INTRODUCTION
In vitro production (IVP) in cattle, encompassing in vitro maturation of eggs (IVM), in vitro fertilization (IVF), and in vitro culture of embryos (IVC), has evolved significantly since the first calf birth via IVP in 1988 [1]. As of 2021, the technique has developed into a substantial industry. The most remarkable increase occurred in cattle. Over 1.5 million IVP embryos were recorded, an increase of 31.5% compared with 2020 (1,521,018 vs. 1,156,422, respectively) [2]. but in vitro matured oocytes exhibit lower implantation rates compared to in vitro oocytes [3]. A critical approach to overcoming these challenges involves enhancing the quality of oocytes and embryos developed in vitro [4,5].
The disparity in embryo cleavage rates and blastocyst formation during IVC is attributed to differences between the IVC and in vitro environments [6]. In vivo, embryos develop in sub-microliter volumes of fluid, while in vitro conditions involve larger medium volumes [6]. This discrepancy leads to the dilution of autocrine factors essential for embryonic development. Cumulus oocyte complexes (COCs) and embryos during IVM and IVC secrete crucial paracrine and autocrine factors [7,8], underscoring the importance of a microenvironment that mimics in vitro conditions.
Given the need to preserve genetic identity in cattle Ovum Pick-Up (OPU)/IVP embryos, individualized culture is essential. The average number of oocytes retrieved via OPU is 17.1 [9], necessitating the culture of small quantities of oocytes and embryos. However, this limited number hinders intercellular interactions, adversely affecting embryonic development [10,11].
This study utilizes the newly developed C9 well dish by MKbiotech.co, Korea, designed to enhance the physical environment of IVC. The C9 well dish, with its nine conically narrowing channels, is specifically tailored for culturing small numbers of oocytes/embryos. Oocytes exhibit positive developmental outcomes under high-density conditions [6,10]. This structure is designed to concentrate oocytes, increasing their density and proximity, which promotes autocrine and paracrine signaling. Its structure promotes autocrine/paracrine secretion, hypothesized to facilitate oocyte maturation and embryonic development [12]. In addition, culturing a large number of oocytes may result in negative effects from unfertilized or dead oocytes. However, by culturing a smaller number of oocytes, these negative effects can be minimized, leading to positive outcomes [13]. The separate channels prevent inter-well mixing and enable individual monitoring and evaluation.
The aim of this study is to assess the C9 well dish’s effectiveness in oocyte maturation, embryonic development, and the expression of mitogen- and apoptosis-related genes, determining its potential as an alternative to conventional cell culture dishes, even when culturing a smaller number of oocytes.
MATERIALS AND METHODS
The use of ovaries in this study was approved by the Institutional Animal Care and Use Committee (IACUC) of Chungnam National University, as indicated by the approval number 202103A-CNU-002.
Unless specified indicated, we purchased chemicals and reagents from Sigma-Aldrich Chemical (St. Louis, MO, USA).
Bovine ovaries were sourced from a local slaughterhouse and transported to the laboratory within three hours. Upon arrival, the ovaries were washed with 1,000 mL of saline enhanced with 0.6 mL of penicillin/streptomycin (P/S), maintained at a temperature of 30°C–32°C. COCs were aspirated from follicles measuring 3 to 8 mm in diameter using a 21-gauge needle attached to a 10 mL syringe. Only COCs with uniform cytoplasm and more than three layers of cumulus cells were selected. These were then washed in Tissue Culture Medium 199 (TCM 199; Gibco, Grand Island, NE, USA). For IVM, COCs were allocated to either a 5-well dish (WTA, Cravinhos, SP, Brazil) containing 500 μL or a C9 well dish containing 200 μL of IVM medium. This medium consisted of TCM 199, 10% fetal bovine serum (FBS; Gibco), 7 μg/mL FSH (Vetoquinol USA, Fort Worth, TX, USA), human chorionic gonadotropin (hCG; Intervet International BV, Boxmeer, Netherlands), 0.2 mM sodium pyruvate, 0.785 mM L-cysteine, and 10 ng/mL EGF. The COCs were then matured in a humidified atmosphere with 5% CO2 at 38.5°C for a duration of 22 hours.
After IVM, COCs were denuded using 0.1% hyaluronidase and gentle pipetting. Subsequently, they were washed in a specially prepared medium. This washing medium was composed of 10 mg/mL TCM 199 powder (Gibco), 2 mM NaHCO3, 10 mM HEPES, and 1% P/S. The maturity of the oocytes was confirmed by the presence of the first polar body.
