INTRODUCTION
Infertility is characterized by the inability of males or females to reproduce successfully and affects both humans and animals, including canines. Studies estimate that male reproductive disorders account for 40–50% of infertility cases in dogs [1]. Despite significant research efforts to address this issue, treatment success remains limited, with only 10% of infertility cases effectively managed [2].
Among various alternative treatments, herbal medicine—particularly Panax ginseng—has garnered significant attention for its potential role in improving reproductive health [3]. Previous studies have demonstrated its beneficial effects on male reproductive function [4]. The first report on ginseng’s influence on spermatogenesis in rats highlighted its ability to enhance sperm production [5]. In human clinical trials, ginseng supplementation significantly improved sperm concentration and motility in patients with oligoasthenospermia and age-related infertility [6]. Beyond its reproductive benefits, ginseng exerts diverse pharmacological effects, supporting the immune [7], cardiovascular [8], and nervous systems [9], while also enhancing physical stamina [10]. Of particular interest, ginsenosides—the primary bioactive compounds in ginseng—share structural similarities with steroid hormones, suggesting their potential role in spermatogenesis and sexual function [11,12].
Panax ginseng is a perennial plant of the Panax genus and has been a fundamental component of traditional medicine in Korea and China [13]. The processing methods of ginseng significantly alter its chemical composition and biological activity, resulting in three main types: white ginseng (WG), red ginseng (RG), and black ginseng (BG). WG is produced by drying fresh roots under sunlight or hot air, whereas RG undergoes steaming before drying, giving it a reddish hue [14]. BG is subjected to multiple cycles of steaming and drying (at least three times), leading to a darker color and a higher concentration of bioactive compounds [15].
Studies have shown that both RG and BG contain a diverse range of ginsenosides with antioxidant and anti-inflammatory properties, including Rb1, Rb2, Rc, Rd, Re, Rf, Rg1, Rg2, 20(S)-Rg3, 20(R)-Rg3, Rg5, Rg6, Rh1, Rh4, Rk1, Rk3, F1, and F4. Repeated thermal processing significantly increases the levels of specific ginsenosides in BG compared to RG, particularly Rg2, Rg3, Rg5, Rg6, Rh1, Rh4, Rk1, Rk3, F1, and F4 [16]. Furthermore, research suggests that this thermal processing enhances the stability and bioavailability of key ginsenosides, including Rg3, Rg5, Rh1, and Rh2 [17,18].
However, the effects of Panax ginseng on canine reproductive physiology remain largely unexplored. Given these knowledge gaps, this study aims to evaluate the impact of ginseng supplementation on sperm motility and reproductive function in dogs. Three different types of processed ginseng (WG, RG, and BG) will be administered, and their effects will be assessed through blood analysis and computer-assisted sperm analysis (CASA). Specifically, this study will examine changes in testosterone levels, biochemical parameters, and sperm motility in response to ginseng supplementation. The findings will provide scientific evidence supporting the potential application of ginseng in canine reproductive health and offer valuable insights into natural supplementation strategies for improving male reproductive function in dogs.
MATERIALS AND METHODS
Unless otherwise indicated, all chemicals and reagents were purchased from Sigma-Aldrich Chemical Company (St. Louis, MO, USA).
The processed Panax ginseng, including WG, RG, and BG, was obtained from Agricultural Corporation, GEUMSAN Black Ginseng Company (Korea), as shown in Fig. 1. The ginseng samples were ground using a blender and encapsulated into hard capsules, each containing 500 mg of ginseng powder [15,19].

Male Beagle dogs weighing 7 to 12 kg and aged 2 to 3 years were used in this study. The animals were fed a commercial diet once daily and provided with ad libitum access to water. The dogs were housed individually in an indoor animal facility [20]. All animal procedures were conducted in accordance with the “Guide for the Care and Use of Laboratory Animals” approved by Chungnam National University (Approval No.: CNU-00618).
Three dogs (A, B, and C) were used in this study. Each dog received a single oral dose of one encapsulated 500 mg ginseng powder capsule per day for 60 days, followed by a 30-day washout period. After the washout period, the dogs were rotated into a different treatment group, ensuring that each dog underwent all three ginseng treatments in a crossover design [21], as illustrated in Fig. 2.
To determine serum testosterone levels, blood samples were collected from the cephalic vein. The samples were centrifuged at 1,600 g for 10 minutes at room temperature. The radioimmunoassay for testosterone quantification was performed by Neodin (Seoul, Korea) [22]. The assay was conducted at 30-day intervals throughout the experimental period.
To assess the general health condition of the experimental dogs, serum biochemical analysis was conducted using the SPOTCHEM EZ SP-4430 (Arkray., Kyoto, Japan) [23]. Blood samples were collected from the cephalic vein and centrifuged at 1,600 g for 10 minutes at room temperature. According to the instrument manual, biochemical analysis was conducted at 30-day intervals throughout the experimental period, following the parameters listed in Table 1.
Semen quality was evaluated using CASA at 20-day intervals throughout the experimental period [24,25]. Semen samples were collected via digital manipulation, with only the second fraction (sperm-rich fraction) used for analysis, while the first and third fractions were discarded.
