INTRODUCTION
Methane gas, a product of anaerobic microbial carbohydrate fermentation in cattle rumen, is second only to carbon dioxide as a prominent greenhouse gas (GHG) impacting global warming [1]. Animal husbandry emissions constitute 16.5% of total GHG emissions, with a continuously increasing global rate [2]. Therefore, developing a methane-mitigation strategy to attenuate ruminant emissions is a worldwide effort and concern. Moreover, the cattle methane conversion factor (MCF) ranges from 2.4% to 9.5% of gross energy intake, depending on diet quality [3]. Thus, reducing enteric methane emissions through dietary methods will ease environmental pressures from beef production and improve cattle efficacy in energy utilization.
Total mixed ration (TMR) is an efficient ruminant feeding system that prevents selective feeding, maintains ruminal pH, and improves carcass yield and quality grade [4–6]. Alternatively, general or separate feeding (SF) systems provide a concentrate mix with forage through individual feeders [7]. SF accounts for 76.22% of beef production systems in Korea, while the remaining 23.78% are TMR [8]. Previous studies have reported that TMR-fed steers significantly increase enteric methane emission levels and alter ruminal microbial populations, such as Coprococcus and Butyrivibrio, without neutral detergent fiber (NDF) intake changes between TMR and SF groups [7,9]. However, Holstein cattle produce similar methane levels when fed with either TMR or SF [10–12]. Although modifying feeding systems can influence enteric methane emission levels without feed additives, further investigation is needed as published results conflict due to varying feed quality, forage-to-concentrate ratio, and particle size. Therefore, feed with the same ingredients must be evaluated through SF and TMR methods for further clarification.
The ruminal microbiome encompasses complex microorganism communities such as archaea, bacteria, fungi, and protozoa [13]. These microbes aid cattle in digestion, provide nutrients, and produce several fermentation products, including methane [14]. Among microbes fermenting feedstuffs in the rumen, bacteria are the most prevalent. Thus, considering methanogen and bacterial populations is imperative when evaluating methane production influences [14], achievable through 16S rRNA gene amplicon sequencing [13,15].
Despite alternative feeding systems being a promising approach for reducing ruminant methane emissions [9], little is known regarding the effects of SF or TMR systems on Hanwoo, beef cattle native to Korea. Therefore, the present study investigates how these two feeding systems (SF and TMR) impact ruminal fermentation characteristics, digestibility, methane emissions, and ruminal microbiota in Hanwoo steers.
MATERIALS AND METHODS
All experimental procedures were approved and performed under the National Institute of Animal Science Institutional Animal Use and Care Committee in Korea guidelines (approval number: NIAS-2018-282). The experiment was conducted in the Livestock Research Building, National Institute of Animal Science, Rural Development Administration in Wanju, Korea.
The crossover design incorporated eight Hanwoo steers with a 507.1 ± 67.4 kg (means ± standard deviation) average initial body weight (BW), approximately 28 months old upon experiment onset. Each experimental period was 29 days long: 14 days in a metabolic cage outside the chamber and 10 days in the chamber for adaptation, and 5 additional days in the chamber for sampling. From Days 15–29, the steers remained inside the chamber all day. Diets were adjusted to 1.5% of the individual BW and consisted of forage and concentrate (F:C = 15:85; dry matter [DM] basis). The steers were randomly assigned to either the SF or TMR group based on BW. SF-group steers were simultaneously fed the concentrate mix and forage in individual feeders. Avoiding selective feeding was not considered, as this experiment mirrored feeding practices at genuine Korean beef farms. TMR feed was obtained using a TMR compounding machine (Horizontal TMR mixer, Daesung ENG, Jeongeup, Korea) with identical feed sources and ratios to SF. A total of 500 kg (as fed) of feed was loaded into the machine and mixed for 10 min. The feed was then dispensed to steers through individual feeders. Table 1 presents all ingredients and chemical compositions of the experimental diets. The animals were fed equal amounts twice daily, at 09:00 and 16:00. Water and mineral blocks were easily accessible.
Feed remaining at the end of the day was recorded and collected before morning feeding. Before each period, feed samples were collected and placed in a drying oven at 60°C for 48 hours. Then, the dried feed samples were ground in a Foss Tecator Cyclotec 1093 Sample Mill (FOSS, Suzhou, China) through a 1-mm screen. The prepared samples were shipped to Cumberland Valley Analytical Services (Waynesboro, PA, USA) for chemical composition analysis. The Association of Official Agricultural Chemists (AOAC) methods [16] were used to analyze DM (#930.15), crude protein (CP; #990.03), acid detergent fiber (ADF; #973.18), ash (#942.05), and calcium and phosphorus (#985.01). Ether extract (EE; #2003.05) was determined using AOAC methods [17]. NDF was analyzed utilizing heat-stable amylase with residual ash (aNDF) [18]. Dry matter intake (DMI) was calculated daily from the as-fed intake of individual steers. CP was calculated by multiplying the nitrogen content by 6.25.
Whole feces were collected daily during the five-day sampling period (Days 25 to 29). Feces were dropped from the caudal region and gathered in an iron plate. After collection, the daily feces were left in a drying oven at 60°C for 48 hours. The dried samples were pooled, and 200 g of fecal subsamples were collected for the apparent total-tract digestibility analysis. Fecal compositions were analyzed following AOAC methods [19]: CP (#942.05), EE (#920.39), and ash (#954.01). The NDF and ADF contents were analyzed using the method proposed by Van Soest et al. [18], and CP and non-fiber carbohydrate (NFC) contents were calculated as previously described. The apparent digestibility of any given nutrient was calculated from the individual DMI and feces excreted.