The entire procedure of IVF and IVC was detailed previously [14]. The IVF process involved the use of frozen-thawed semen from fertile cattle. To elaborate, 1 semen straw was thawed for 1 minute in a water bath at 36°C–38°C. The thawed sperm were then subjected to 2 rounds of centrifugation at 288 g for 6 minutes each, using a modified Tyrode’s albumin lactate pyruvate (TALP) medium. This medium included 10 μg/mL heparin, 5 mM caffeine, 1.387 mM D-glucose, and 6 mg/mL bovine serum albumin (BSA). The sperm concentration was subsequently adjusted to 1 × 107 sperm/mL in the same modified TALP medium. Following IVM, the COCs were washed in the modified TALP medium. Next, 15 COCs were placed into 90 μL droplets of the sperm-containing medium (1 × 107 sperm/mL) under mineral oil (SAGE, Malov, Denmark) and incubated in a humidified 5% CO2 atmosphere at 38.5°C for 6 hours. After incubation, sperm and cumulus cells around the presumptive embryos were gently pipetted out using a washing medium. The presumptive embryos were then transferred to an IVC medium, which was a combination of modified CR1aa (C1) and CR2 (C2) media [15], and cultured in a humidified atmosphere of 5% CO2, 5% O2, and 90% N2 at 38.5°C for 7 days. The embryos were kept in C1 medium for the initial 1–3 days and then transferred to C2 medium for the remaining 4–7 days.
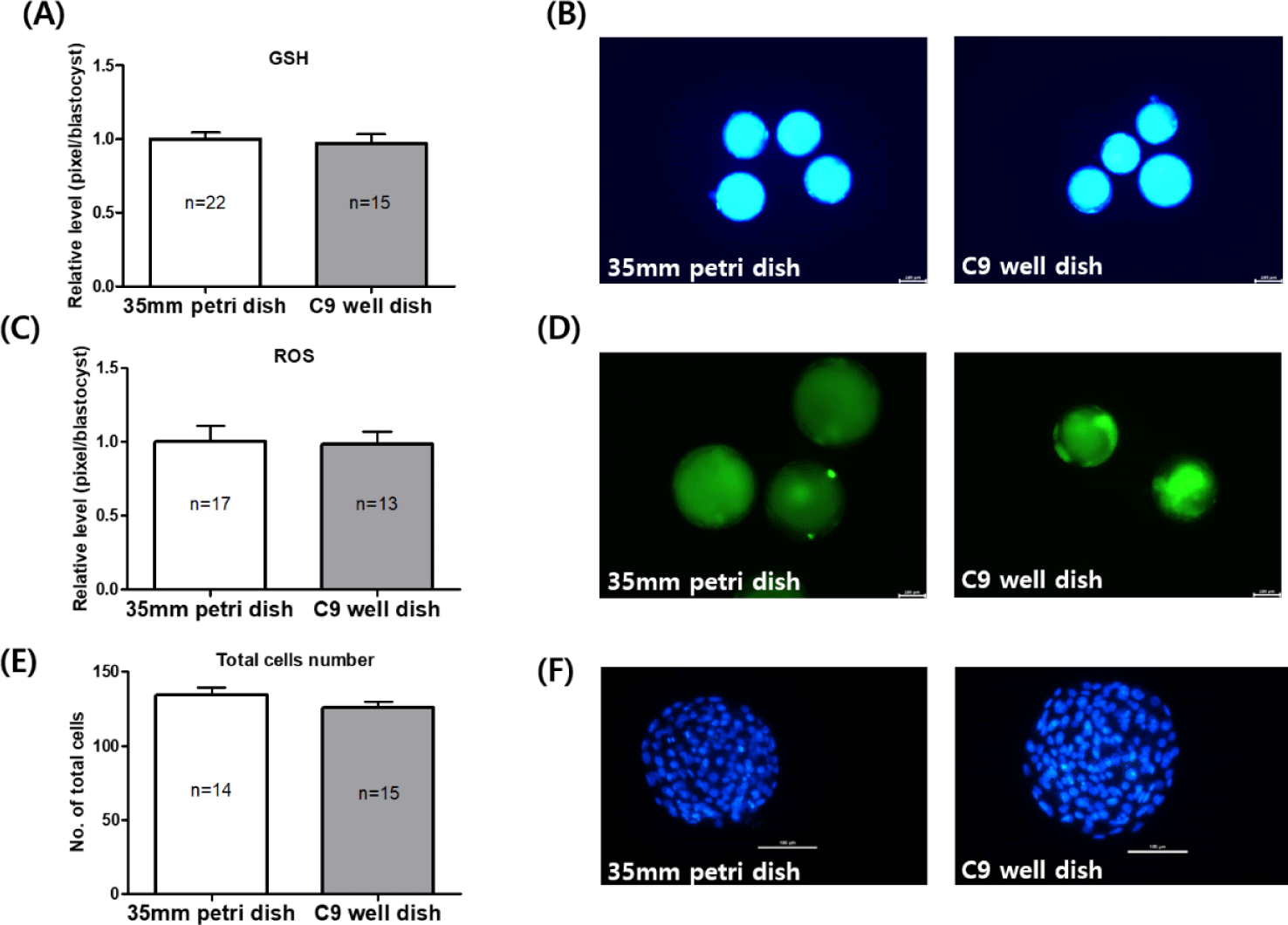
Observations of the cleaved embryos were performed using a stereomicroscope on Day 2. The evaluation of blastocyst formation and the counting of total cell numbers took place on Day 7. To aid in this counting process, the blastocysts collected on Day 7 were stained for 10 minutes at 38.5°C in a washing medium containing 1 μg/mL Hoechst 33342. This staining technique is employed to label DNA, thereby simplifying the visualization of cell nuclei. Post-staining, the blastocysts were rinsed with phosphate-buffered saline (PBS) and mounted on glass slides using 1,2 μL of glycerol, followed by covering with cover slips for detailed examination. Finally, the total cell counts within these blastocysts were determined using a fluorescence microscope (DMi8, Leica Microsystems CMS GmbH, Wetzlar, Hesse, Germany), at a 100× magnification.
