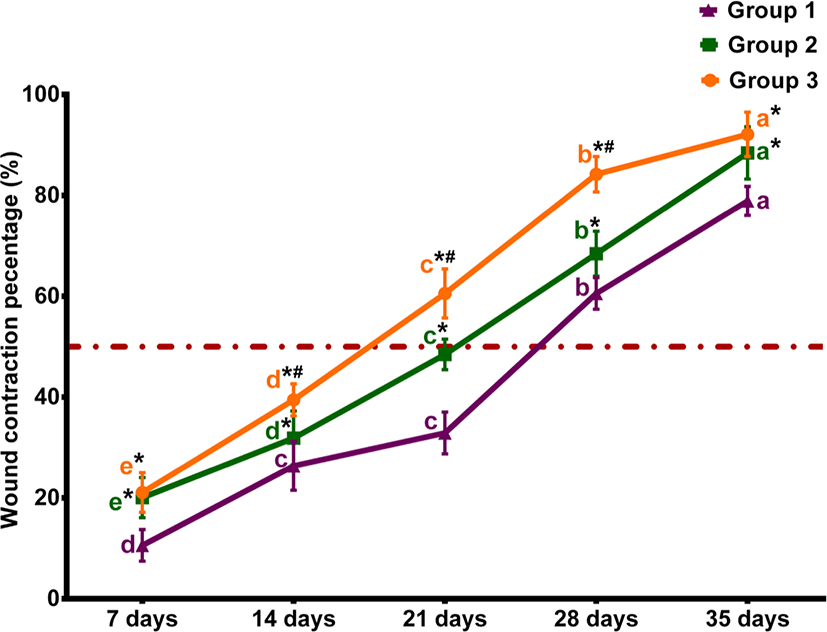
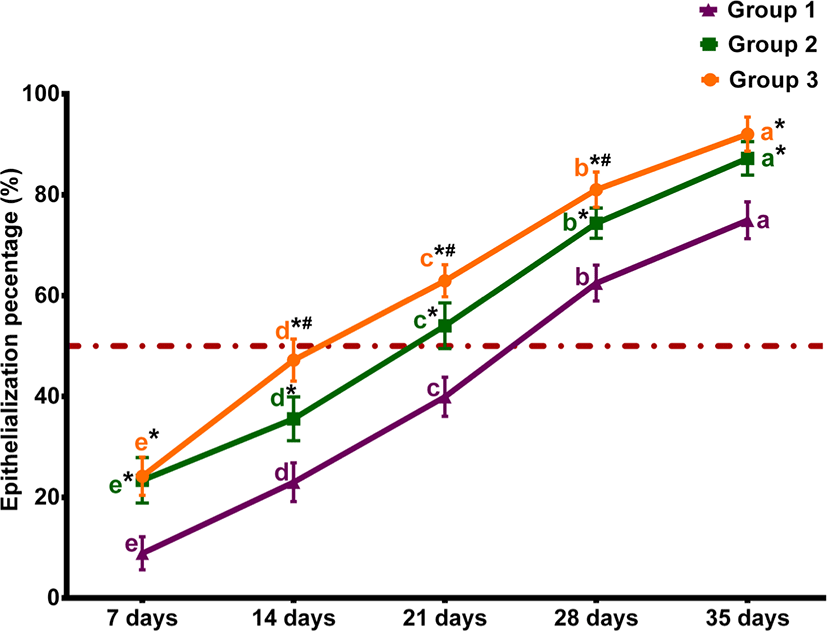
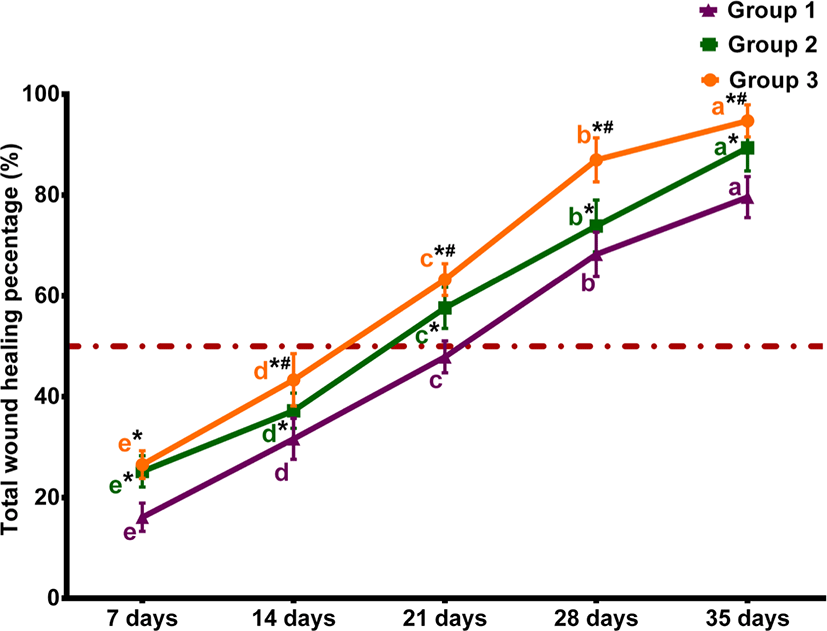
The percentages of macroscopic wound contraction showed no significance between groups 2 and 3 on days 7 and 35 (p > 0.05) (Fig. 2). The wound area contracted fast in horses treated with Multidex® in groups 2 and 3, compared to horses treated with BETADINE® only in the control group. As presented in Fig. 3, the epithelialization rates increased in different groups during the observation period. However, group 3 demonstrated the greatest increase in epithelialization rates compared to the control group by the end of the study. The mean epithelization percentage between groups by the end of the study was 74.96 ± 1.67, 87.23 ± 2.33, and 92.03 ± 3.38 for groups 1, 2, and 3, respectively. Regarding the total healing time, wounds irrigated with BETADINE® before the application of Multidex® healed faster than other wounds. The mean total wound healing percentage by the end of the study in different groups was 79.59 ± 4.16, 89.36 ± 2.6, and 94.71±3.2 for groups 1, 2, and 3, respectively (Fig. 4). The total wound healing percentage for group 3 was significantly increased (p < 0.05) than that for group 2 and the control group by the end of the study period.
DISCUSSION
Wound healing by second intention aims to restore the tissue defect by the creation of granulation tissue, which provides a matrix for epithelial cell migration [38]. Although the benefits of maltodextrin/ascorbic acid gel have been established for long, research or clinical studies performed on its use in horse wounds are limited. This clinical study aims to assess the healing efficacy of maltodextrin/ascorbic acid gel on distal limb chronic non-granulating wounds in horses.
We chose to focus on distal limb chronic non-granulating wounds in horses since wounds on the distal limb do not appear to heal or contract as well as other kinds. According to several studies, the rates of wound contraction differed depending on the location of the wound on the horse. Clinical findings have demonstrated that horse limb wounds recover at around half the pace of body wounds [39,40]. Furthermore, small wounds on the equine distal limb that are expected to heal without any significant contraction may fail to heal [5]. Horses in this research ranged in age from 3 to 15 years, with geriatric horses being excluded to avoid reduced fibroplasia and an increased infection risk [41].
In this study, it was found that using maltodextrin/ascorbic acid gel in chronic wounds in the equine distal limb, either alone or in conjunction with povidone-iodine 1%, enhanced granulation tissue formation and epithelialization, which is consistent with prior research results in experimental models or human clinical cases [13,22,26,27,30,32,42,43]. There were no clinical consequences detected during the maltodextrin/ascorbic acid gel application or healing period, which might be attributed to the fact that maltodextrin/ascorbic acid gel is manufactured from natural components such as maltodextrin and ascorbic acid [30,43].
