INTRODUCTION
The production and transfer of embryos in the bovine species has been widely used as an effective method for genetic improvement in many livestock-producing countries This trend is also increasing in Korea, where awareness of embryo transfer for breed improvement and genetic resource conservation is growing. Moreover, reproductive efficiency is strongly correlated with the economic viability of dairy and beef industries [1–7]. Consequently, assisted reproductive technologies such as in vitro embryo production (IVEP) using ovum pick-up (OPU), have been globally adopted to rapidly obtain genetically superior traits in cows [8]. The number of large-scale farms and intensive production systems for Hanwoo (native Korean) and Holstein cattle is increasing in Korea. To achieve successful breeding, the importance of embryo production as a valuable trait is increasing, not only in traditional breeding programs such as artificial insemination, but also in enhancing the pace of genetic improvement. In Korea, cows with excellent carcass characteristics or high genetic value are preferred for breeding, and multiparous cows are primarily used as valuable donors. Factors such as growth rate, pedigree, market situation, temperament, and meat quality are prioritized as criteria for selecting donor cows, often neglecting indicators related to the inherent oocyte or embryo production capacity [9–13]. Moreover, recent reports have indicated significant variability in the response to superstimulation treatments and the quantity of oocytes retrieved via OPU [14–16]. Recent studies have suggested that in cows, anti-Müllerian hormone (AMH) measurements and antral follicle count (AFC) through ovarian ultrasound scanning can serve as predictive variables for the quantity of oocytes collected, thereby assisting in forecasting the ovarian response to superstimulation treatment [17,18]. However, there is a lack of research exploring the correlation between AMH levels and AFC with respect to the quantity of oocytes retrieved and transferable embryos in Bos taurus breeds, such as Hanwoo. Also, the efficiency of donor selection using the superstimulation method, which has become a global trend, needs to be demonstrated. Consequently, the development and application of predictive methods to determine the inherent oocyte or embryo production capacity of donor cows are essential.
During male fetal differentiation, Sertoli cells in the testes secrete AMH, leading to regression of the Müllerian ducts. In the ovaries, granulosa cells from preantral or antral follicles also produce AMH [19–22]. The physiological functions of AMH are not completely understood; however, it is thought to regulate follicular recruitment and selection [23]. Serum AMH concentration is closely associated with the quantity of AFC and remains relatively stable throughout the estrous cycle [24]. Serum AMH levels have recently been proposed as a good indicator of ovarian reserve, showing a strong correlation with the quantity of oocytes retrieved by OPU [25]. However, recent studies have emphasized the need for reliable research on the use of AMH and AFC as selection indicators of the quantity of oocytes by superstimulation, rather than OPU in a random ovarian cycle state without superstimulation treatment in cows [26–29]. In Hanwoo cattle, recent research has shown a correlation between AMH concentrations and the quantity of embryos retrieved from donors with normal ovarian cyclicity [30]. This suggests the value of early evaluation of AMH concentrations when selecting potential Hanwoo embryo donors. Based on these findings, the measurement of AMH concentration and AFC in Hanwoo cows during superstimulation, along with follicle-stimulating hormone (FSH) treatment, could be a valuable predictive tool for assessing the reproductive potential of donor cows.
The quantity of embryos produced by the OPU-IVEP varies according to the quantity of oocytes retrieved [31]. Choosing donors specifically for the OPU-IVEP appears to be most effective strategy for enhancing the yield of superior oocytes and embryos [30]. Thus, our study aimed to improve genetics and embryo production efficiency through OPU sessions. Hanwoo donors were selected using AMH concentration measurements as a basis for commercial farms. To correlate AMH as an indicator of ovarian function in superstimulated donors in this study, we measured AMH hormone concentrations in a total of 42 donors, and 12 donors with concentrations close to the mean were used as non-superstimulated controls. The 30 donors were used as the superstimulated group, and the group was divided into high, medium, and low groups according to concentration. Additionally, we evaluated the correlations between AFC measurements before OPU testing and the quantity of oocytes retrieved, embryos, and embryo-to-oocyte ratio after OPU testing among the groups.