Sperm motility and kinematic parameters were assessed after appropriate dilution using the SAIS Plus system (Medical Supply, Wonju, Korea). A 5 μL aliquot of diluted semen was placed onto a pre-warmed Makler chamber (New York Microscope Company, Hicksville, NY, USA) at 37°C. Sperm motility characteristics were examined under a 100× objective of an Eclipse E600 microscope (Nikon, Tokyo, Japan) equipped with a CCD camera (HVR-2000C, HVS, Seongnam, Korea). The following sperm motility parameters were measured using CASA: total motility (MOT, %), curvilinear velocity (VCL, μm/sec), straight-line velocity (VSL, μm/sec), average path velocity (VAP, μm/sec), linearity (LIN, %) = (VSL/VCL) × 100, straightness (STR, %) = (VCL/VAP) × 100, and wobble (WOB, %) = (VAP/VCL) × 100. Each measurement was performed in triplicate.
The results were expressed as means ± SE. The Shapiro-Wilk test was used to assess normality, while Levene’s test evaluated the homogeneity of variances. Data analysis was performed using R software (version 4.3.2, USA). One-way analysis of variance (ANOVA) was conducted, followed by Tukey’s post hoc test for multiple comparisons. Pearson correlation analysis was used to assess the relationship between serum P4 levels. Statistical significance was set at p < 0.05.
RESULTS
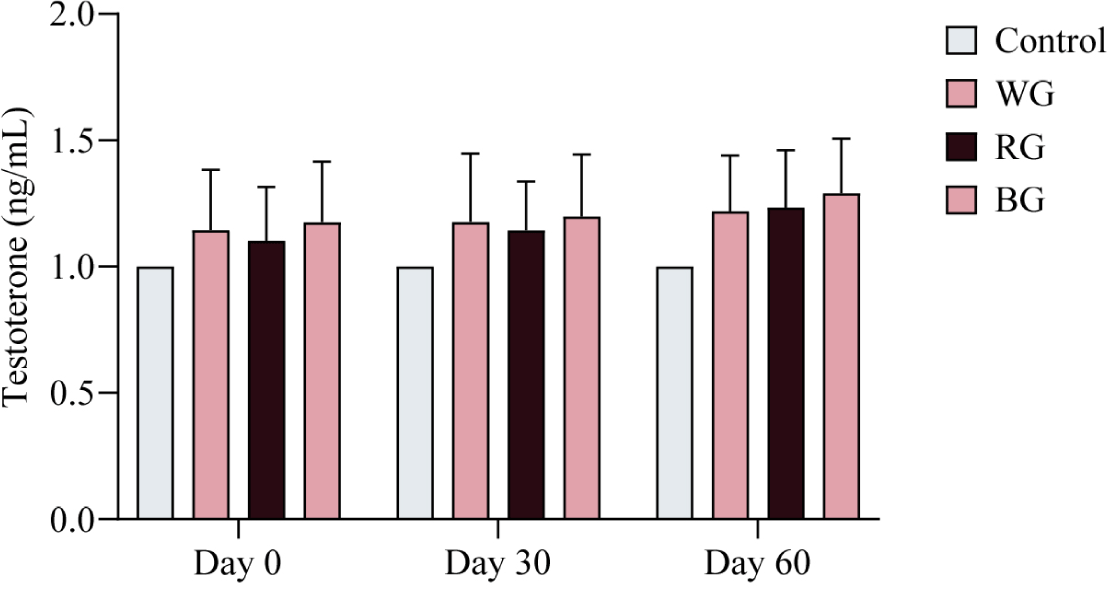
To assess variations in T4 levels among the experimental groups (WG, RG, and BG), hormone analysis was conducted at three time points: Day 0, Day 30, and Day 60 of the experimental period. No significant differences in T4 levels were observed among the treatment groups over the 60-day period (Fig. 3). However, a trend of increasing T4 levels was noted in the ginseng-administered groups compared to the control group throughout the study.