Wound debridement and washing with an isotonic saline solution were conducted before treatment application to eliminate any necrotic tissue or debris that might serve as a potential source for infection and prevent wound contraction and epithelialization [23,44]. Although chronic wounds should be considered infected [44], the use of an antiseptic to lessen the wound’s estimated bacterial population may be beneficial [45]. The general consensus is that using antiseptic dressings sparingly can be a valuable alternative for controlling infection and promoting healing in open wounds [8,46,47]. Povidone-iodine 1% [28] was selected since it is the most commonly used iodine in clinical settings, which retains broad-spectrum antibacterial, anti-fungal, and anti-viral effects while reducing tissue toxicity as recommended in contaminated wounds [8,12,45].
In this study, wounds treated with maltodextrin/ascorbic acid gel had no septic complications. This could be attributed to the use of diluted povidone-iodine, which showed a faster healing rate than equivalent wounds treated with isotonic saline solution [48,49]. In the same context, local antibiotics were not used with caution owing to bacterial resistance risk [45]. Regardless, antibiotics were not used in horses with distal limb wounds, instead of relying on the antibacterial activity of povidone-iodine as an antiseptic and the action of maltodextrin/ascorbic acid gel, which reduces wound pH and causes an osmotic effect that promotes bacterial lysis [26,50]. Furthermore, nonsteroidal anti-inflammatory drugs were specifically avoided since they would have interfered with the treatment’s effectiveness [11,51].
Following each treatment, bandages were used to keep the area clean, protect the newly granulated tissue from drying out too rapidly, and keep the product on the wound surface [52]. Once a healthy layer of infection-resistant granulation tissue had formed, the bandages were removed as soon as possible to prevent the formation of exuberant granulation tissue [8].
A substantial improvement in overall wound contraction and epithelialization percentage, as well as total wound healing percentage, was recorded throughout the study period when using maltodextrin/ascorbic acid gel alone or in conjunction with 1% povidone-iodine compared to using 1% povidone-iodine alone. On the other hand, povidone-iodine alone in the control group, delayed the development of granulation tissue and epithelialization process [23]. In agreement with previously reported findings, maltodextrin seemed to alleviate discomfort and induce granulation and epithelialization in an equine model when compared to topical antibiotics [13,29].
Our study was in contrast to the study by Howard et al. [26] who found no differences in assessed parameters between maltodextrin treated and control wounds throughout time or at any one-time point in the experimentally created wounds in equines. The difference in overall wound healing percentage between using maltodextrin/ascorbic acid gel alone and in combination with povidone-iodine could be attributed to the combined antibacterial activity of both compounds on bacterial infection because clinically relevant infections caused by bacterial infection can cause delayed wound healing [23].
In the current clinical investigation, maltodextrin/ascorbic acid gel increased wound filling with granulation tissue; however, unlike other experimental models using sugar-based dressings [7,26], the granulation tissue did not extend above the level of the epithelium, and the proliferative phase of wound healing was followed by epithelization.
Finally, regarding the regularity with which horses incur wounds and the inherent challenges associated with distal limb wound healing, maltodextrin/ascorbic acid gel exerts both host and antimicrobial effects, holding tremendous potential as an equine wound care product. The present clinical study proved the ability of maltodextrin/ascorbic acid gel to achieve better wound-healing capacity and cosmetic appearance under field conditions when compared with the conventional treatment of equine distal limb wounds.
The limitations of this research include the inability to identify the numerous bacterial species that developed on the wounds using bacterial cultures and the authors’ reliance only on the clinical appearance of the wounds. Another limitation of the study is that it did not include larger and deeper cutaneous wounds and different age groups of horses (geriatric animals).
CONCLUSION
The use of maltodextrin/ascorbic acid gel, alone or in conjunction with 1% povidone-iodine, results in the healing of chronic non-granulating wounds of the distal limb in horses when compared to the use of povidone-iodine alone. This combination provides a better mix for hastening and enhancing the healing process. Therefore, maltodextrin/ascorbic acid gel is being advocated as a safe, rapid, and effective alternative therapy for long-term non-healing wounds in equines.