MATERIALS AND METHODS
All procedures involving animals in this study were in accordance with relevant national laws and guidelines for animal care and use. Approval for the study was obtained from the Institutional Animal Care and Use Committee of the Gyeongsangbukdo Livestock Research Institute (Approval No. GAEC/161/23, approved on 14 December 2022). The experiment was carried out with Hanwoo donors (n = 42). The Hanwoo donors had a normal cycle and were 4.1 ± 0.6 (mean ± SEM) years of age and were kept on a commercial farm in Gyeongsangbukdo, Korea, from March to September 2023. The mean body condition score was 3.0 ± 0.2 (mean ± SEM) on a scale of 1 to 5 (1 = very thin; 5 = very fatty, respectively) [32]. They had unrestricted access to water and mineralized salts. Venous blood samples were obtained from the donor’s jugular vein immediately before determining their eligibility as oocyte donors. The blood was drawn into tubes and centrifuged at 1,314 ×g for 10 min to separate the plasma. The recovered plasma was preserved at a temperature of −80°C until subsequent AMH testing was conducted.
A bovine AMH ELISA kit (Ansh Labs, Webster, MA, USA) was used to assess usual plasma AMH concentrations prior to oocytes retrieval, in accordance with a previous report [33]. Donors were categorized into three treatment groups and one control group based on their AMH concentrations. Group H included the top 30%, Group M included the next 30%, and Group L included donors with the lowest concentration at 40%. The control group comprised 12 donors whose AMH concentrations were closest to the mean value of the 42 donors. Nine donors from group H, 9 from group M, and 12 from group L were selected as on-farm donors for OPU.
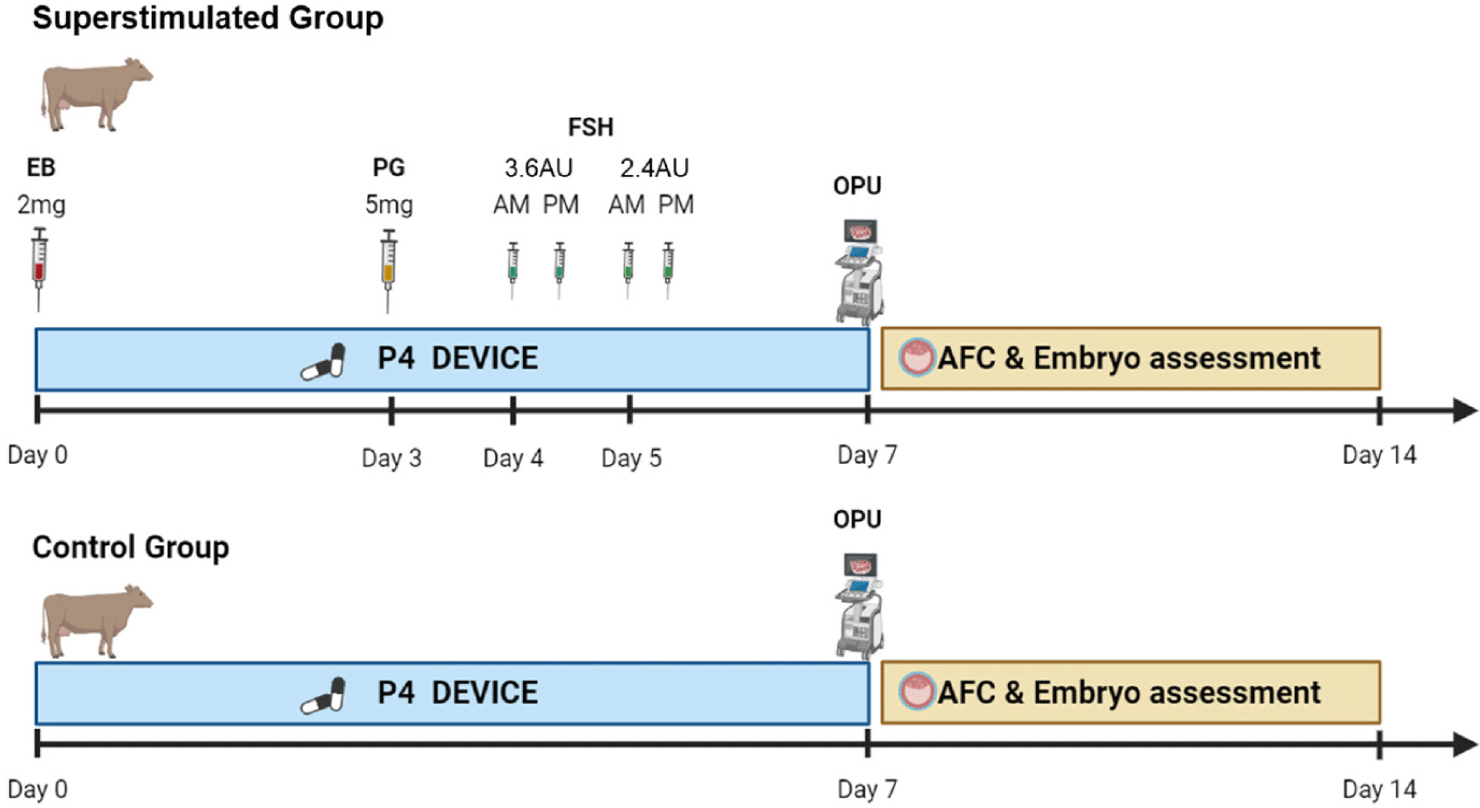