The matured oocytes and Day 7 blastocysts were sampled to determine intracellular GSH and ROS levels, which were measured by previously described methods [16]. The denuded matured oocytes and blastocysts from each group were incubated (in the dark) for 30 minutes in washing medium containing 10 mM H2DCFDA and 10 mM CellTracker Blue at 38.5°C. After incubation, oocytes and blastocysts were washed three times with PBS with 5% FBS. The fluorescence was observed under a fluorescence microscopy (DMi8; leica microsystems) with UV filters (460 nm for ROS and 370 nm for GSH). The fluorescence intensities were analyzed using ImageJ software (version 1.53, National Institutes of Health, Bethesda, MD, USA) and normalized to control samples.
Blastocysts from each experimental group were individually harvested for gene expression analysis. These samples were then stored at -80°C until analysis was conducted. For RNA extraction, over 40 blastocysts from each group were processed using the RNAqueous-Micro Kit (Ambion, Austin, TX, USA), following the manufacturer’s guidelines. The quality and concentration of the extracted RNA were determined using a spectrophotometer (Biospec-nano, Shimadzu, Kyoto, Japan), a crucial step to ensure the integrity of the RNA. Next, complementary DNA (cDNA) was synthesized from the extracted RNA, utilizing the Maxime RT PreMix Kit (Intron Biotechnology, Seongnam, Korea) with 20 μL of total RNA. The synthesized cDNA was then preserved at −80°C until it was utilized for quantitative real-time polymerase chain reaction (qRT-PCR) amplification.
The levels of gene expression for TGFα, PDGFβ, IGF-1, Bcl-2, and Bax in the blastocysts were evaluated using qRT-PCR. For the reaction mix, 1 μL of cDNA was combined with 10 μL of SYBR Green Supermix (Bio-Rad, Hercules, CA, USA) a reagent that detects accumulating PCR products along with 8.2 μL of nuclease-free water (Invitrogen, Grand Island, NY, USA), and 0.4 μL of both forward and reverse primers (10 pmol/μL) specific to each gene. This mixture was then placed into a PCR plate (Bio-Rad). The amplification process involved using the CFX96 Touch Real-Time PCR system (Bio-Rad) and followed a specific protocol: initial denaturation at 95°C for 3 minutes, 40 cycles consisting of 10 seconds at 95°C for denaturation, 45 seconds at 55°C for annealing, and 1 minute at 72°C for extension. Details of the primer sequences are provided in Table 1. Gene expression was quantified in relation to the internal control gene, GAPDH. The relative expression levels were calculated using the formula R = 2−(ΔCt_sample − ΔCt_control), in accordance with the methodology described by Park et al. [16].
The C9 well dish features a square design, with each side measuring 6.5 cm in both length and width, and a height of 1 cm (Fig. 1). It is partitioned into a total of 9 channels, designed for the individual cultivation of samples in each. These channels are uniquely conical in structure, meaning their cross-sectional area tapers from top to bottom. At the top, the diameter of each channel measures 1.5 cm, which gradually narrows to about 0.3 mm at the bottom, with a depth of approximately 0.8 cm. This design plays a pivotal role in ensuring physical separation between the channels, which is essential for preventing cross-contamination and maintaining cellular isolation, especially against external disturbances during the culturing process. Moreover, the conical shape of each channel is instrumental in directing cells towards the center, creating optimal conditions for cell growth.