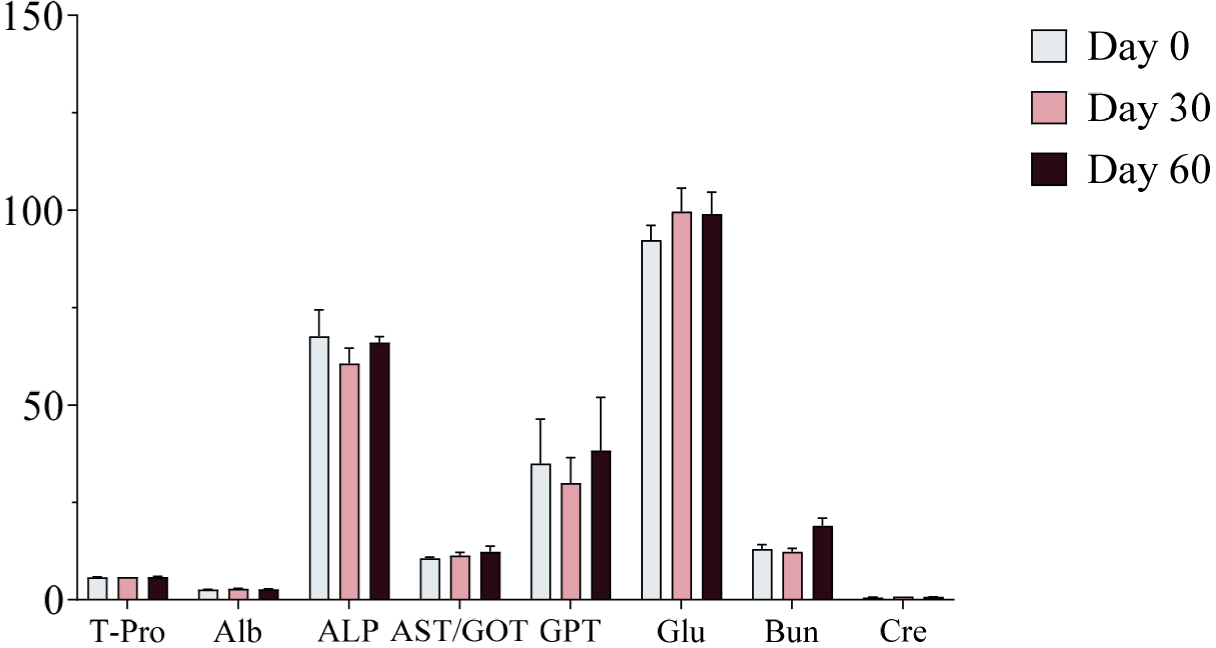
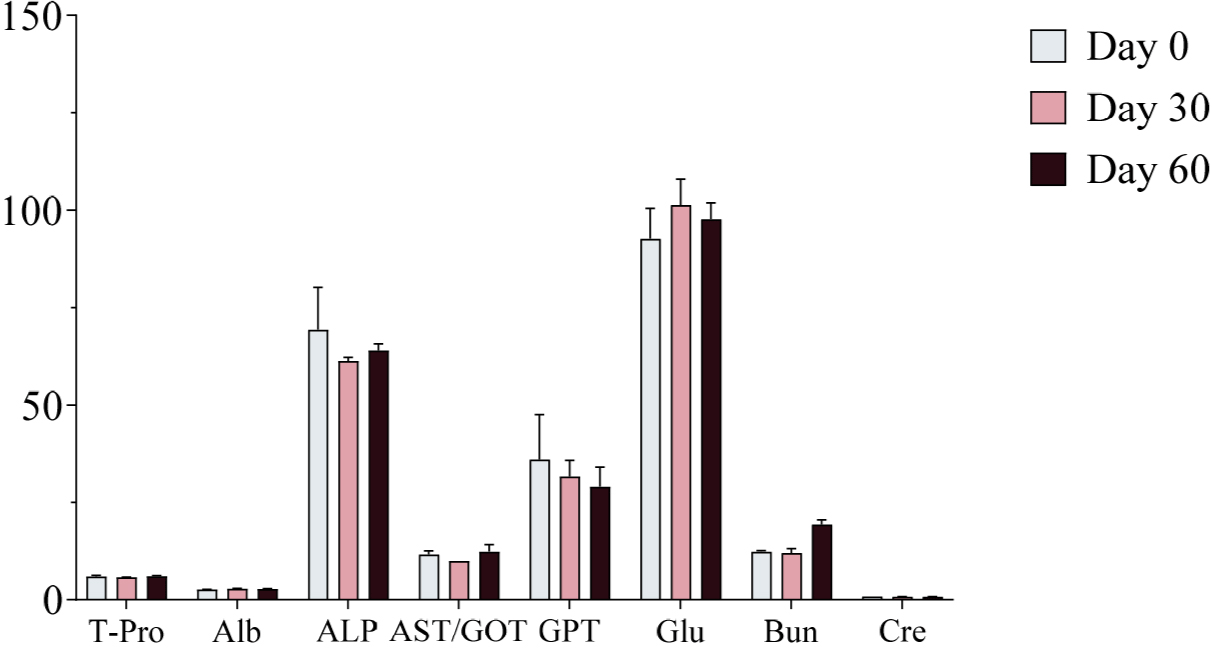
Serum samples were collected on Days 0, 30, and 60 of the experimental period and analyzed using a serum analyzer to assess changes in biochemical markers following ginseng supplementation. No significant changes in biochemical markers were observed, and all values remained within the normal physiological range (Figs. 4, 5, and 6). Furthermore, no adverse health effects were detected in any of the experimental groups (WG, RG, and BG).