OPU was performed on 42 selected cows six times every two weeks by two skilled technicians. Prior to OPU handling, each donor’s follicular waves were synchronized with controlled intravaginal drug-release (1.38 g of Progesterone, CIDR DEVICES ®, Zeotis, Rhodes, New South Wales, Australia) insertion and simultaneous administration of estradiol benzoate (2.0 mg/cow, Esron®, Samyang-Anipharm, Seoul, Korea) to induce follicular wave present in the ovary on day one. On Day 3, 5.0 mg of intramuscular prostaglandin F2α (PGF2α) (Lutalyse®, Zoetis, Brussels, Belgium) was administered. Starting four days after insertion, FSH (Antrin R-10®, Kyoritsu Seiyaku, Tokyo, Japan) was given twice daily over a span of two days, with decreasing doses given at 12-hour intervals. The dosage regimens used were 3.6, 3.6, 2.4, and 2.4 AU on days four and five. On day seven, OPU was conducted 36 h after the final FSH injection, following removal of the progesterone (P4) device (Fig 1). The follicle count was assessed by ultrasound immediately before oocyte retrieval, specifically by counting the quantity of antral follicles ranging in diameter from 1 to 15 mm. Ovarian ultrasound scanner (4Vet Slim, Draminski Tech, Olsztyn, Poland) were conducted with a 6.5 MHz OPU endovaginal probe (BLUE, Draminski Tech) on day seven, immediately before the OPU session. During the OPU, the aspiration medium (MK_OPU®, MK biotech, Daejeon, Korea) used to retrieve the oocytes was anticoagulated with heparin to prevent blood clotting. A 19-G disposable hypodermic needle was used to perform the follicular puncture. A vacuum pump was used to maintain the vacuum for aspiration between 45 and 60 mmHg (BV-003, WTA, Cravinhos, Brazil) during the OPU procedure in both ovaries. Follicular contents were recovered using a 100 cm long tube with an internal diameter of 1.1 mm (WTA). The recovered cumulus–oocyte complexes (COCs) were washed once in wash medium (MK_WM®, MK biotech, Daejeon, Korea) with an oocyte filter (100 cm nylon screen; Mini IVF Filter, WTA) and classified four groups: 1 (excellent), 2 (fair), 3 (poor) and 4 (dead). Immediately after aspiration, a single technician evaluated the COCs using the most common criteria used to select and classify a standard collection of bovine oocytes [34–36].