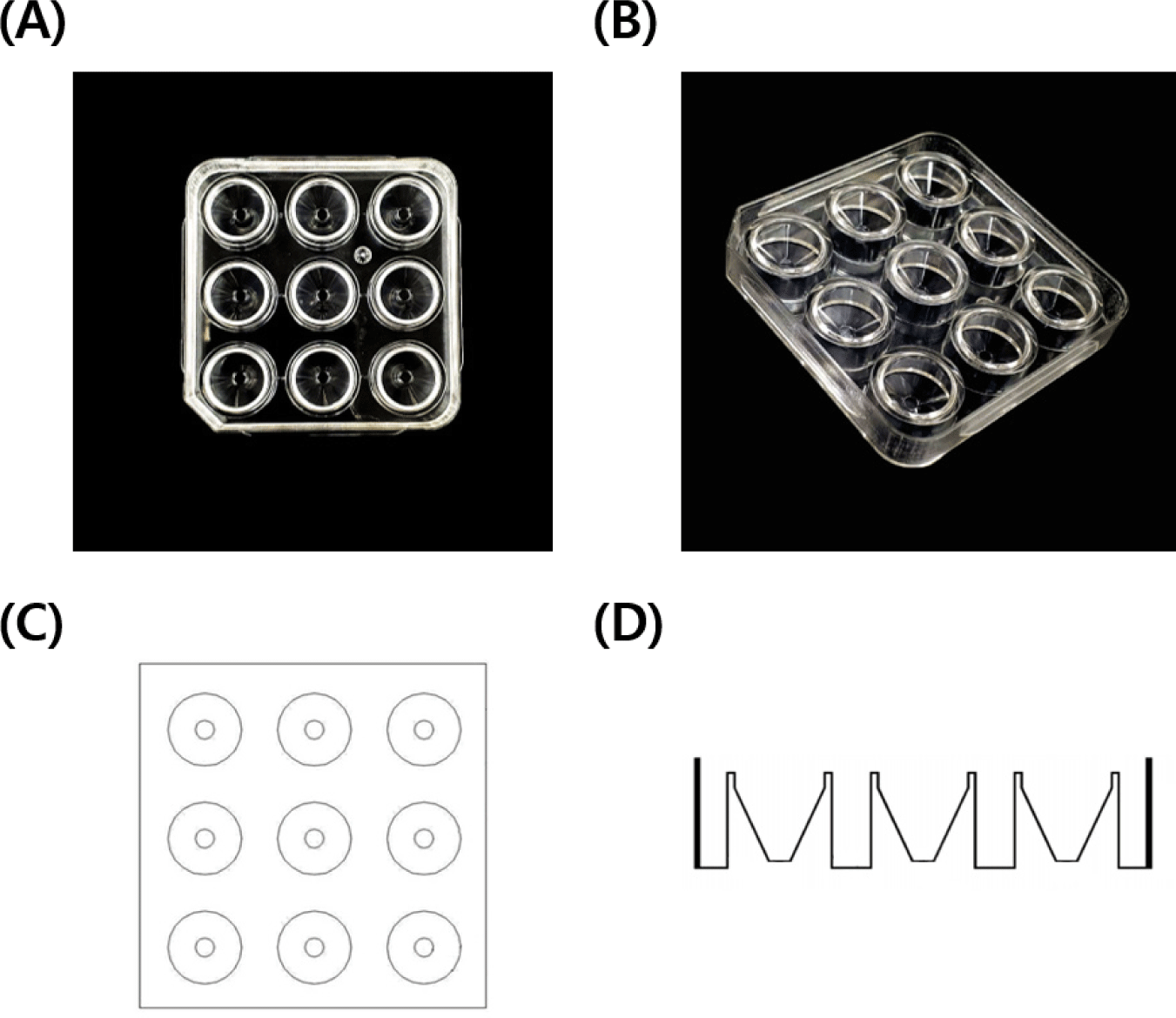
Experiment 1 was designed to determine the more effective dish for IVM of oocytes. This determination was based on the analysis of nuclear maturation, as well as intracellular levels of GSH and ROS, in oocytes matured in both 5-well and C9 well dishes. To achieve this, COCs were evenly distributed into two groups. In order to ensure similar oocyte densities across the groups, 30–40 COCs were cultured in 500 μL of IVM medium per well in the 35es, while 13–15 COCs were placed in 200 μL of IVM medium per well in the C9 well dishes.
Experiment 2 followed, using oocytes cultured in C9 well dishes as per the findings of Experiment 1. After IVF, the resulting presumptive embryos were randomly allocated into two groups for further culture. One group was cultured in a standard 35 mm petri dish (Corning, NY, USA), while the other was placed in a C9 well dish. The objective was to assess the impact on embryonic development, intracellular GSH and ROS levels, and mRNA expression in the blastocysts. In the 35 mm petri dish, 13-15 embryos were cultured in 50 μL of IVC medium, covered with 4.5 mL of mineral oil. Conversely, in the C9 well dish, 7-8 embryos were cultured in 50 μL of IVC medium, with a covering of 100 μL of mineral oil. Both groups were incubated under the same conditions: a humidified atmosphere containing 5% CO2, 5% O2, and 90% N2 at 38.5°C for a duration of 7 days.
To ensure the reliability of the results, each experiment was repeated a minimum of 3 times. The data collected from these iterations were consolidated and are presented as mean values ± standard error of the mean. This approach amalgamates the findings from the repeated trials for a more comprehensive analysis. For statistical comparison of factors such as nuclear maturation, intracellular levels of GSH and ROS, embryo development, and gene expression, the Student’s t-test was utilized. This analysis was performed using GraphPad Prism 5 (GraphPad Software, San Diego, CA, USA), a software specifically tailored for biomedical statistical evaluations. A p-value of less than 0.05 was set as the threshold for statistical significance.