Methane emissions from eight Hanwoo steers within each period were measured using four respiratory chambers with two batches of four animals. Each chamber had a volume of 25.4 m3 (3.9 × 2.6 × 2.5 m, L × H × W, Changsung Engineering, Gwangju, Korea), concrete outer walls, and a front door fixed with a transparent window (300 mm × 150 mm) for observation. In addition, a metabolic cage made of steel pipes (1,400 mm × 2,950 mm × 2,120 mm) was fixed within the chamber for keeping animals in one place. Four 24-V air circulation fans were installed at 45° angles on each side of the chamber ceiling for even air circulation. A PVC (Φ100) tube was installed at the center of the ceiling, and an air motor was attached to the PVC end behind the chamber for continuous air exhaust. In addition, three non-woven profiler layers were installed at the air outlet on the front PVC pipe to prevent dust and animal hair from entering the pump. An identical PVC pipe was inserted through the ceiling at the front of the chamber for fresh air flow.
Air samples were vented through an infrared methane sensor (Horiba VIA-510 gas analyzer, Horiba, Kyoto, Japan) to measure methane emissions within a 0–200 ppm detectable range (± 0.2 ppm resolution). Furthermore, a dehumidifier (KAFM251-03, KCC, Jeonju, Korea) was installed for more precise methane analysis, and an Oxymax system consisting of an air pump, a flow meter, a sample pump, and a gas drying device (incorporating an Oxymax sample max, system sampling pump, Paramax-101, and carbon dioxide sensor; Columbus Instrument International, Columbus, Ohio, USA) for gas analysis.
A standard methane-recovery rate was performed thrice before the experiment and thrice after to evaluate the accuracy of the four chambers. First, 5 L of methane gas (99.95% purity) was released into the chamber at a 900 L/min rate and measured in five-second intervals until the methane gas concentration in the air discharged from the chamber reached 0 ppm. The average methane gas recovery rate was 92.45% (SD = 9.27), and the recovery rate of each chamber was used to calculate methane emissions after the experiment. Next, airstreams from the chambers were sequenced to an analyzer at five-minute intervals in a 20-minute cycle for each chamber. The sampled gas was stabilized for 4.5 minutes, and the air sample was then quantified for 30 seconds from each chamber to measure the gas levels. Sample stream sequencing to the analyzer was controlled using a CI-Bus serial interface (Columbus Instrument International).
Methane emissions were measured for four consecutive days (Days 25 to 28), and the data generated during 1 hour after feeding (2 hours a day total) were not included in calculations due to interruptions from open doors. Methane generation during the open-door period was estimated through interpolation. After measuring methane emissions at 0900 hours, the doors were opened for approximately 10 minutes to feed the animals, clean the metabolic cage, and check equipment. This process was repeated at 1600 hours. Methane emission calculations considered chamber temperature and relative humidity, wind speed of the air discharged through the main discharge pipe, and analytical gas concentrations (Table 2). The chamber program maintained a 20°C, 50% humidity, and 900 L/min wind speed, and real-time data and methane detection were automatically recorded simultaneously.
The average methane emissions of each chamber from Days 25 to 28 were utilized for the statistical analysis. The MCF was determined as the gross energy percentage of feed converted to methane [1]. Similarly, the methane emission factor (MEF; kg of methane/head/year) was determined by the gross energy intake (MJ/head/d) × (MCF ÷ 100) × 365 ÷ 55.65 (MJ/kg of methane) [1].
Ruminal fluid was collected from each animal before morning feeding on Day 29 with a stomach tube that we previously developed [20]. The stomach tube includes a head segment (length 13 cm, diameter 3 cm), a flexible tube (length 210 cm, diameter 1 cm), and a vacuum pump (Welch & Thomas, Mount Prospect, IL, USA) to obtain the ruminal fluid. The stomach tube was thoroughly washed with warm water between sampling to prevent cross-contamination. Additionally, the first 200 mL of the ruminal fluid was discarded to reduce any contamination from the saliva [21,22]. The sampled ruminal fluid was filtered through a four-layered cheesecloth. A pH meter (Pinnacle pH meter M540, Corning, NY, USA) measured the sampled inoculum pH immediately after collection. Then, the ruminal fluid was sealed in a tube and frozen in liquid nitrogen. The samples were stored at -80°C until volatile fatty acids (VFA), ammonia nitrogen (NH3-N), and metagenomic DNA extraction were analyzed.
VFA and NH3-N concentrations were determined as described by Erwin et al. [23] and Chaney and Marbach [24] with minor modifications. Briefly, the ruminal fluids were centrifuged at 14,000×g for 10 minutes at 4°C, and 5 mL of the supernatant was mixed with 500 μL of 50% metaphosphoric acid (MPA; Catalog number 239275, Sigma-Aldrich, St. Louis, MP, USA) for VFA or 500 μL of 25% MPA for NH3-N. Then, the mixture was further centrifuged at 14,000×g for 10 minutes at 4°C for VFA analysis, and the supernatants were distributed to gas chromatograph (GC) analysis vials (6890N, Agilent Technologies, Wilmington, DE, USA) with a capillary column (Nukol™ Fused silica capillary column, 15 m × 0.53 mm × 0.5 µm, Supelco, Bellefonte, PA, USA) and analyzed. Next, the standard curve was generated using a VFA standard solution (Catalog number 46975-U; Sigma-Aldrich). The inoculum and 25% MPA mixtures were centrifuged at 14,000×g for 5 minutes at 4°C for NH3-N analysis. After centrifugation, 20 μL of the supernatant was mixed with 1 mL of a phenol color reagent (50 g/L of phenol plus 0.25 g/L of nitroferricyanide) and 1 mL of an alkali-hypochlorite reagent (25 g/L of sodium hydroxide and 16.8 mL/L of 4%–6% sodium hypochlorite). Finally, the mixture was colored in a 37°C water bath for 15 minutes, 8 mL of distilled water was added, and a UV spectrophotometer (Bio-Rad, US/benchmark plus, Tokyo, Japan) measured the NH3-N concentration at 630-nm absorbance. All analyses were conducted thrice, and the mean values were established.
