INTRODUCTION
Atopic dermatitis (AD) is a common inflammatory skin disease that affects humans and dogs [1–3]. Recent studies have focused on understanding the clinical and immunological signs of AD in dogs. The common signs of this condition include itching, scratching, rubbing, licking, skin redness, and hair loss [4–6]. When the skin is damaged, it is more susceptible to infection, and the body increases the amount of cortisol in response to fight the infection.
Cortisol as stress hormone secreted in blood stream is a natural response to both external and internal stimuli in animals [7]. However, when animals are exposed to stimuli over a long period, excessive concentrations of cortisol are produced, which can have detrimental effects [8–10]. Poor environmental conditions and infection can cause stress in animals, leading to physical, physiological, psychological, and behavioral responses [9,11,12].
The relationship between stress and diseases or behavior has been widely studied in dogs and is often assessed by monitoring cortisol concentrations [1,13,14]. One preferred method for chronic stress assessment involves monitoring hair cortisol concentration (HCC), as cortisol is incorporated into the hair shaft during hair growth, allowing for analysis over weeks or months [15,16]. Previous studies have examined HCC in healthy and unhealthy dogs [1,14], as well as dogs displaying various social behaviors [13], body conditions, and nutritional statuses [17].
Recently, the health and welfare of companion dogs, including those with AD, have received increasing attention. Understanding the relationship between stress levels and AD can improve companion dog care. Thus, we hypothesized that assessing HCC as a biomarker of chronic stress might be useful for understanding the role of AD in stress in companion dogs. Therefore, the aim of this study was to explore stress levels in companion dogs with AD through HCC analysis and to compare them with non-AD dogs whose owners reported that the dogs showed different behaviors.
MATERIALS AND METHODS
The experimental procedures and methods were approved by the Institutional Animal Care and Use Committee (IACUC) of Kangwon National University, Chuncheon, Korea (KW-220915-1). The experiment was noninvasive and did not cause pain or suffering.
In this study, 202 companion dogs in Korea were examined from 2019 to 2023 (Table 1). The owners provided information about their dogs, including their lifestyle, such as diet, bathing, exercise routines, living in single or multidog households, any medical treatments (e.g., steroid drugs) they received, and details about AD and normal or abnormal skin. Our analysis was based on the information provided by the dog’s owners that were diagnosed AD by veterinarians. However, we initially focus on finding a correlation between AD with HCC and the management for companion dogs in Korea are generally similar, therefore, we did not acquire such management data and objective skin score.
Hair samples were carefully harvested once by dog owners from the neck region of the dogs using scissors and placed in foil bags labeled with the general identifying name, age, sex, date, and skin condition. The samples were sent to the laboratory for processing and analysis. HCC was measured using commercial enzyme-linked immunosorbent assay (ELISA) kits (1-3002– Salimetrics LLC, State College, PA, USA) and a plate reader (450 nm measurement wavelength; SpectraMax absorbance reader, Molecular Device LLC, San Jose, CA, USA) as previously described by Ghassemi Nejad et al. [18].
RESULTS AND DISCUSSION
In this study, HCC in dog breeds with and without AD, including bichon frise, maltese, and poodle breeds living in Korea, was evaluated using ELISA (Fig. 1).