RESULTS
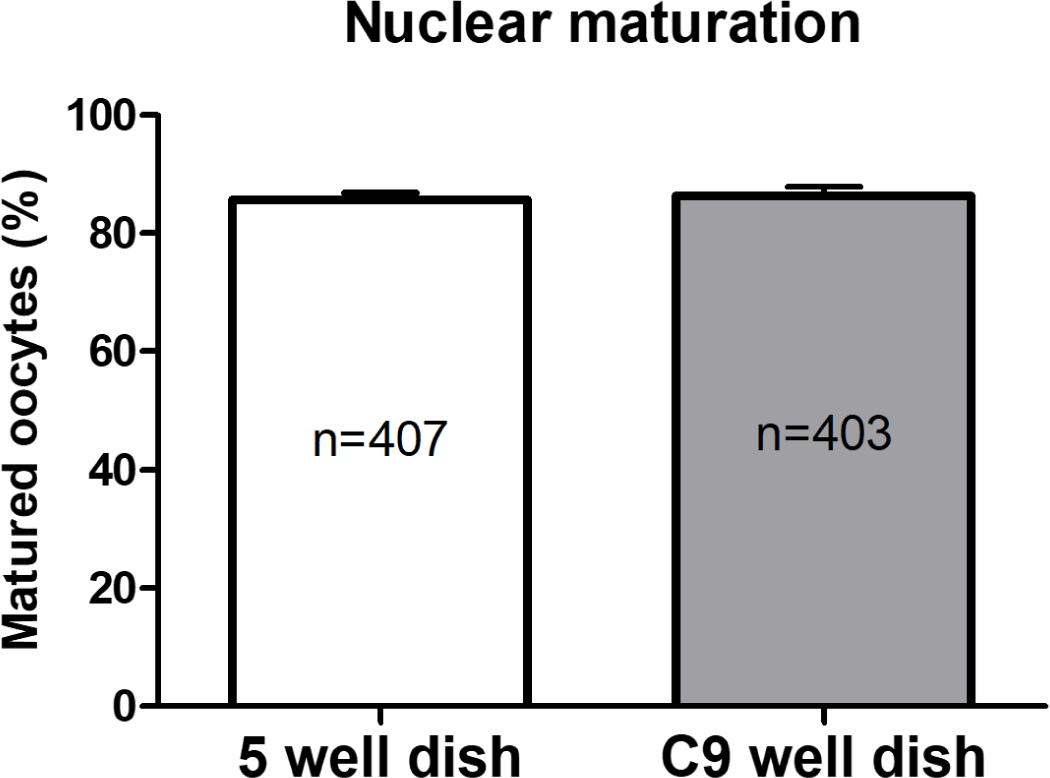
In Experiment 1, the nuclear maturation of oocytes cultured in both 5-well and C9 well dishes during the IVM phase was evaluated. This assessment was based on the presence of the first polar body, a key indicator of successful oocyte maturation. As illustrated in Fig. 2, the findings revealed comparable rates of nuclear maturation between the two dish types, with no statistically significant differences noted. Specifically, the maturation rate in the 5-well dishes was recorded at 86.34 ± 1.57%, whereas it was 85.68 ± 1.18% in the C9 well dishes.
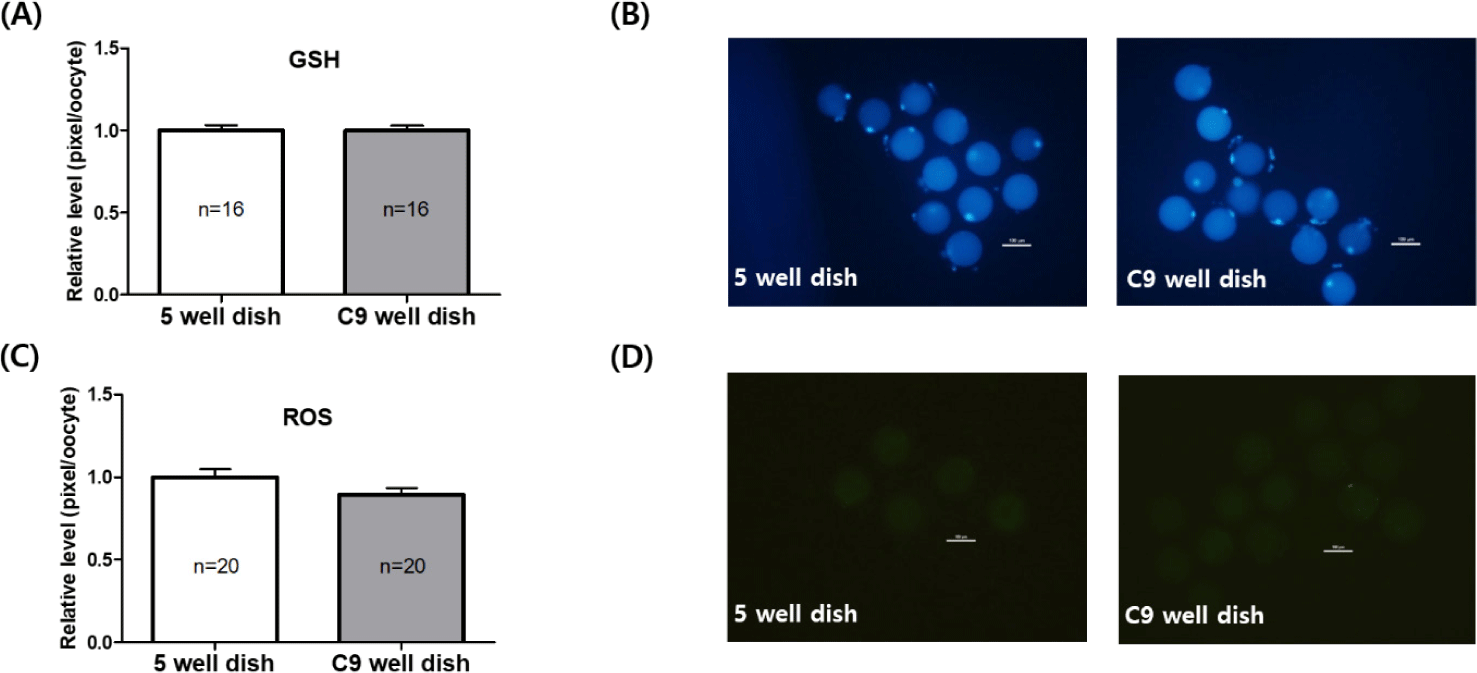
In Experiment 1, we assessed the levels of intracellular GSH and ROS in oocytes that were matured in both 5-well and C9 well dishes. The evaluation of GSH, which is a crucial antioxidant, revealed similar concentrations in oocytes from both dish types. Specifically, the GSH levels were 1.00 ± 0.03 in the 5-well dish and 1.00 ± 0.02 in the C9 well dish, as depicted in Fig. 3A. In terms of ROS, recognized as markers of oxidative stress, a marginal reduction was noted in oocytes from the C9 well dish (0.89 ± 0.03) compared to those in the 5-well dish (1.00 ± 0.04). However, this observed difference was not statistically significant, as outlined in Fig. 3C.
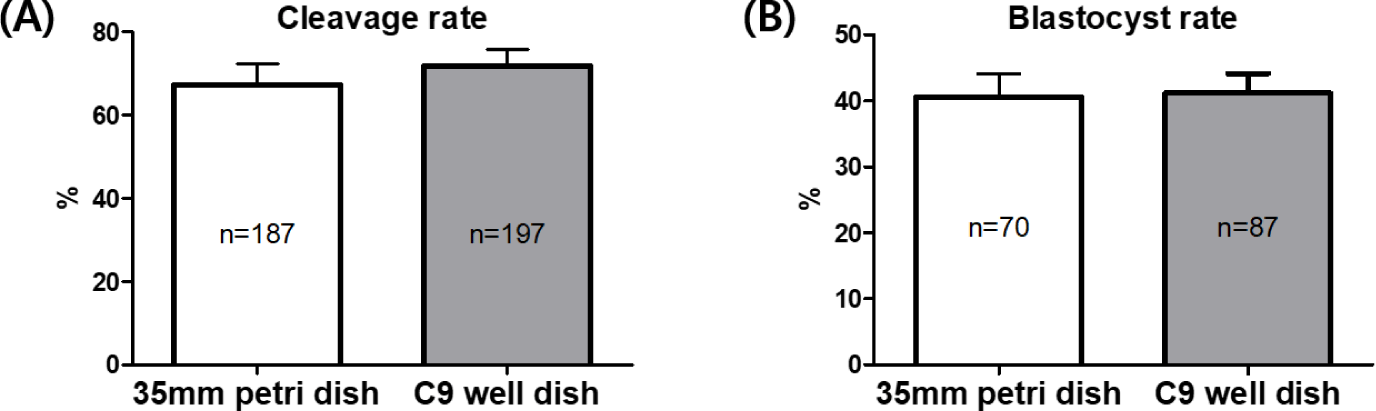