For in vitro maturation (IVM), the COCs were cultured for 22 h in 450 μL of TCM-199 media that contained 0.005 AU/mL FSH (F2293, Sigma-Aldrich, St. Louis, MO, USA), 10% fetal bovine serum ([FBS] GIB16000-044, Thermo Fisher, Waltham, MA, USA), 1 μg/mL 17β-estradiol (E4389, Sigma-Aldrich), and 100 μM cysteamine (M6500, Sigma-Aldrich). The IVM cultures incubated under a humidified environment with 5% CO2 at 38.5°C. For in vitro fertilization (IVF), sperm preparation was conducted using BoviPure® Gradient following the manufacturer’s instructions (Nidacon, Gothenburg, Sweden) [37]. Layering 2 mL of BoviPure® bottom medium with 2 mL of BoviPure® top medium was meticulously done in a 15 mL centrifuge tube. Following this, thawed semen (500 µL) was mixed with BoviPure® extender in a warm test tube at a 1:1 ratio. The prepared semen (800 µL) was then gently loaded onto the top of the gradient and centrifuged at 1,500 rounds per minute (RPM) for 20 minutes. After centrifugation, the liquid above the sperm pellet was carefully removed. Subsequently, the pellet was resuspended in 5 mL of BoviWash and centrifuged at 1700 RPM for 5 minutes. The resulting pellet was resuspended in 100 µL of IVF medium (VitroFert™, ART Lab Solutions, Adelaide, Australia). The supplements found in the IVF medium consist of 10 IU/mL of heparin, 25 mM of penicillamine, 12.5 mM of hypotaurine, and 1.25 mM of epinephrine. Finally, on day 0, the oocytes were inseminated with 1–2 × 106 spermatozoa/mL for 18 hours in an IVF medium within a humidified atmosphere of 5% CO2 at 38.5°C. Following co-culture of COC with sperm, referred to as day 1, potential zygotes were mechanically cleared of cumulus cells by repeated pipetting into wash medium. They were then washed once in cleavage medium (VitroCleave™, ART Lab Solutions) and six embryos were placed in 20 μL drops of pre-conditioned cleavage medium covered with paraffin oil. On day 5, embryos were washed once in blastocyst medium (VitroBlast™, ART Lab Solutions) and groups of six embryos were transferred to 20 μL drops of pre-equilibrated blastocyst medium, also covered with paraffin oil. Embryos were then cultured until day 8. All maintained at 38.5°C in an atmosphere consisting of 5% O2, 5% CO2 and 90% N2.