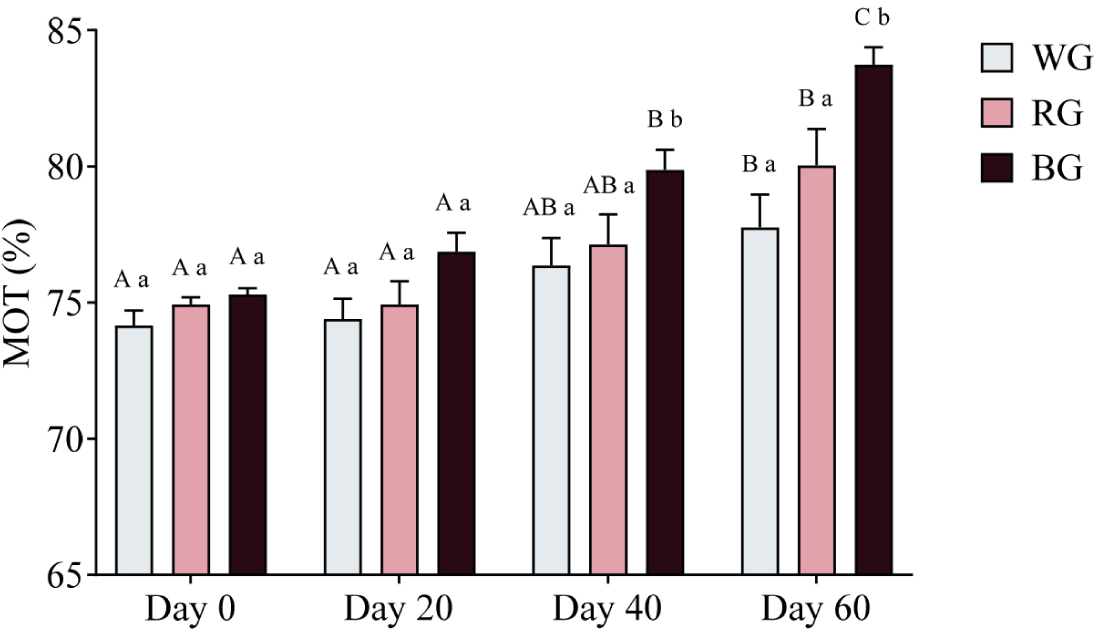
A two-way ANOVA was conducted to assess the effects of different treatments and time points on sperm motility (Fig. 7). On Day 40, motility in the BG group was significantly higher than in the WG group (p < 0.05). By Day 60, the BG group exhibited significantly higher motility than both the WG (p < 0.001) and RG groups (p < 0.05). Within-group comparisons revealed that motility in the WG group increased significantly on Day 60 compared to Day 0 and Day 20 (p < 0.05). Similarly, motility in the RG group showed a significant increase on Day 60 (p < 0.01). In the BG group, motility was significantly higher on Day 40 (p < 0.01) and peaked on Day 60 (p < 0.0001), with a further increase from Day 40 to Day 60 (p < 0.05).
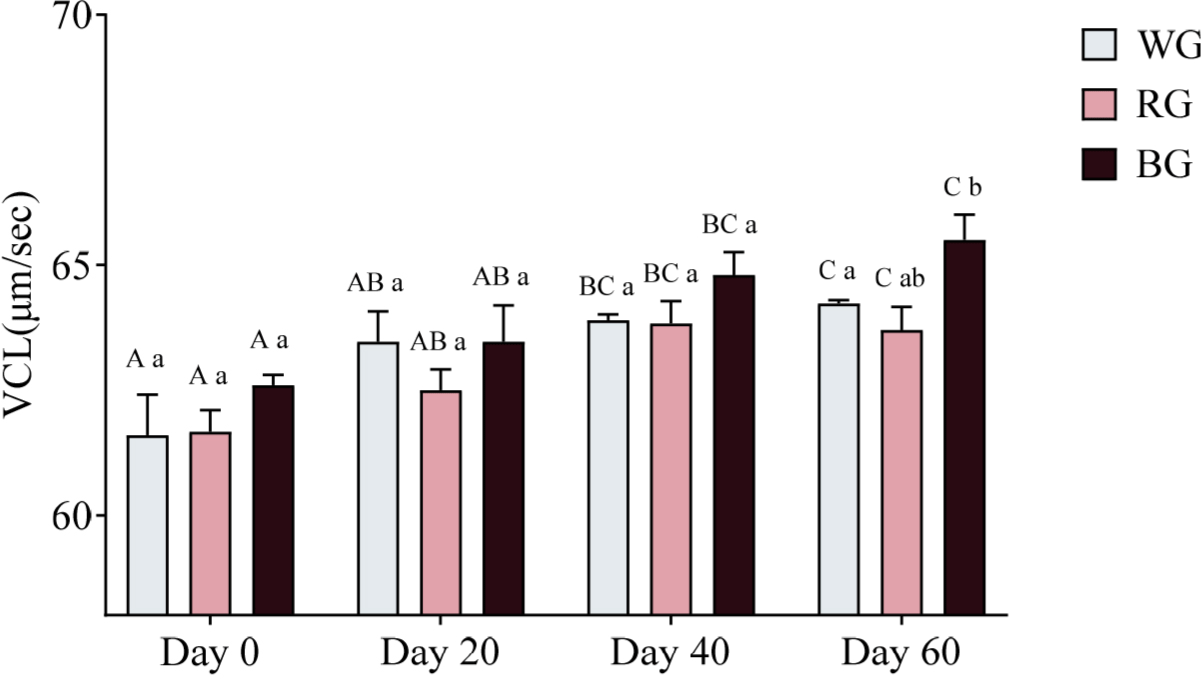
Sperm velocity parameters were measured using CASA. A two-way ANOVA was conducted to assess the effects of different treatments and time points on sperm VCL (Fig. 8). On Day 60, only the BG group exhibited significantly higher VCL than the RG group (p < 0.05). Within-group comparisons over time showed that WG VCL increased significantly on Day 40 (p < 0.05) and Day 60 (p < 0.01) compared to Day 0. Similarly, RG VCL was significantly higher on Day 40 and Day 60 compared to Day 0 (p < 0.05). In the BG group, VCL significantly increased on Day 40 (p < 0.05) and Day 60 (p < 0.01) relative to Day 0, with Day 60 also significantly exceeding Day 20 (p < 0.05).