After conducting IVF on mature oocytes from C9 well dishes, the resultant embryos, referred to as putative embryos, were cultured in both 35 mm petri dishes and C9 well dishes. This was done to perform a comparative analysis of their embryonic development. The analysis indicated a slightly higher cleavage rate in the C9 well dishes by 4.6% compared to the 35 mm petri dishes (67.14 ± 5.23% vs. 71.74 ± 4.06%). However, this difference was not statistically significant, as demonstrated in Fig. 4A. Similarly, the rates of blastocyst formation were found to be nearly equivalent between the two types of dishes, recorded at 40.60 ± 3.48% in the 35 mm petri dishes and 41.20 ± 2.95% in the C9 well dishes, as illustrated in Fig 4B.
In Experiment 2, the levels of intracellular GSH and ROS were evaluated in blastocysts cultured in both 35 mm petri dishes and C9 well dishes. The GSH levels in blastocysts derived from C9 well dishes were found to be similar to those from 35 mm petri dishes, with measurements of 1.00 ± 0.04 in the C9 well dish versus 0.96 ± 0.06 in the 35 mm dish, as illustrated in Fig. 5A. Similarly, the ROS levels did not show a significant difference between the two types of dishes (Fig. 5C). Additionally, the total cell count of blastocysts on day 7 of IVC was slightly lower in the C9 well dish (125.9 ± 3.86) compared to the 35 mm petri dish (134.1 ± 5.25), but this difference was not statistically significant, as shown in Fig. 5E.