GraphPad Prism (version 10.2.0, GraphPad Software, Boston, MA, USA) was applied for statistical analysis in this study. Quantity of follicles, retrieved oocytes and transferable embryos according to the AMH level were analyzed by two way-ANOVA, and the level of significance was p < 0.05, then the outcomes was displayed as the mean ± SEM. Regression analysis was used for the correlation between total quantity of follicles and plasma AMH concentration, and the larger the slope of the regression curve.
RESULTS
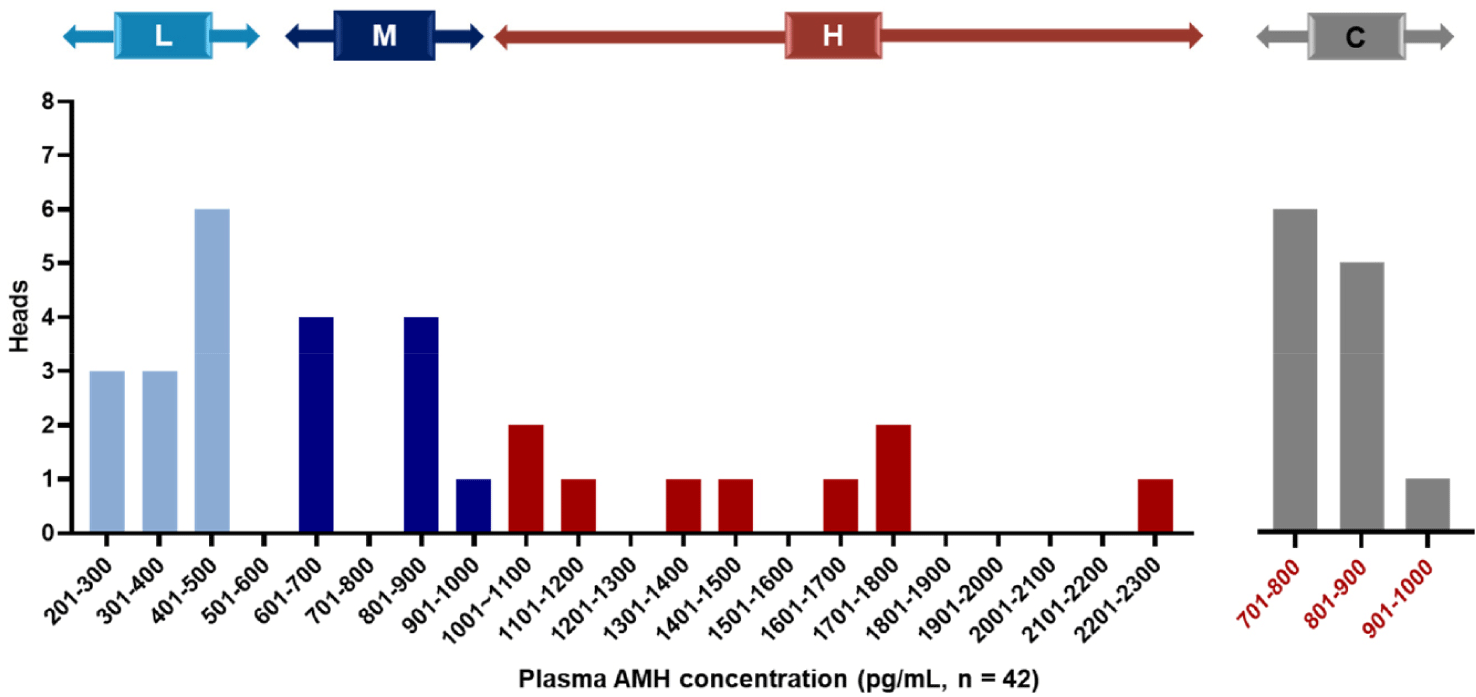
Among the 42 Hanwoo donors, concentrations of AMH in plasma varied between 276.5 and 2,212.5 pg/mL, with a mean AMH concentration (± SEM) of 820.8 ± 172.4 pg/mL (Table 1). Based on these concentrations, we categorized the donors into three groups: the H group (top 30% of donors, n = 9), M group (next 30% of donors, n = 9), and L group (lowest 40% of donors, n = 12), with the control group being the 12 donors closest to the mean concentration. The AMH concentrations for each group were as follows: H group (1,484.9 ± 129.2 pg/mL), M group (775.5 ± 39.0 pg/mL), L group (371.6 ± 17.8 pg/mL), and control group (806.1±16.6 pg/mL). The range of AMH concentrations in each group of Hanwoo donors is shown in Fig. 2. Furthermore, the age of donors in each group was as follows: H group (age, 4.1 ± 0.7 years), M group (age, 4.3 ± 0.8 years), L group (age, 4.1 ± 0.6 years), and control group (age, 3.9 ± 0.6 years) with age differences between groups not significant.
| No. of donors | No. of session | AMH (pg/mL) | AFC (n) | COCs (n) | TE (n) | TE rate (%) |
|---|---|---|---|---|---|---|
| 42 | 252 | 820.8 ± 172.4 | 16.8 ± 2.1 | 11.8 ± 1.7 | 5.1 ± 1.0 | 40.9 ± 4.2 |
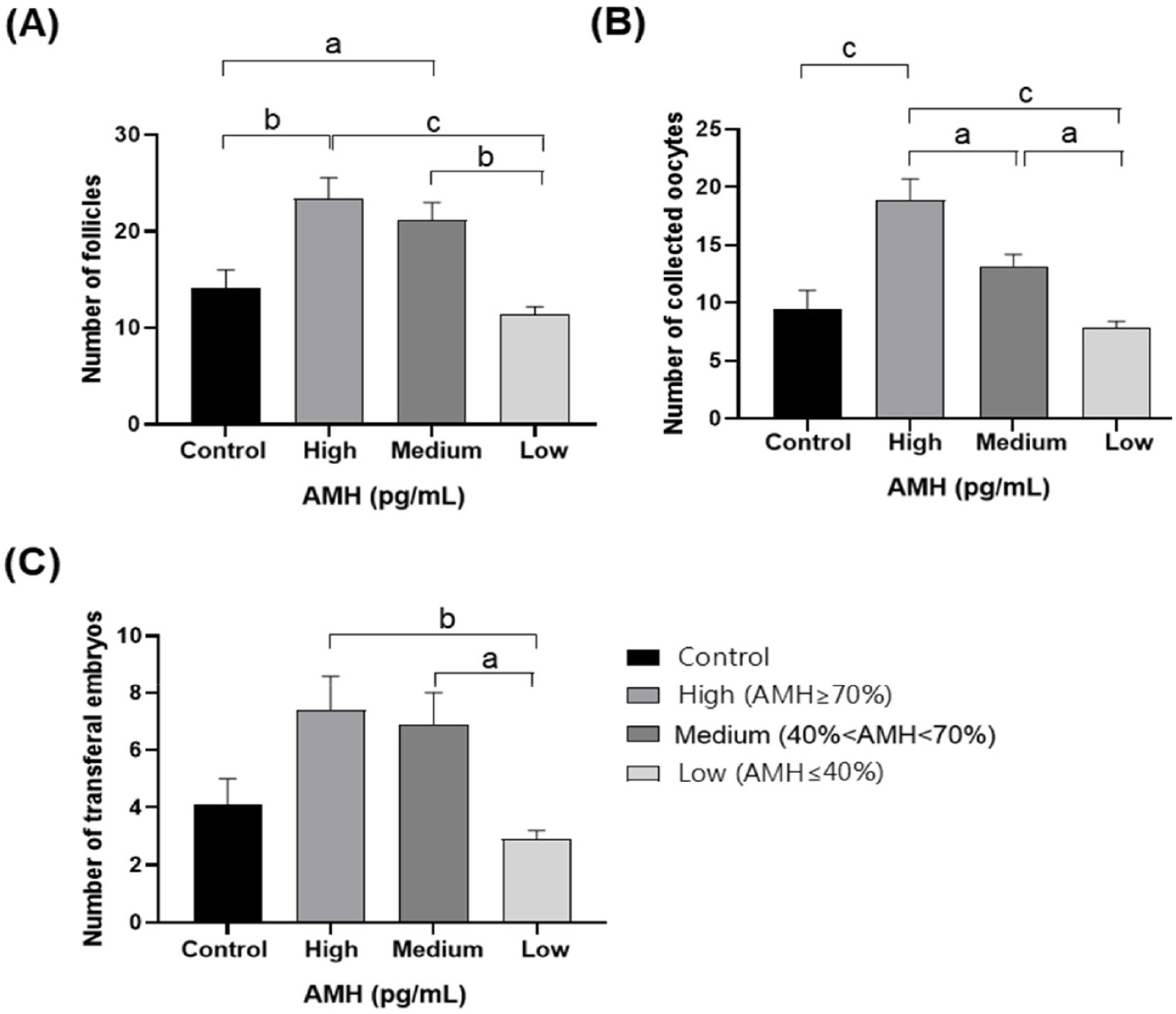
In total, 252 OPU sessions were conducted across both the superstimulated and control groups. AMH concentration, AFC, oocyte retrieval, and transferable embryo production by donors are provided in Table 2. We divided the cows into control and superstimulation groups. The AMH concentration was 806.1 ± 16.6 pg/mL in the control group and 826.8 ± 172.4 pg/mL