Metagenomic DNA was extracted from the ruminal fluid samples collected on Day 29. Frozen samples were thawed at room temperature, and DNA was extracted following the RBB+C bead-beating method [25]. The V3–V4 region of 16S rRNA genes from each DNA sample was amplified with the universal primers 341F (5’-CTACGGGNGGCWGCAG-3’) and 805R (5’-GACTACHVGGGTATCTAATCC-3’) for bacterial analysis [26]. In addition, the V6–V8 region of 16S rRNA genes was amplified using primers 915F (5’-AGGAATTGG CGGGGGAGCAC-3’) and 1386R (5’-GCGGTGT GTGCAAGGAGC-3’) for methanogen analysis [27]. The primer sets produced approximately 450 and 470 base paired-end protocols with the MiSeq platform (Illumina, SanDiego, CA, USA) at the Macrogen Sequencing Facility (Macrogen, Seoul, Korea).
Raw sequences were pre-processed, quality filtered, and analyzed using QIIME2 (version 2019.1), a next-generation microbiome bioinformatics platform, adhering to the developer’s recommendations [28, 29]. The amplicon sequence variants (ASVs) were generated using the DADA2 algorithm [30] to denoise and remove chimeric sequences. Then, a bacterial analysis was accomplished using the “SILVA_132 99% OTUs full-length sequences” database for taxonomic determination and the Rumen and Intestinal Methanogen (RIM)-DB as a reference [31]. Data sets were then transferred and analyzed through various R packages, such as phyloseq [32], vegan [33], Ampivis2 [34], DESeq2 [35], and ggplot2 [36], for relative abundance, microbial diversity matrix, and correlation calculations.
Data were analyzed by the SAS PROC MIXED (Enterprise Guide 7.1, SAS Institute, Cary, NC, USA) for crossover design. Before data analysis, we conducted the normality test by the Shapiro–Wilk test using the XLSTAT statistical software (Addinsoft, New York, NY, USA) and confirmed that the normality assumption was met. The experimental unit was an individual steer, and the fixed effects were period and diet. However, the period effect is not displayed because there were no statistical differences. Data are presented as least-squares means. Significant differences were defined at p < 0.05, and tendencies were determined at 0.05 ≤ p < 0.1.
Statistical microbiome analysis was conducted with various R packages as previously described. Alpha diversity indices, such as Shannon’s index and Chao1, were calculated with the phyloseq R package, and ANOVA test significance. The principal coordinate analysis (PCoA) assessed beta diversity on Bray–Curtis dissimilarity with the ADONIS permutational multivariate analysis. Correlations between the Shannon Index and various factors such as DMI, methane production, and MEF were completed using a linear regression model.
RESULTS
DM and gross energy intake did not vary by the feeding method (p > 0.10; Table 3). The feeding system type did not affect DM, CP, and NFC digestibility (p > 0.10). Although NDF digestibility (NDFD) differed between the feeding groups, the NDFD of the TMR group was 4.73% higher than the SF (p = 0.013).
The different feeding methods did not affect methane production (g/d), DMI (g/d/kg), digestible (d) DM (g/d/kg), dNDF (g/d/kg), or MCF yields (p > 0.10; Table 4). Consequently, the MEF increased and was more elevated in the TMR group than in the SF (p = 0.089). There were no ruminal fermentation parameter differences between the groups (p > 0.05; Table 4).
Methane conversion factor = gross energy percent in feed converted to methane [1].
Methane emission factor = (MJ/head/d of gross energy intake) × (MCF ÷ 100) × 365 ÷ (55.65 MJ/kg of methane) [1].
Illumina sequencing detected 172,902 bacterial and 140,210 archaeal sequences. Notably, the Shannon Diversity Index of ruminal bacteria was significantly higher in TMR-fed steers (p = 0.038; Fig. 1A), while ruminal archaea levels did not differ between the groups (p = 0.87). The Chao1 Index of both ruminal bacteria and archaea did not significantly differ between the two feeding systems (Fig. 1A). The PCoA plots did not indicate a relationship between feeding methods and ruminal microbes (p > 0.10; Fig. 1B).