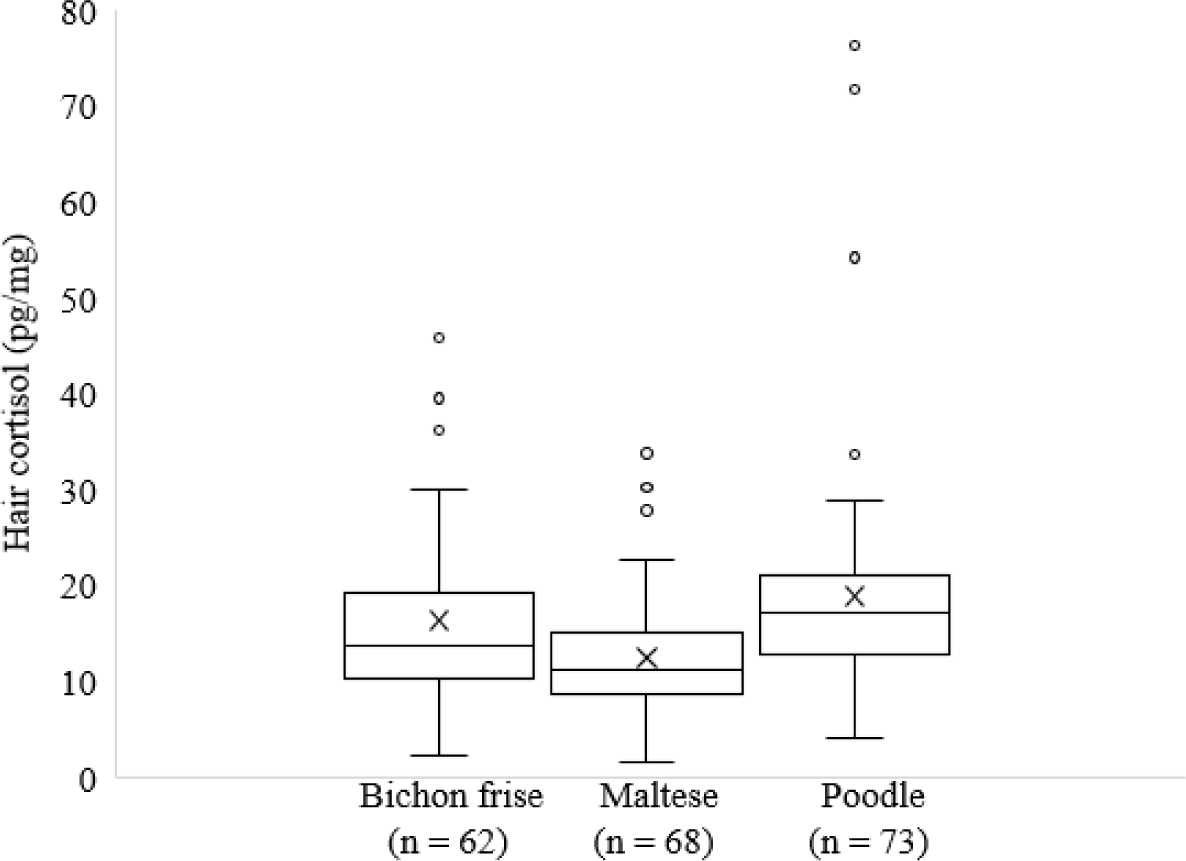
In this study, we investigated the HCC in dog breeds with and without AD. HCC provides information about how cortisol concentrations change over time. This makes it a useful biomatrix for monitoring animal stress, health, and deficiencies in minerals and vitamins [16]. Hair has long been used as a biomatrix for cortisol measurements in both wild and domestic animals [19]. Our study indicated that HCC was significantly lower in maltese dogs than in bichon frise and poodle dog breeds. In dog breeds, hair follicles have a lifelong capacity for hair growth [20]. Hormonal changes, seasonal variations, and breed-specific factors may affect the hair cycle, causing different patterns of hair growth and molting and subsequently affecting HCC [20,21].
The HCC was compared between AD and non-AD breeds in bichon frise, maltese, and poodle dog breeds (Fig. 2). The HCC in bichons frises and poodles with AD were higher than those in non-AD (p < 0.05). However, no significant differences in HCC were observed between AD and non-AD maltese (p > 0.05).