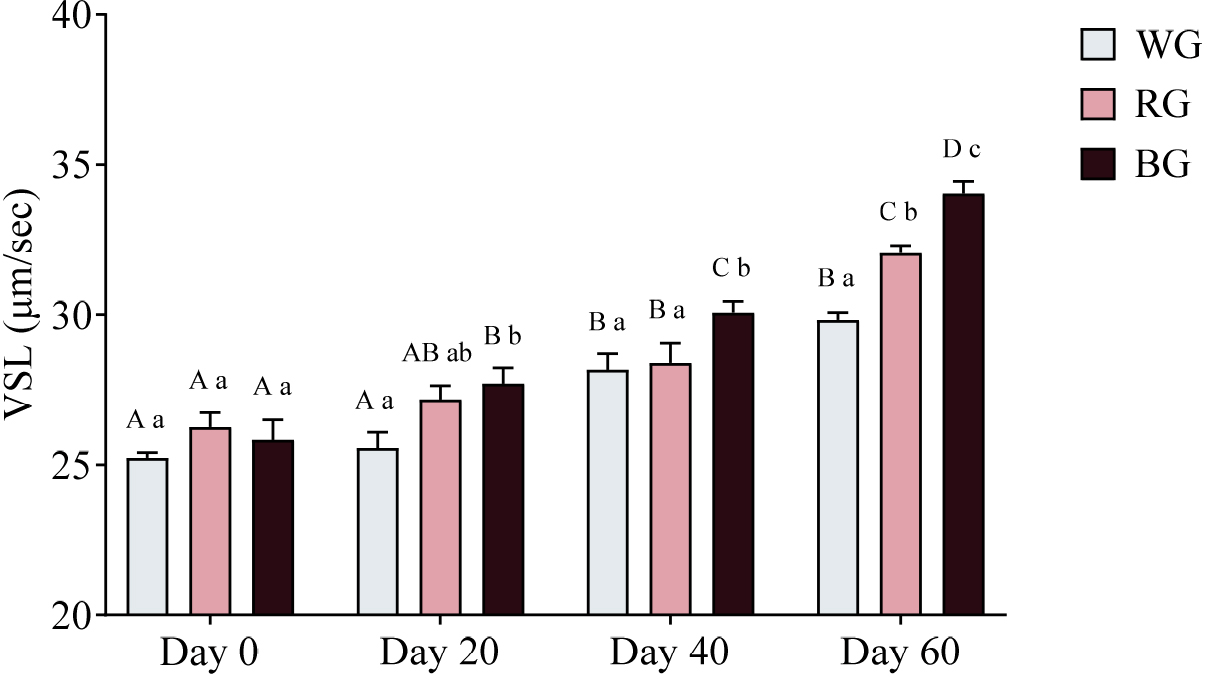
A two-way ANOVA was also conducted to assess the effects of different treatments and time points on sperm VSL (Fig. 9). The BG group exhibited significantly higher VSL than the WG group on Day 20 (p < 0.01). On Day 40, VSL in the BG group was significantly higher than in both WG (p < 0.05) and RG (p < 0.05). By Day 60, the RG group showed significantly higher VSL than the WG group (p < 0.01), while the BG group exhibited the highest VSL, significantly exceeding both WG (p < 0.0001) and RG (p < 0.05). Within-group comparisons showed that WG VSL increased significantly on Day 40 (p < 0.001) and Day 60 (p < 0.0001) compared to Day 0, with Day 60 also exceeding Day 20 (p < 0.0001). RG VSL was significantly higher on Day 40 (p < 0.05) and Day 60 (p < 0.0001) compared to Day 0, with Day 60 also exceeding Day 20 (p < 0.0001) and Day 40 (p < 0.0001). The BG group exhibited the most substantial increase, with significantly higher VSL on Day 20 (p < 0.05), Day 40 (p < 0.0001), and Day 60 (p < 0.0001) compared to Day 0. Additionally, BG VSL on Day 60 was significantly higher than on Day 20 (p < 0.0001) and Day 40 (p < 0.0001).