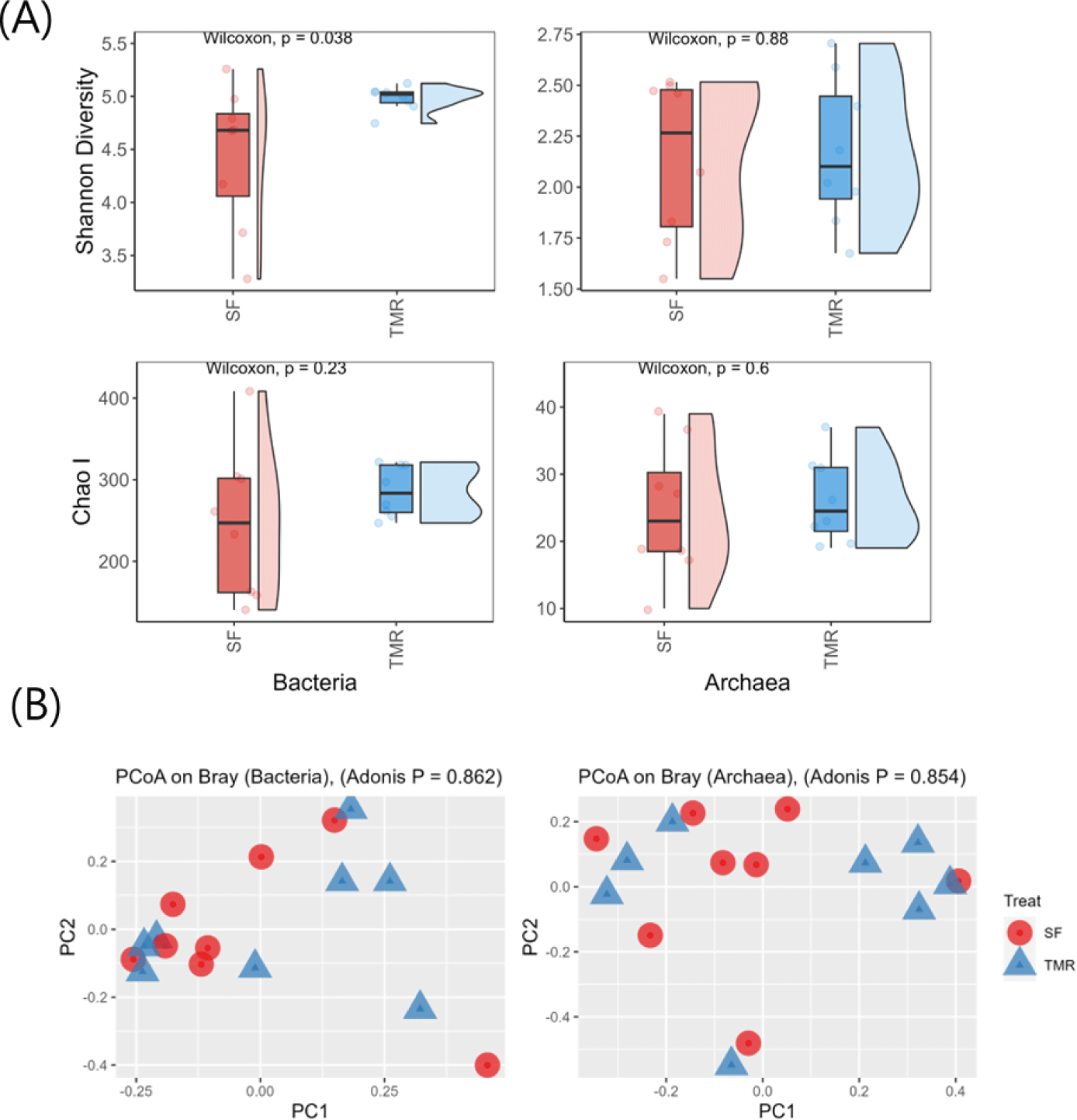
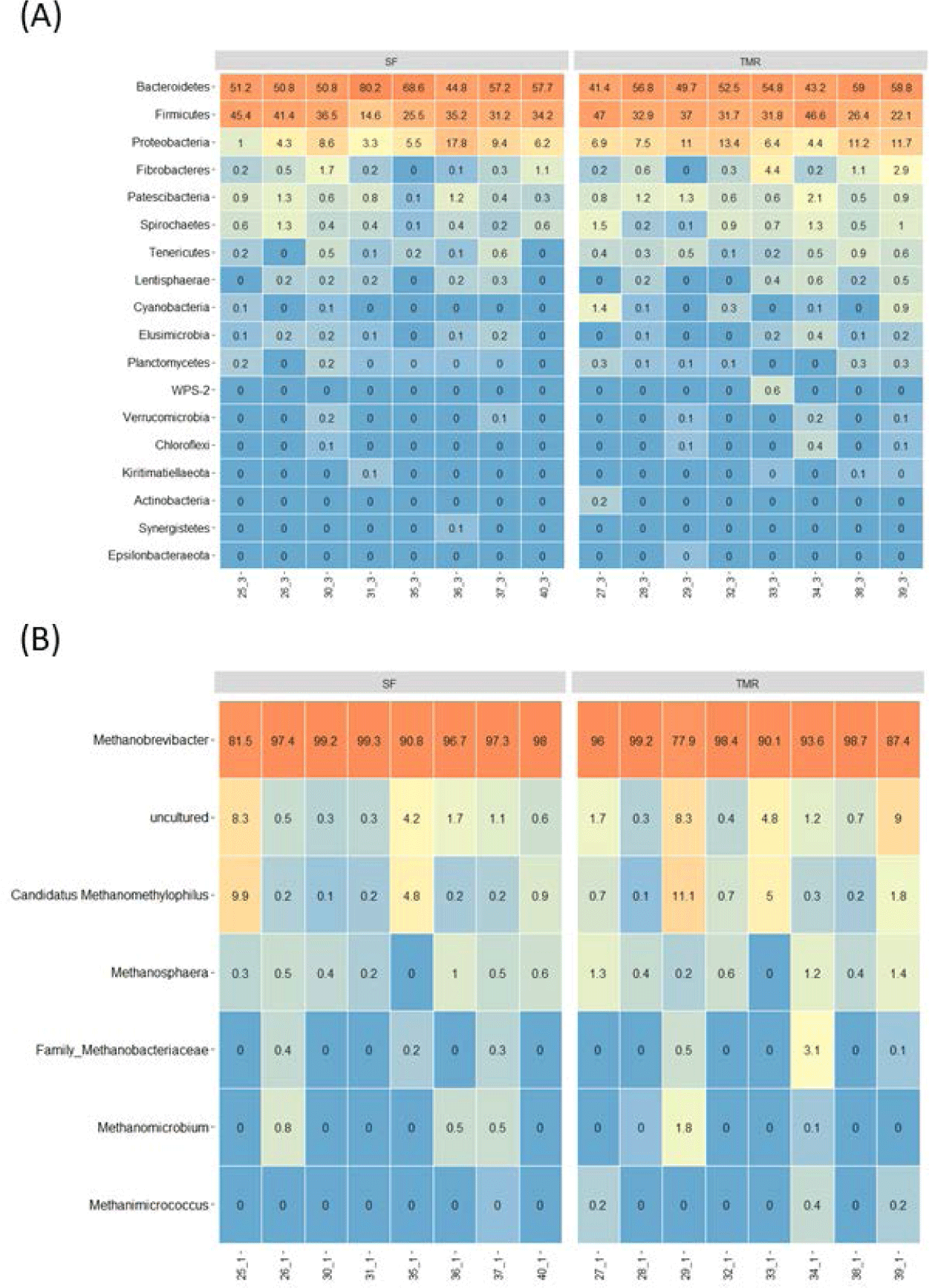
Fig. 2 displays the relative abundance of bacterial phyla and archaeal genera in Hanwoo steer rumens. The most dominant ruminal bacterial phylum in both feeding groups was Bacteroidetes (SF: 57.66%; TMR: 52.03%), followed by Firmicutes (SF: 33.72%; TMR: 34.44%) and Proteobacteria (SF: 8.04%; TMR: 9.06%; Fig. 2A). Bacteroidetes and Firmicutes comprised 80.0%–96.6% of the total taxonomic profile. Fourteen minor phyla were also detected: Fibrobacteres, Patescibacteria, Spirochaetes, Tenericutes (or Mycoplasmatota), Lentisphaerae, Cyanobacteria, Elusimicrobia, Planctomycetes, WPS-2, Verrucomicrobia, Chloroflexi, Kiritimatiellaeota, Actinobacteria, and Synergistetes. The Methanobrevibacter genus was the most prevalent among the ruminal archaeal genera (77.9%–99.3%; Fig. 2B). The other sorted genera included uncultured archaea families Methanobacteriaceae and Methanomethylophilus: Candidatus methanomethylophilus, Methanosphaera, Methanomicrobium, and Methanimicrococcus. However, there were no significantly different bacterial phyla and archaeal genera between the feeding groups.