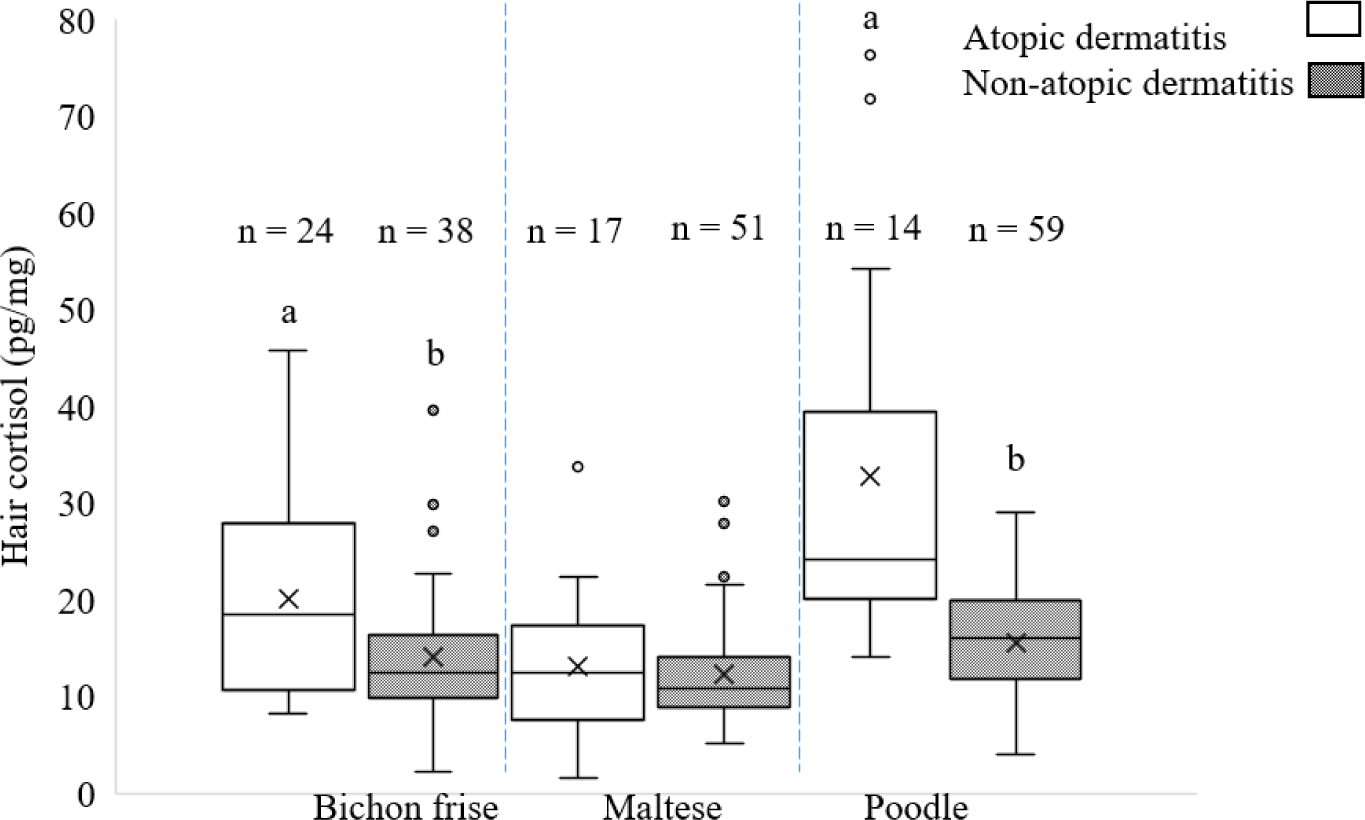
The comparison of HCC in bichon frise, maltese, and poodle dogs with and without AD was a crucial aspect of this study. HCC may be influenced by intrinsic and extrinsic factors, such as lifestyle, seasonal fluctuations [22], medications, environmental conditions, and behavioral problems [23]. The explanation for the differences in HCC in this study could be that each breed has its own genetic and behavioral characteristics and sensitivity to allergens. The breed allergy test showed that maltese, bichon frise, and poodle breeds had the highest percentages of dogs with AD [24]. Previous studies have reported that age and sex do not affect HCC [14,25]. The results indicated that HCC in the bichon frise and poodle with AD was significantly higher than that in the bichon frise and poodle without AD. This could be related to the severity of AD symptoms, the dog’s response to discomfort, or the overall stress sensitivity of the breed. In contrast, the lack of a significant difference in HCC between maltese dogs with and without AD may suggest that the presence of AD did not significantly induce stress in maltese dogs, at least as measured using HCC. The observed differences in HCC between breeds may be attributed to a combination of factors beyond the presence of AD. The degree of skin abnormalities caused by AD can be mild, moderate, or severe, and the degree of systemic inflammation may vary [26,27]. Park et al. [14], did not observed differences in HCC between normal and mild AD cases when compared to moderate and severe groups of dogs affected by AD. While, we observed a higher HCC among female maltese with AD. Consequently, it appears that the lower degree of AD may not significantly impact the observed HCC in maltese results. In addition, Park et al. [14] explained clinical severity of AD were positively correlated. We acknowledge a limitation in this study that the AD dogs were not separated based on levels of AD severity. Additionally, there is a recognition of influential factors that may alter HCC The existence of outliers in the HCC data may be related to individual characteristics, health status, or other unknown factors.