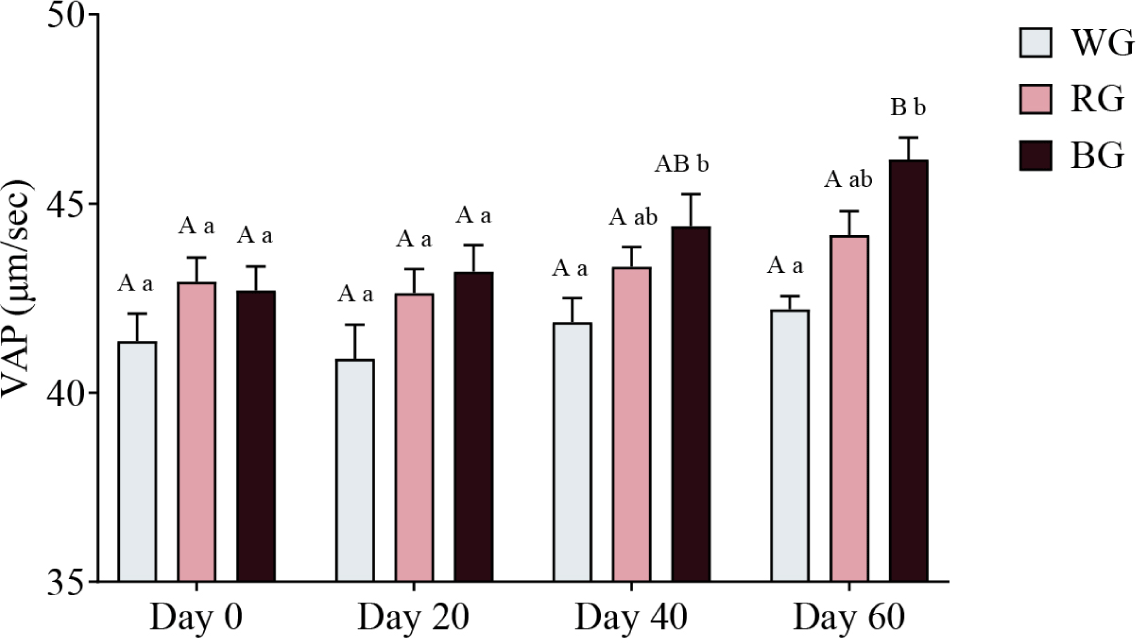
A two-way ANOVA was conducted to assess the effects of different treatments and time points on sperm VAP (Fig. 10). On Day 40, the BG group exhibited significantly higher VAP than the WG group (p < 0.05). By Day 60, VAP in the BG group was significantly higher than in the WG group (p < 0.001). Within-group comparisons showed a significant increase in BG VAP on Day 60 compared to Day 0 (p < 0.01) and Day 20 (p < 0.05). No other within-group comparisons reached statistical significance.
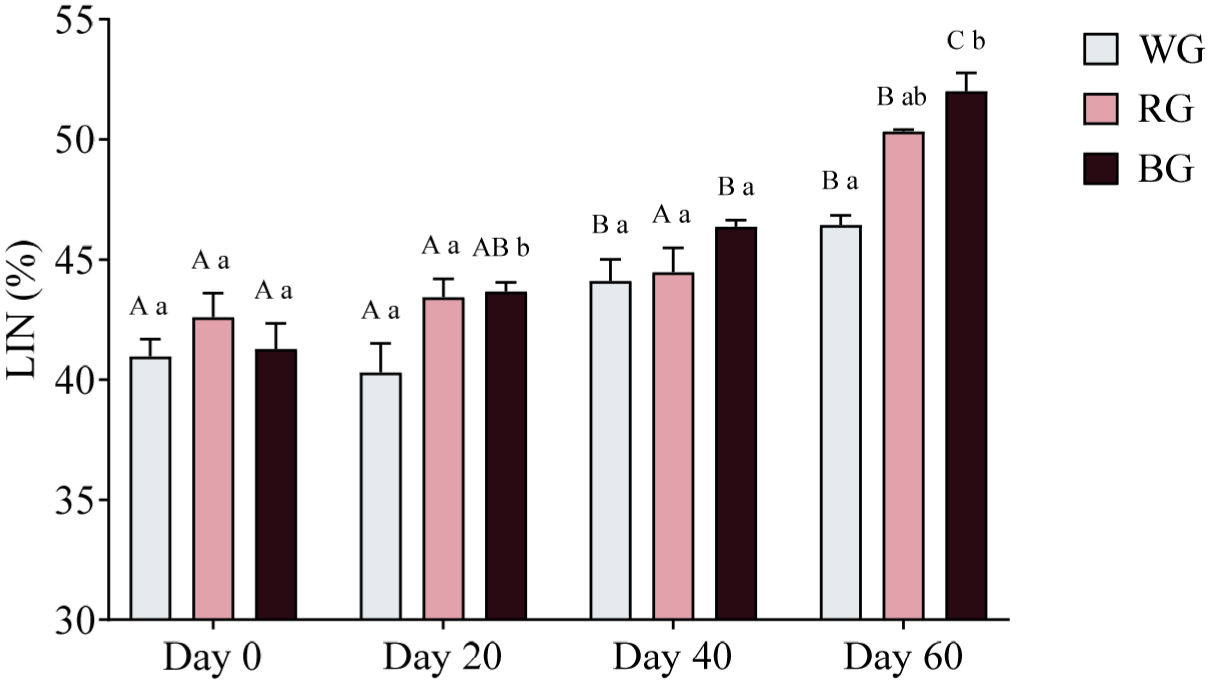
Sperm trajectory was assessed by measuring LIN, STR, and WOB. A two-way ANOVA was conducted to evaluate the effects of different treatments and time points on sperm LIN (Fig. 11). On Day 20, both the RG and BG groups displayed significantly higher LIN compared to the WG group (p < 0.05). By Day 60, LIN was significantly higher in the RG group than in the WG group (p < 0.01), while the BG group exhibited the highest LIN, significantly exceeding WG (p < 0.001). Within-group comparisons showed that LIN in the WG group increased significantly on Day 40 (p < 0.05) and Day 60 (p < 0.001), with a further increase on Day 60 compared to Day 20 (p < 0.0001). In the RG group, LIN was significantly higher on Day 60 compared to Day 0 (p < 0.0001), Day 20 (p < 0.0001), and Day 40 (p < 0.001). Similarly, in the BG group, LIN was significantly higher on Day 40 (p < 0.001) and Day 60 (p < 0.0001) compared to Day 0, with further increases on Day 60 compared to Day 20 (p < 0.0001) and Day 40 (p < 0.001).