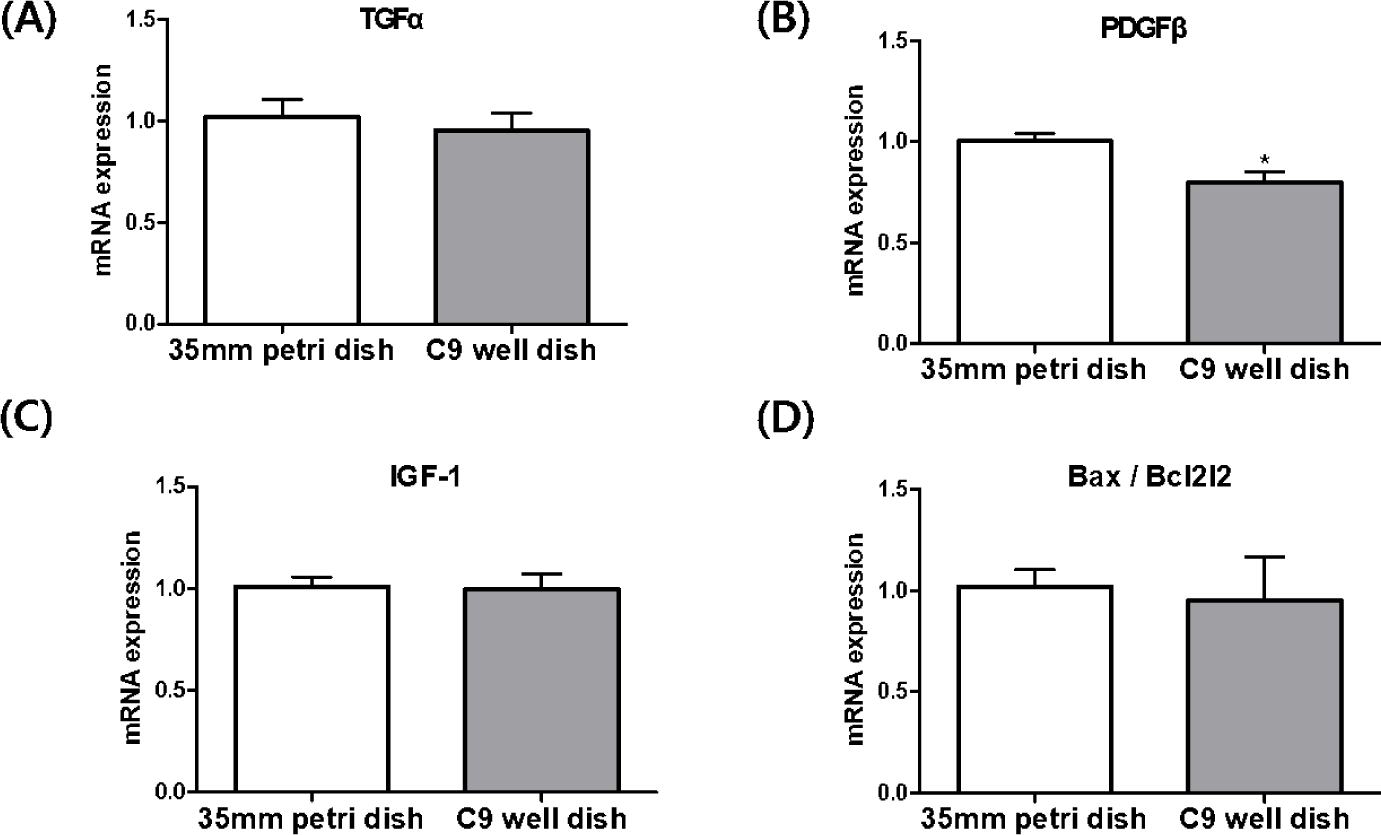
In the analysis of blastocysts derived from IVF in both 35 mm petri dishes and C9 well dishes, we investigated the relative expression levels of genes such as TGFα, PDGFβ, IGF-1, and the Bax/Bcl2l2 mRNA transcripts (Fig. 6). Our focus was particularly on genes associated with mitogens. The results revealed that PDGFβ mRNA transcript levels were notably lower in the blastocysts from the C9 well dish compared to those from the 35 mm petri dish, a difference that was statistically significant (p < 0.05) However, the expression levels of TGFα and IGF-1 mRNA did not show significant differences between the two groups. In addition, the expression ratio of the apoptosis-related genes, Bax and Bcl2l2, exhibited similar patterns in blastocysts from the C9 well dish group and those from the 35 mm petri dish group.