In this study, pooled data showed a higher HCC (p < 0.05) in dogs with AD compared to dogs without AD (Fig. 3).
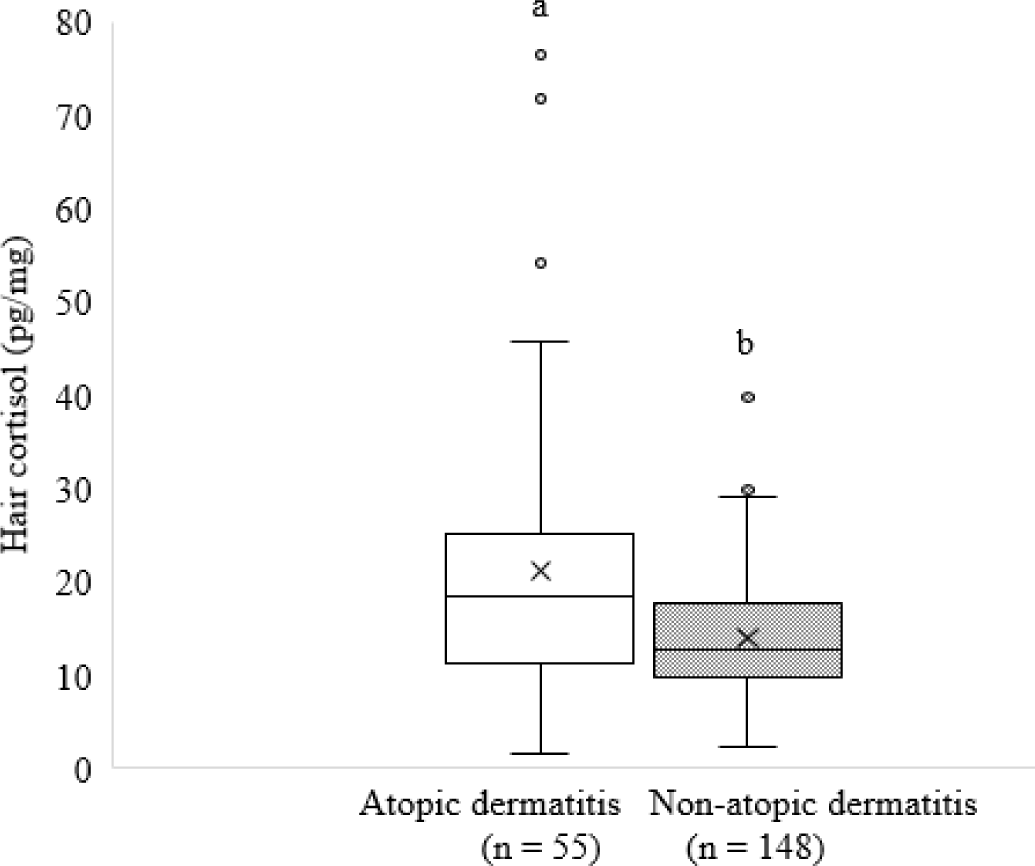
The higher HCC across dog breeds in this study suggests that AD may have a substantial effect on stress levels in dogs. Similarly, previous studies have shown that dogs with AD have higher HCC rates than those without AD [14]. Dog owners may need to provide additional care and support for dogs with AD to manage their stress levels effectively.
The HCC in female and male bichon frise dogs with and without AD are shown in Fig. 4. Values for AD females were significantly higher than those for non-AD female bichon frise dogs (p < 0.05). However, no differences were observed between AD and non-AD male bichon frise dogs (p > 0.05).