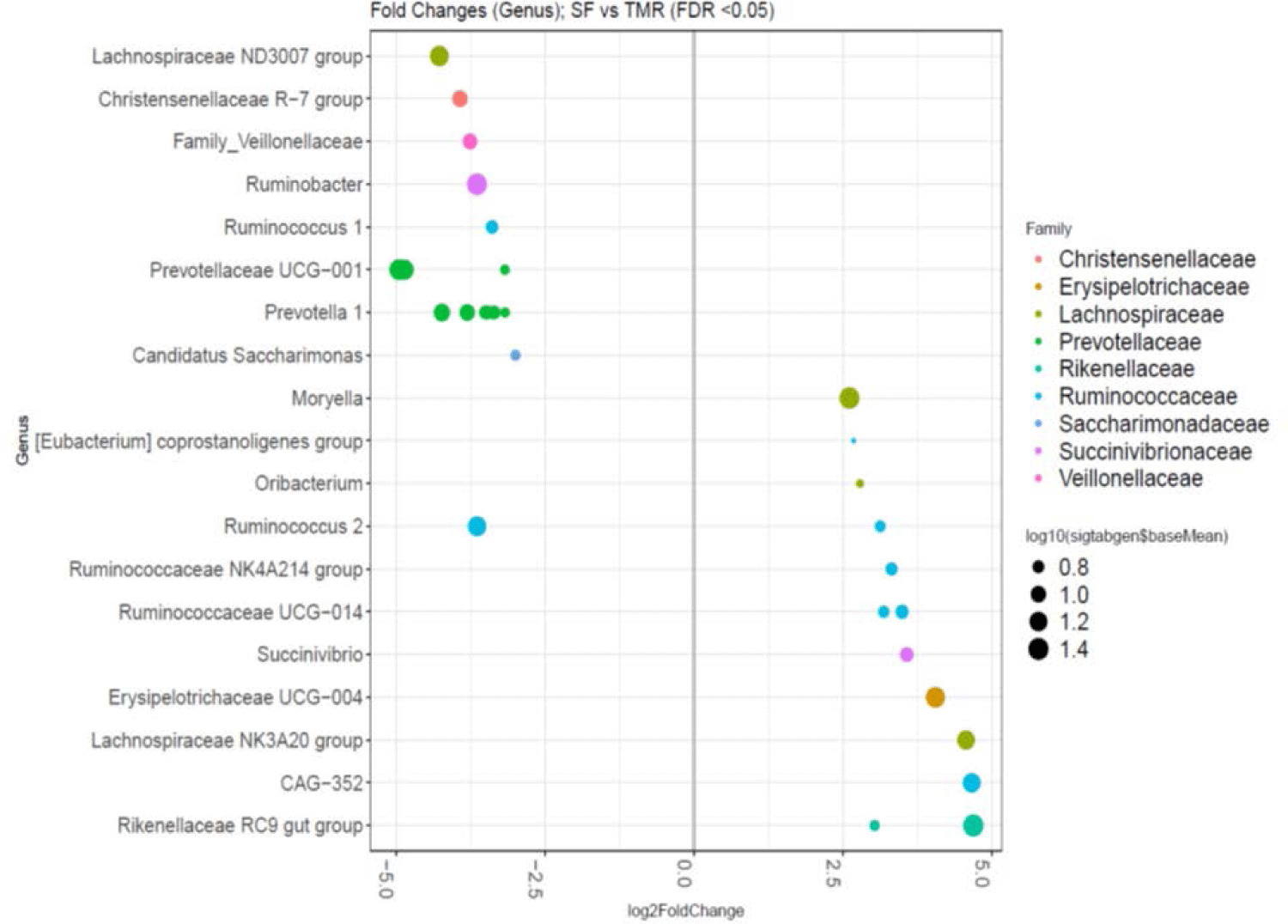
The SF group expressed higher abundances of the Ruminococcaceae family genera (p < 0.05): CAG-352, Ruminococcaceae UCG-014, Ruminococcaceae NK4A214 group, Ruminococcus 2, and Eubacterium coprostanoligenes. Compared to TMR-fed cows, the bacterial abundance of SF group was more enriched with the following genera: gut Rikenellaceae RC9, Lachnospiraceae NK3A20, Erysipelotrichaceae UCG-004, Succinivibrio, Oribacterium, and Moryella (p < 0.05). Comparatively, the TMR group exhibited relatively higher levels of the Prevotellaceae family (genera Prevotellaceae UCG-011 and Prevotella1) than the SF group (Fig. 3). Moreover, the bacterial abundance of the TMR group included: Lachnospiraceae ND3007, Christensenellaceae R-7, Ruminobacter, Ruminococcus 1, Candidatus saccharimonas, and an unidentified Veillonellaceae family genus (p < 0.05).