DISCUSSION
This research was carried out to assess the effectiveness of the newly designed C9 well dish in the processes of bovine IVM and IVC. The findings revealed that in terms of nuclear maturation, as well as GSH and ROS levels, oocytes matured in the C9 well dish showed results comparable to those in the widely used 5-well dish. This equivalence suggests that the C9 well dish is proficient in facilitating the IVM of cattle oocytes. Moreover, during the IVC phase, embryos cultured in the C9 well dish reached the blastocyst stage, exhibiting a developmental efficiency similar to that of embryos cultured in standard 35 mm petri dishes. Taken together, these outcomes suggest that the C9 well dish is a viable and effective option for both the IVM and IVC stages in bovine embryonic development.
In Experiment 1, which aimed to compare the maturation of oocytes in C9 well and 5-well dishes during IVM, two key aspects were assessed: nuclear maturation, indicated by the extrusion of the first polar body, and cytoplasmic maturation, evaluated through the measurement of intracellular levels of GSH and ROS. The results demonstrated no significant differences in either nuclear or cytoplasmic maturation between the two types of dishes. This outcome is particularly noteworthy in light of prior studies that reported adverse effects on oocyte development when cultured in smaller groups [6,10,11]. Despite its design, which narrows towards the bottom and accommodates fewer oocytes [13–15], the C9 well dish showed an average nuclear maturation rate of 85.68% (Fig. 2). This rate aligns with the 80%–90% range recently reported for bovine IVM [17, 18]. The study hypothesizes that this high maturation rate, despite the smaller oocyte number, may be attributed to effective autocrine/paracrine interactions enabled by the unique structural characteristics of the C9 well dish.
In terms of cytoplasmic maturation, the GSH levels were found to be comparable in both dish types. While the ROS level in the C9 well dish was slightly lower, the difference was not statistically significant (Fig. 3). It’s important to note that excessive ROS production can trigger oxidative stress, leading to detrimental outcomes such as DNA damage, impaired oocyte growth and quality, and reduced ATP synthesis capability [18–20]. Moreover, oocyte-derived paracrine factors like TGF and FGF play a vital role in regulating apoptosis, ROS levels, and meiotic resumption [21]. The study suggests that the design of the C9 well dish might contribute to maintaining controlled ROS levels through efficient regulation of paracrine secretion, thereby supporting adequate nuclear and cytoplasmic maturation, even with fewer oocytes.
In Experiment 2, we compared embryo development, blastocyst levels of GSH and ROS, total cell number, and the expression of genes related to mitogenesis and apoptosis between embryos cultured in C9 well dishes and standard 35 mm petri dishes. On the second day of IVC, the cleavage rate in C9 well dishes were observed to be 4.6% higher than in 35 mm petri dishes; however, this difference did not reach statistical significance, as shown in Fig. 4A. Similarly, blastocyst formation rates on day 7 of IVC were comparable between the two dish types, aligning with the 30%–40% efficiency reported in prior bovine IVF embryo studies using commercialized cell culture dishes [22–24]. In our study, blastocyst formation in IVF embryos cultured in C9 well dishes averaged 41.20%, as depicted in Fig. 4B, underscoring their efficacy in embryo development.
Further analysis focused on the expression of mitogen and apoptosis-related genes in the blastocysts. Mitogens such as TGFα, IGF-1, and PDGFβ are crucial in inducing cell division [6,25]. TGFα, structurally similar to EGF, binds to the EGF receptor, triggering an increase in cyclic adenosine monophosphate (cAMP) and promoting embryonic development [26]. Autocrine/paracrine activation of TGFα has shown benefits in embryonic development [27], with its supplementation during bovine IVC linked to increased blastocyst formation [28]. IGF-1, essential for cell proliferation, is produced in the fallopian tubes and uterus of cattle during early estrus [29] and has been linked to improved cell numbers in the inner cell mass (ICM) and trophectoderm (TE) of embryos when supplemented during IVC [30,31]. Our data showed similar expression levels of TGFα and IGF-1 between the two dish types, correlating with their comparable embryonic development and blastocyst formation rates. However, the expression of PDGFβ, a gene involved in the 8-16 cell stage of embryonic development [28], was significantly lower in blastocysts derived from C9 well dishes. Despite varying reports on the influence of PDGFβ on blastocyst formation [28,32], our results demonstrate its downregulation in C9 well dish-derived blastocysts without adversely affecting blastocyst formation rates. This finding suggests that PDGFβ may not be a critical factor in blastocyst formation in cattle.
This study has demonstrated that the C9 well dish offers efficiency comparable to commercialized cell culture dishes, even when used for culturing a limited number of oocytes or embryos. This indicates that the C9 well dish is a viable alternative for the culture of bovine oocytes and embryos, offering a practical and economically beneficial substitution for standard commercialized dishes. Recommended for culturing 7-15 oocytes per well, the C9 well dish is particularly effective for handling the small quantities of oocytes typically retrieved from cattle via OPU. Its design, which requires reduced volumes of culture medium and mineral oil, presents significant economic advantages by minimizing resource use while maintaining high standards of oocyte and embryo development. Furthermore, it is more cost-effective than a 5-well dish or a 35 mm petri dish. Lastly, the physically separated structure is user-friendly, even for beginners.