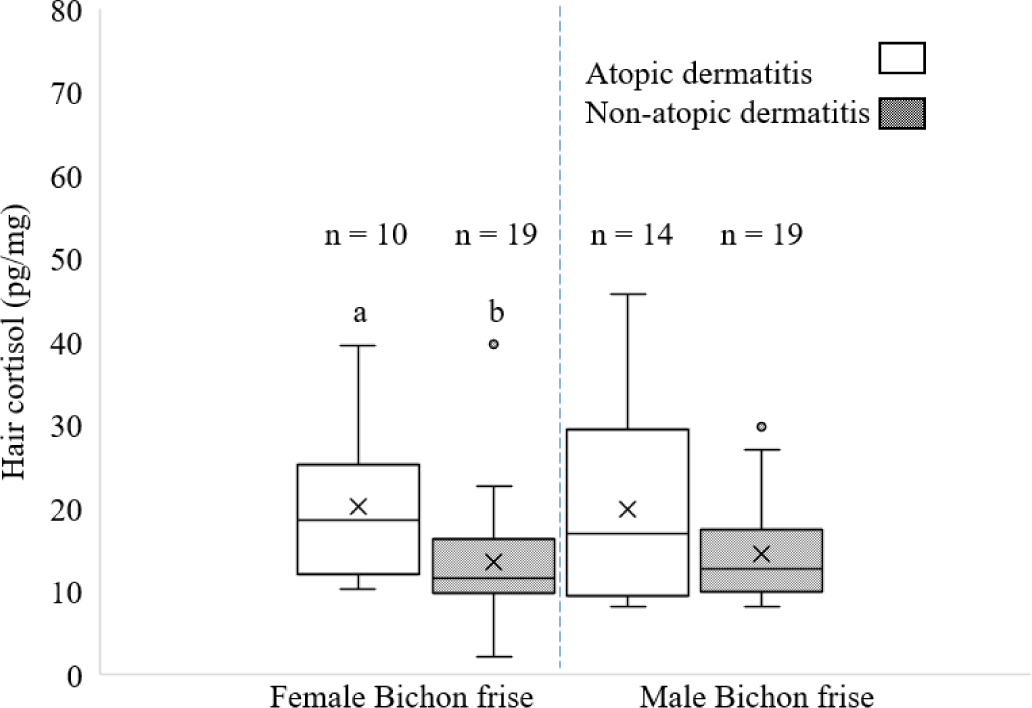
It was found in this study that AD may have induced a higher cortisol response, as evidenced by elevated levels in the hair of female bichon frise dogs. This could be related to differences in hormone regulation or behavioral responses to allergies or stressors between sexes. In contrast, the study found no significant differences in the HCC between male bichon frise dogs with and without AD. This suggests that AD may not significantly influence cortisol levels in male dogs. Sundman et al. [21] examined the factors that influence dogs’ stress levels, specifically focusing on the owner-dog relationship and the presence of other dogs. The researchers also investigated the impact of different seasons and lifestyle on dogs’ stress levels, as well as the effect of the dogs’ gender. Their findings indicated that both male and female dogs’ stress levels were related to their owners’ stress levels, but this association was stronger in females. Female dogs showed higher cortisol levels than males, suggesting that they are more emotionally reactive. In the present study, the female dogs were more susceptible to stress than male according to observed high HCC in those female with AD than without AD. Despite this difference, between the reference study and our present research the importance of various stressors such as gender, owner-dog relationship, and environmental influences in understanding and managing dog stress levels.