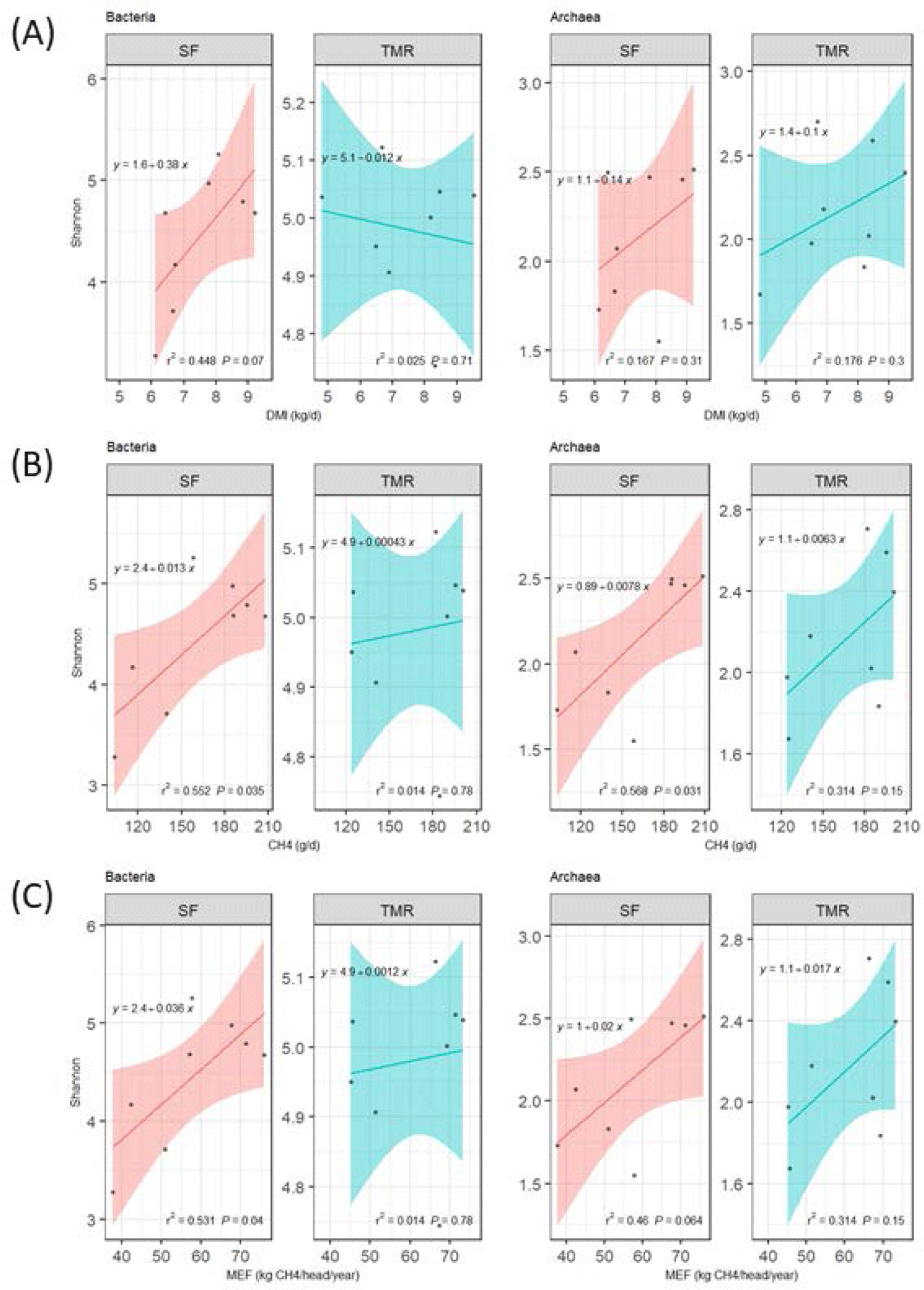
A linear regression analysis was conducted between the bacterial or archaeal Shannon Diversity Index and DMI, methane production, and MEF (Fig. 4) (Table 5). Positive bacterial diversity and DMI correlations were noted in the SF group (R2 = 0.448; p = 0.07); however, statistical archaea differences were not observed (p > 0.10; Fig. 4A). The Shannon Diversity Index (bacteria and archaea) and methane production indicated positive correlations in the SF group (Figs. 4B and 4C); bacterial and archaeal diversities had significantly different correlations with methane production in SF-fed steer (R2 = 0.552 and 0.568 and p < 0.05) (Fig. 4B). In addition, the MEF diversity of the SF group regression models exhibited substantial bacteria (R2 = 0.531; p = 0.04) and an archaea tendency (R2 = 0.46; p = 0.064; Fig. 4C). In contrast, no significant differences were observed between bacterial diversity and methane or MEF in the TMR group (p > 0.10).