in the superstimulated group, which was not significantly different between the two groups. However, the difference in the number of AFCs increased by 3.8, from 14.1 ± 1.9 in the control group to 17.9 ± 1.3 in the superstimulated group, and the difference in the number of COCs increased by 3.3, from 9.5 ± 1.6 in the control group to 12.8 ± 1.0 in the superstimulated group. The difference in the number of transferable embryos increased by 1.3, from 4.1 ± 0.9 in the control group to 5.4 ± 0.6 in the superstimulated group, but the difference in the transferable embryo rate was not statistically significant, from 40.0 ± 4.5 in the control group to 41.3 ± 2.6 in the superstimulated group. The superstimulated group was divided into three groups according to AMH concentration to evaluate correlation of AFC, oocyte retrieval, and transferable embryo production with AMH concentration. The AFC confirmed by ultrasound monitoring during OPU in groups H, M, L, and the control group are presented in Fig. 3A. Significantly higher average quantities of follicles with diameters ranging from 1 to 15 mm were observed in groups H and M compared to the control group (p < 0.05). However, no significant difference was noted between the control group and group L. Furthermore, the average quantity of follicles in groups H and M was significantly higher than that in group L (p < 0.05), with no significant difference between groups H and M. Fig. 3B displays the number of oocytes recovered after OPU. The average quantity of retrieved oocytes was significantly higher in group H than in the control group (p < 0.05), whereas no significant difference was noted between control and groups M and L. Moreover, there was a significant difference in the average quantity of retrieved oocytes among groups H, M, and L (p < 0.05). Transferable embryos derived from oocytes retrieved after OPU are shown in Fig. 3C. No significant difference was observed in the quantity of transferable embryos between the three AMH level groups and the control group. However, in the groups in which AMH levels were measured, the H and M groups demonstrated a significant difference from the L group (p < 0.05).