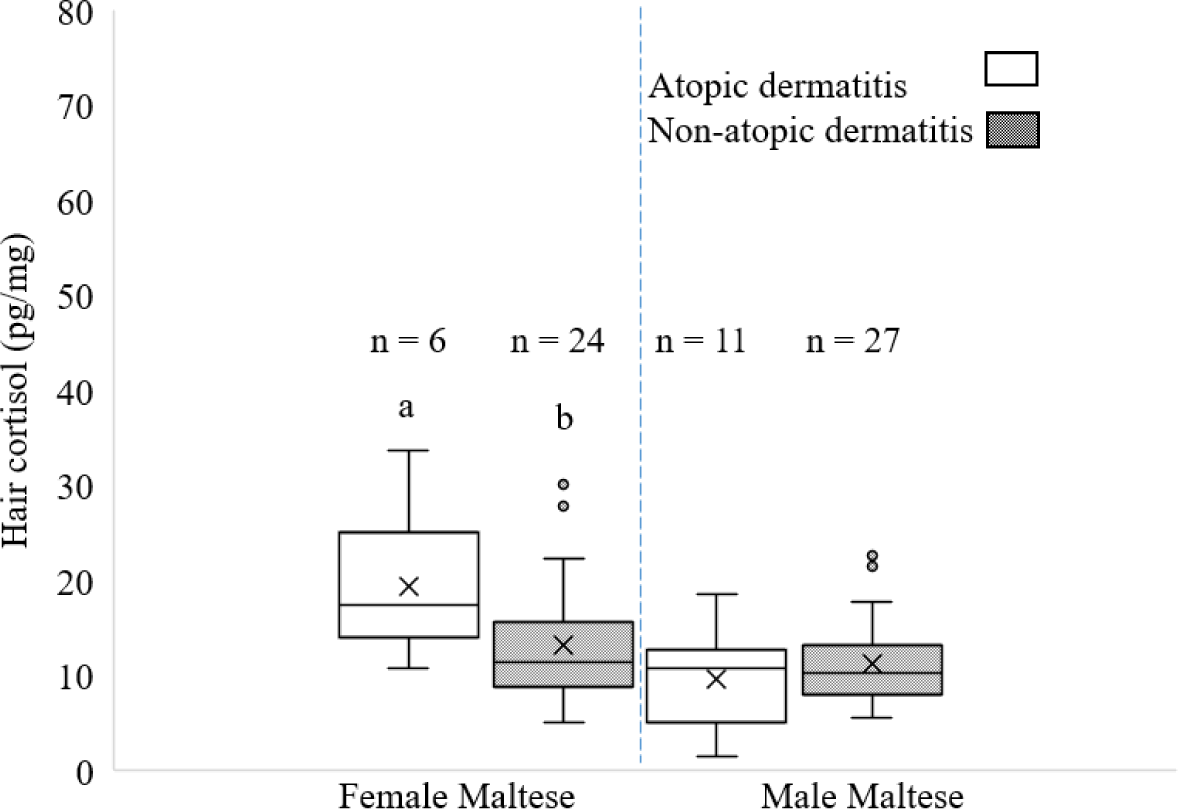
The analysis of HCC in female and male maltese dogs with and without AD are shown in Fig. 5. Similar previous results in bichon frise dogs, which showed sex-related differences in HCC in response to AD, this study revealed that the HCC in female maltese with AD was higher (p < 0.05) than that without AD. Female maltese dogs released more cortisol than male maltese in their stress response to AD condition, and male maltese dogs may be infected with a very low or mild degree of AD that was not influenced HCC. Sung and Huang [28] reported that Golden Retrievers, mongrels, Beagles, Labrador Retrievers, and maltese Terriers were among the breeds most frequently presenting with AD. Additionally, they noted that male dogs were more susceptible to AD. Further research specifically focusing on susceptibility of maltese dogs to AD would provide valuable insights into their dermatological health and contribute to a more comprehensive understanding of breed-specific susceptibilities.
HCC in female and male poodles with and without AD are shown in Fig. 6. Higher (p < 0.05) HCC were observed between the AD and non-AD in female and male poodles.