DISCUSSION
Ruminal fermentation, microbiota, and methanogenesis are most impacted by diet, followed by breed, host, and other feeding system factors [7,9,15,37]. However, research on how different feeding systems impact ruminal fermentation and methanogenesis in Hanwoo cattle is severely limited. Therefore, we compared the ruminal fermentation, methane emissions, and microbiota of Hanwoo steers when provided with the same amount of feed through SF or TMR systems. Steers were allowed to express selective feeding to mirror Korean beef farm conditions. Moreover, restricted feeding in which steers can entirely consume feed was chosen to compare the exact methane yields by feeding methods.
Previously reported results on feeding method-induced NDFD are inconsistent. One study identified higher fiber digestibility in TMR-fed Hanwoo steers [38], corroborating similar studies that observed improved NDFD in TMR-fed Holstein steers [39]. However, synonymous results were obtained through different feeding systems [7,9]. The apparent factors influencing NDFD are forage particle size, forage maturity, passage rate, and feed intake [40–42]. This study noted that the TMR group exhibited higher NDFD without DMI or ruminal pH fluctuations. Therefore, NDFD alterations may be caused by ruminal bacteria shifts based on the feeding system. Bekele et al. [43] identified Prevotella as the dominant genus in the rumen; many Prevotella members are uncultured and could be involved in fiber degradation. Similarly, the present study revealed that the Prevotellaceae family was abundant in the TMR-diet group, and the presence of unknown Prevotella strains in this family may contribute to fiber degradation, increasing the NDFD of the TMR group. Kononoff et al. [44] indicated that reduction of forage particle size led to increased NDFD. Therefore, the increase in the NDFD in the TMR group may be attributable to the reduction in the particle size during TMR manufacturing. In this study, the improved NDFD in the TMR group could likely be because of the increase in the surface area attacked by the Prevotella species. However, several studies have shown that reduced particle size is associated with decreased fiber digestibility when the increase in the passage rate exceeds that in the digestibility rate [45,46]. In this study, the distribution of feed particle size in the TMR group was > 19 mm (25%), 8–19 mm (25%), and < 8 mm (50%). These particle sizes in the TMR group does not seem to lead to a considerable increase in the passage rate.
In addition, ruminal pH may correlate with methane production; as ruminal pH increased from 5.7 to 6.5, methane production potentially increased as well [47,48]. Crossbred beef heifers’ ruminal pH and methane production associations have been previously reported [49], evidenced by decreased activity of methanogens when ruminal pH is lowered from the dietary concentrate elevation [50]. In this study, the dietary F:C ratios of the SF and TMR group were equally set, demonstrating no changes in the ruminal pH and ruminal methanogen abundance of either group. Thus, methane production may remain unchanged between the treatment groups.
Methane production and MCF between the groups were not significantly different. However, MEF did tend to differ relative to treatment (p = 0.067) because MEF calculations consider gross energy intake and MCF as factors. Previous study results varying from those in this experiment may be due to disproportional forage quantities in the feed. Feed in other studies contained 27% [9] and 25% [7] of roughage as the DM basis; however, in the present study, the feed only contained 15% of roughage. Alterations in the F:C ratio affect the ruminal fermentation environment and determine the feed nutritional levels [51]. The F:C ratio is adjusted relative to the cattle growth stage. The present study selected the F:C ratio for the Hanwoo fattening stage based on Korean feeding standard recommendations [52]. Therefore, in this study, smaller roughage amounts might be responsible for the different results from previous studies.
Furthermore, previous studies have divulged higher methane production and yield in TMR-fed Holstein and Hanwoo steers [7,9]. However, additional studies proclaimed no statistical differences between SF and TMR feeding systems concerning methane emission from Holstein cows [11] and steers [10,12]. Studies that recounted no differences in the methane yield based on feeding methods are consistent with the results of this study. The inconsistent methane production among studies is due to variations in the nutritional level, forage type, and F:C ratio feed. These factors affect ruminal fermentation, subsequently impacting ruminant methane emission [53,54].
The Intergovernmental Panel on Climate Change (IPCC) formed the MEF to estimate methane generated during livestock feed digestion and utilize these findings to establish country-specific emission statistics [1]. Korea has also developed emission factors for beef (Hanwoo) and dairy cattle (Holstein). The Korean ruminant MEF is lower than the IPCC value (over 1-year-old Hanwoo, 61 kg/methane/head/year; IPCC, 64 kg/methane/head/year) because of the country’s unique feeding system [55]. In this study, the MEF was 57.59 in the SF group and 61.18 in the TMR, comparable to the Korean inherent emission factor. Jo et al. [56] analyzed several MEF prediction methods; Hanwoo steers MEF at the finishing stage was predicted as 33.9 when the IPCC Tier 2 method was used. However, Bharanidharan et al. [7] reported that Hanwoo steers MEF measured through respiration chambers was 35.1 in SF and 49.4 in TMR, higher than those predicted by Jo et al. [56] and lower than the measured values in this study. This difference can be explained by experimental animal BWs, which averaged 292 kg in the previous study [7] and 507 kg in this one.