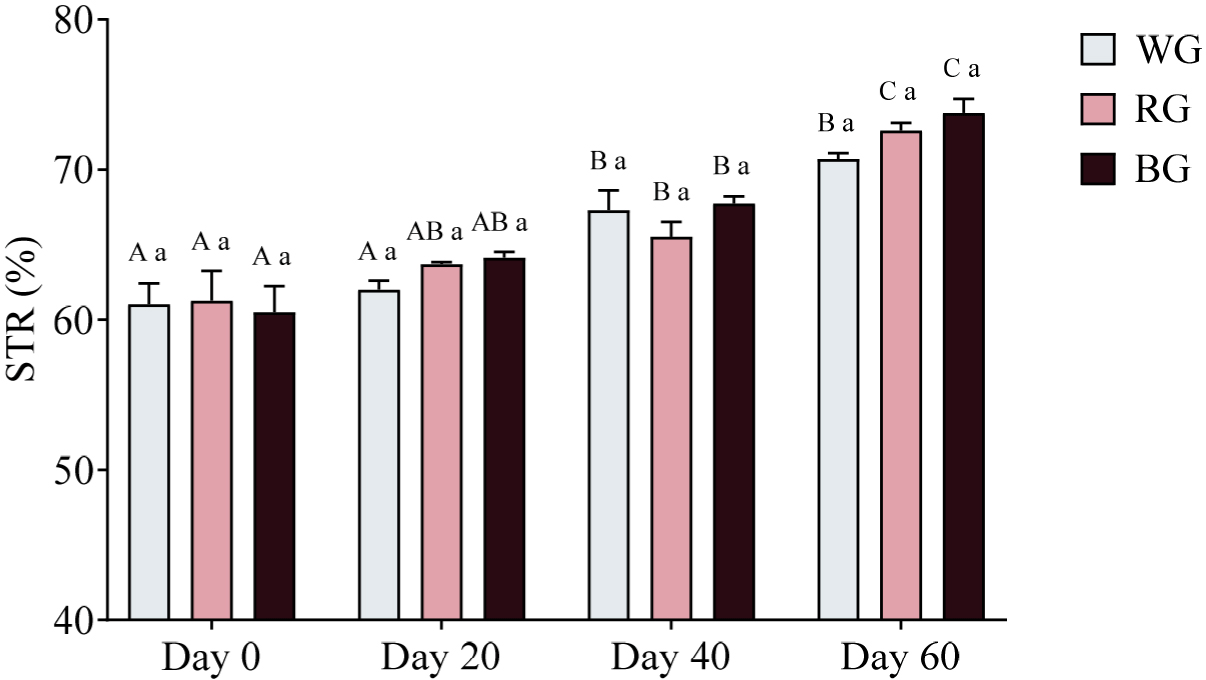
A two-way ANOVA revealed no significant differences in STR among groups at any time point (p > 0.05; Fig. 12). However, within-group comparisons showed significant increases over time. In the WG group, STR was significantly higher on Day 40 (p < 0.01) and Day 60 (p < 0.0001) compared to Day 0, with an additional increase on Day 40 relative to Day 20 (p < 0.01). Similarly, in the RG group, STR was significantly higher on Day 40 (p < 0.05) and Day 60 (p < 0.0001) compared to Day 0, with further increases on Day 60 relative to Day 20 (p < 0.0001) and Day 40 (p < 0.001). The BG group exhibited the most pronounced increases, with significantly higher STR on Day 40 (p < 0.001) and Day 60 (p < 0.0001) compared to Day 0, as well as significant increases on Day 60 relative to Day 20 (p < 0.0001) and Day 40 (p < 0.01).