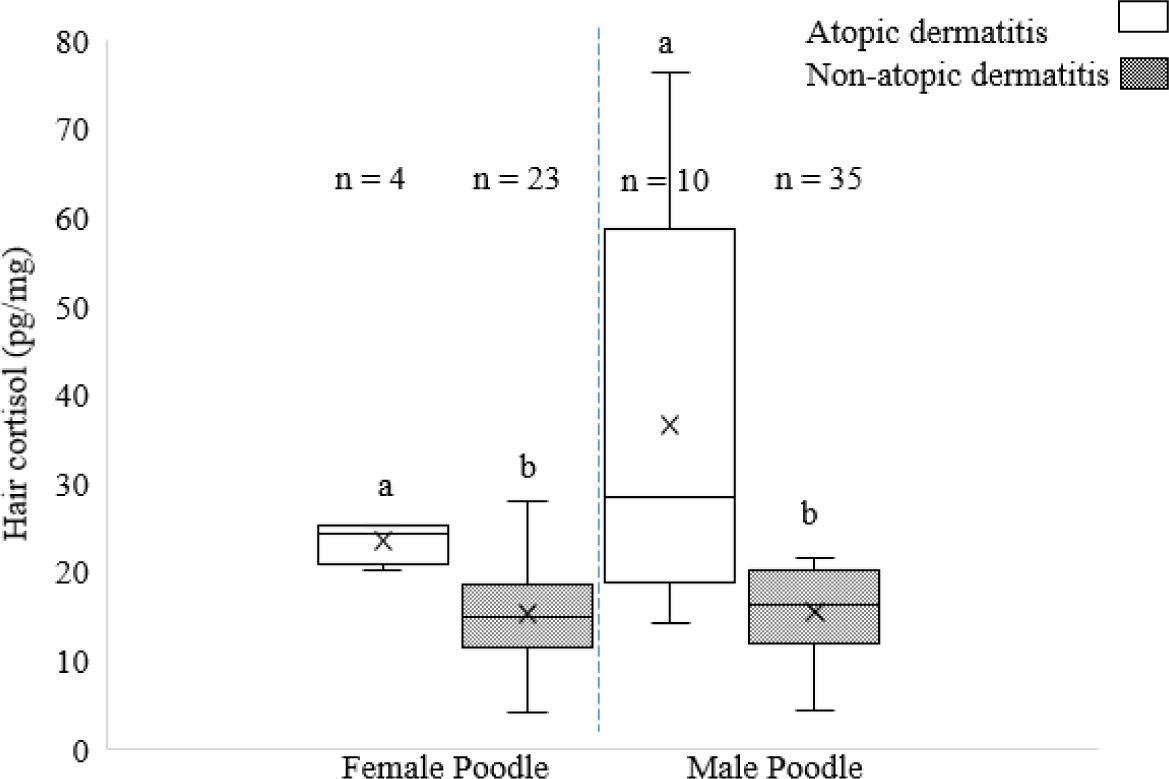
Significant differences in HCC in poodles with and without AD in both female and male suggested that AD have a notable impact on HCC. This suggests that AD may have induced a higher cortisol response, as evidenced by elevated levels in the hair of poodle dogs. These findings emphasize the need to consider breed-specific and sex-specific factors when managing AD in poodles. However, Počta and Svoboda [29] reported that AD was not significantly different between female and male dogs. Although no significant difference was found in the analysis of HCC in some breeds affected by AD in this study, AD can cause a chronic pruritic condition in dogs that is associated with chronic stress [30].
We could not analyze the relationship between HCC in dogs with AD and aggressive behaviors towards the environment, animals, owners, or themselves. The clinical signs of AD and skin allergies can vary and occur in different body parts of all dog breeds, such as the neck, face, back, and abdomen. AD usually occurs in dogs between six months and three years of age, and clinical signs have been reported in individuals younger than six months and in dogs aged seven years old. However, some breeds are more at risk of AD than others. [29]. In skin allergies such as AD, dogs react differently until the AD condition is controlled. Anti-inflammatory drugs such as steroids are commonly used to block allergic reactions and reduce scratching [31].
CONCLUSION
Analysis of HCC can help to better understand the chronic stress of dogs with AD. Dogs with AD demonstrated higher HCC compared dogs without AD, despite being in similar living conditions. This suggests that dogs with AD were more likely to experience increased stress. Consequently, it is advisable to provide additional care to manage stress in dogs affected by AD. Caution is advised in interpreting the results of this study, as the severity of AD and other influential factors on HCC were not considered. Future studies should be conducted with addressing other potential influential factors and severity of AD to validate and enhance the obtained results.