Very few studies have investigated how feeding systems impact ruminant methane emissions. A previous study indicated that an SF diet reduced methane production from Holstein steers even more than the TMR diet did [9]. However, methane production did not differ between TMR and SF strategies in the present study. Bharanidharan et al. [7] elucidated that methane emissions deviated between different breeds fed the same diet (TMR or SF) under identical management conditions. Therefore, this contradictory result is potentially due to breed differences. Yurtseven et al. [57] demonstrated that diet composition impacts methane emissions. Similarly, these contrasting methane production findings could be from varying diet compositions between studies [9]. In a previous study, roughage was fed to animals first, followed by concentrate after 40 minutes [9]; however, avoiding SF was not considered in the present study and may have also contributed to the different production calculations. Thus, the abovementioned factors should be considered in future studies using different feeding systems as a methane-mitigation practice in ruminants.
Although the different feeding practices in this study did not shift prominent microbes, some minor bacterial abundance fluctuations were observed. Ruminal Rikenellaceae have reported a negative relationship with the NDFD, ADFD, and methane yield (L/kg metabolic BW) in sheep [58], corroborating the current study’s findings that the NDFD of the SF group was lower than that of the TMR group. However, Rikenellaceae abundance was also prevalent when yaks were fed fiber-rich diets [59] or when Holstein cows were fed low-starch diets [60]. Erysipelotrichaceae, subsuming the genus Erysipelotrichaceae UCG-004, exhibited a relatively high abundance in sheep rumen with a low methane yield, similar to the present study results where its relative abundance was high in SF with a low MEF [61].
Succinivibrio ferments starch to dextrin in animals [62,63], and some strains possess enzymes that dismantle plant cell walls [64]. Studies using cashew nut shell supplements to attenuate ruminal methane have confirmed reduced methane production or yield with a higher Succinivibrio dextrinosolvens abundance [65,66]. Moreover, previous reports have revealed that lower methane-emitting cows had a higher Succinivibrio spp. ruminal abundance [67,68]. In this study, the SF group did exhibit some bacterial species causing low methane emissions; however, the bacterial community of the TMR group conveyed contradictory results. This observation suggests that methanogens and further bacterial species identifications are required to clarify the methane emission and ruminal microbe relationship.
Prevotellaceae UCG-011 and genus Prevotella1 ASVs were higher in the TMR group than in the SF group. Prevotellaceaeis is a bacterial family that degrades hemicellulose, pectin, starch, and protein in the rumen [69–71]. Despite Prevotella being a prominent bacterium abundant in the rumen, the functions of only some identified species (P. ruminicola and P. bryantii) are known. Although the present study signified that the TMR group with more Prevotellaceae microbes also expressed more MEF, previous studies convey contradictory results. In a cohort study, Colombian buffalos had abundant ruminal Prevotella species in a low methane-emitting group [72]. Moreover, heifers fed with low-forage-containing diets (F:C = 30:70) indicated intensified Prevotella species prevalence [73]; however, Prevotella species dominated high-forage diet-fed cow rumen (F:C = 65:35 and 50:50) compared to low-forage diets (F:C = 35:65) [74]. Another study certified that the Prevotella species was positively correlated with methane yield, NDFD, and ADF digestibility (ADFD) [58], which complements the present study results. These conflicting findings suggest that further studies are required to understand the effect of Prevotellaceae on ruminant methane emissions.
The TMR group, which had a higher NDFD than the SF group, also had relatively higher levels of the Christensenellaceae R-7 genus and the Veillonellaceae family. The Christensenellaceae R-7 group is abundant in high-forage diets and positively correlates with the DMD, NDFD, ADFD, and methane yield [58,59], partially coinciding with our results. The Veillonellaceae family produces propionate as their fermentation end-product. Thus, Veillonellaceae levels are consistently higher in Holstein dry cows fed with high-starch diets [60]. Methane emission was also reduced through encapsulated nitrate supplementation in Nellore steers [68]. A previous study observed Boer goats with a low NDFD [75]. However, Veillonellaceae bacterial family abundance shifts could not be confirmed in this study. Thus, the influence of unidentified ruminal bacteria needs careful investigation.
Although there were no observable statistical DMI and methane emission differences between SF and TMR, the microbial diversity index and DMI, methane production, and MEF linear regression differed by the feeding system. The SF method presented linear regression models applicable for bacteria and archaea approximation; however, none were suitable for the TMR group. Therefore, it is assumed that maintaining a stable ruminal TMR feed environment contributed to maintaining consistent microbial diversity. Previous studies have reported that the TMR systems maintain ruminal pH and acetate-to-propionate ratios, as TMR provides a more balanced and uniform roughage-to-concentrate ratio [76,77].
TMR feeding decreases selective feeding behavior and maintains a stable ruminal environment. Hasty changes in the rumen from the SF strategy can relocate microbes, potentially affecting the DMI and bacterial diversity relationship. However, studies resembling the present experiment did not report a Shannon Diversity Index and DMI correlation [7,9]. Hence, further studies are required to verify the feed intake and bacterial diversity association. Nonetheless, the positive correlation between bacterial diversity, methane production, and MEF is linked to specific bacteria shifts (Fig. 3). Furthermore, an archaeal diversity and methane production association was observed as most ruminal archaea belong to Methanogens [78].
CONCLUSION
This study concluded that different feeding systems for Hanwoo steers given F:C = 15:85 diets did not affect methane production. The overall microbial composition based on the PCoA plot was analogous between feeding systems, although some ruminal microbes did shift. Based on the current data, feed ingredient factors must be considered for further study using different feeding systems to reduce ruminant methane generation. Our results will aid future studies in developing novel feeding systems that reduce methane production by manipulating the ruminal microbiota composition.