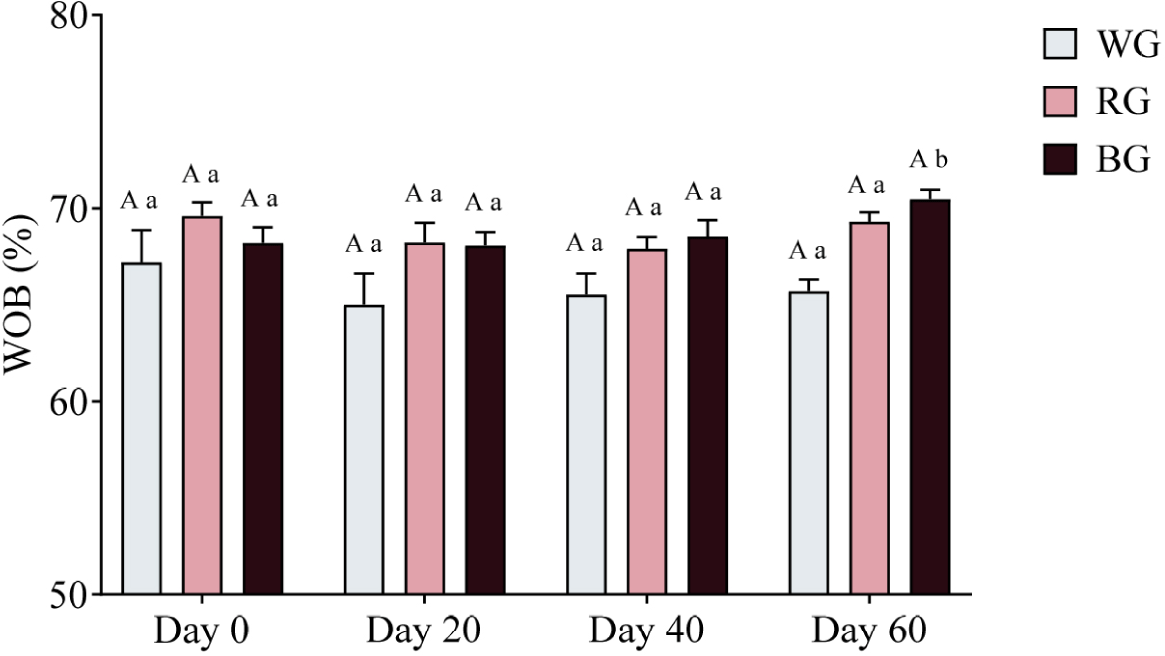
A two-way ANOVA was conducted to assess the effects of different treatments and time points on sperm WOB (Fig. 13). The RG group exhibited significantly higher WOB than the WG group (p < 0.05), while the BG group had significantly higher WOB than the WG group (p < 0.01). No significant differences were observed between the BG and RG groups at any time point. Within-group comparisons showed no significant changes in WOB over time for either WG or RG.
DiSCUSSION
The present study investigated the effects of three types of processed ginseng (WG, RG, and BG) on male canine reproductive physiology. This was evaluated through testosterone (T4) measurement, biochemical serum analysis, and sperm quality assessment.
In 2007, a study reported that a dosage of 1 g of ginsenoside was used in human trials, with no adverse effects [19]. However, no prior research has examined the effects of ginseng supplementation in dogs. Therefore, in this study, the dosage was set at 500 mg per day, half of the dosage used in humans.
Testosterone, a primary male steroid hormone, is produced by Leydig cells in the testes and plays a crucial role in libido regulation [26]. Previous studies have demonstrated that feeding rats a diet containing 5% Panax ginseng for 60 days significantly increased serum testosterone levels [27]. Additionally, supplementation with ginsenoside Rg1 (10 mg/kg) significantly increased serum testosterone levels and enhanced sexual behavior in animal models [28]. However, in this study, no significant differences in testosterone levels were observed. Despite this, no adverse effects were detected in any of the experimental groups (WG, RG, or BG). Biochemical analysis of serum samples indicated that all measured markers remained within normal physiological ranges throughout the study. No significant deviations were observed, suggesting that oral administration of WG, RG, and BG did not negatively affect liver, kidney, or general health functions.
Semen quality is influenced by various factors, including health, nutrition, and oxidative stress [29,30]. Panax ginseng has long been studied for its reproductive benefits, particularly its ability to enhance sperm motility [4]. BG, which undergoes repeated steaming and drying, contains higher levels of secondary ginsenosides, such as Rg3, Rg5, and Rk1, compared to WG and RG, contributing to its greater bioactivity [16]. Recent studies suggest that ginsenosides improve sperm function by enhancing mitochondrial activity and reducing oxidative stress [31]. In particular, BG may protect sperm from oxidative damage by activating the Nrf2/ARE pathway, which upregulates antioxidant enzymes such as SOD and GPx [32]. Additionally, ginsenosides regulate lipid metabolism, a key factor in maintaining sperm membrane integrity and motility [33]. El-Shimi et al. reported that ginseng mitigates reproductive toxicity caused by environmental pollutants like bisphenol A, preventing mitochondrial dysfunction and sperm DNA damage [11]. Moreover, Zhang et al. demonstrated that ginseng-derived soluble dietary fiber enhances spermatogenesis via the MAPK signaling pathway, indicating a role in cellular energy regulation [34]. These mechanisms align with our findings, where CASA analysis showed that BG significantly improved sperm motility and trajectory parameters.
Despite these promising findings, several limitations should be considered. Although ginseng supplementation improved sperm motility, it did not lead to significant changes in testosterone levels. This suggests that its mechanism of action may not be directly testosterone-mediated, warranting further investigation. Additionally, while no adverse effects on liver, kidney, or overall health functions were observed, the long-term effects remain unclear. Future studies should evaluate prolonged supplementation to determine potential cumulative effects.
The role of specific ginsenosides requires further investigation. While Rg3 has been shown to exhibit bioactive properties [35], this study did not isolate individual ginsenosides. Future research should focus on identifying key active compounds and elucidating their mechanisms of action. Additionally, varying dosages and supplementation durations should be tested to optimize reproductive benefits.
Given the structural similarities between ginsenosides and steroid hormones [36], potential endocrine interactions should also be explored. While this study provides valuable insights, further research is needed to validate these findings and refine the applications of ginseng in reproductive medicine.