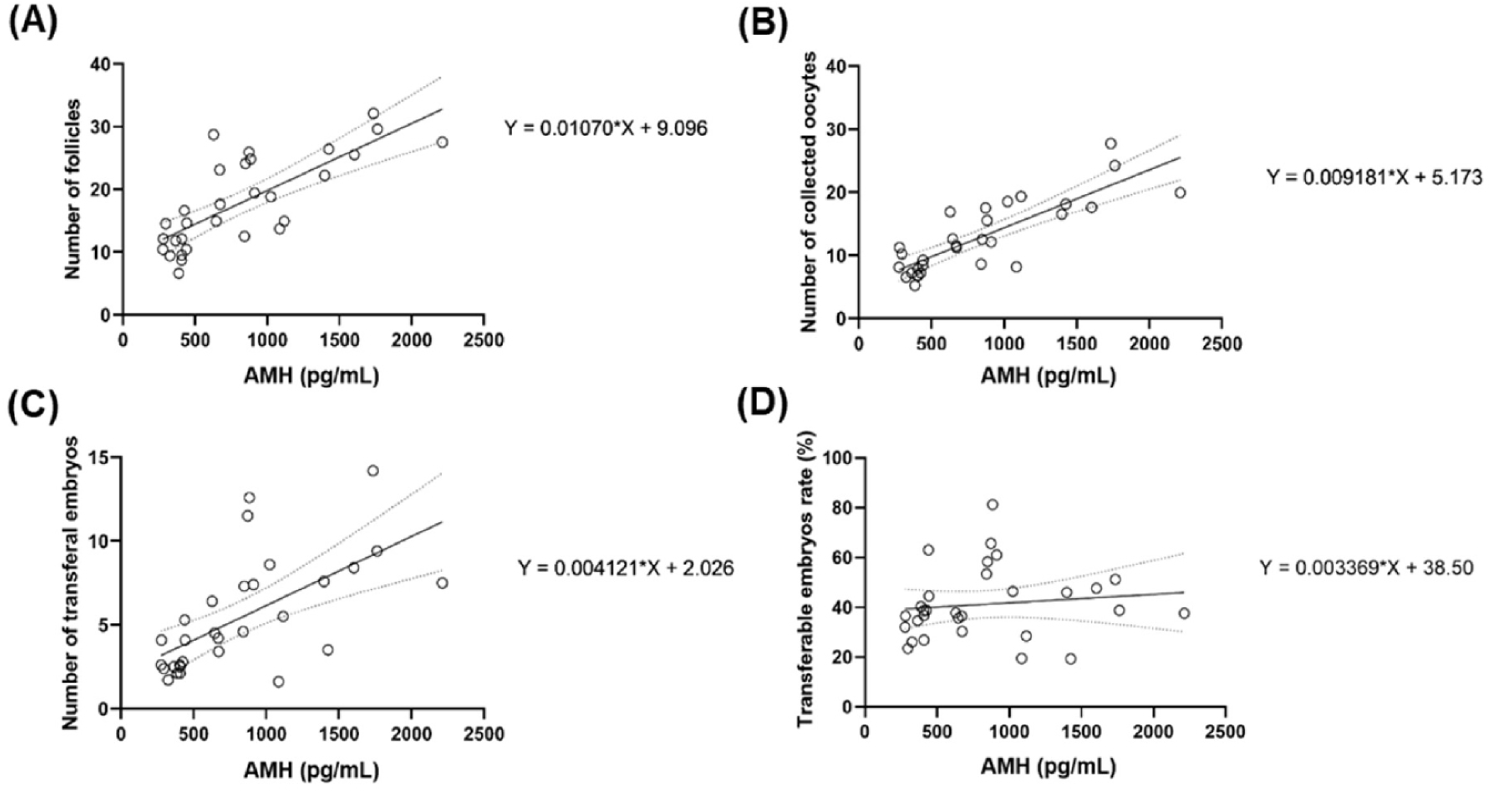
Individuals exhibiting higher levels of AMH tend to possess a greater quantity of antral follicles, as evidenced by a strong positive correlation observed between AMH level and follicle quantity. This correlation was statistically significant (R2 = 0.5785, p < 0.0001;) as shown in Fig. 4A. Similarly, a strong positive correlation was evident between AMH concentrations and the quantity of retrieved oocytes, suggesting that individuals with higher AMH levels tend to have a greater quantity of retrieved oocytes. This correlation was statistically significant (R2 = 0.6857, p < 0.0001;), as shown in Fig. 4B. The total number of transferable embryos and plasma AMH concentration were also correlated. (R2 = 0.4049, p < 0.0001), as shown in Fig. 4C. When the correlation between AMH levels and the proportion of transferable embryos was assessed, a weak positive correlation was observed in individuals with higher AMH levels. This correlation was not significant (R2 = 0.1476, p = 0.5225) as shown in Fig. 4D.
DISCUSSION
Efficient embryo production and optimal donor selection for OPU in beef cattle are crucial to save labor and time. Recently, Ghanem et al. [30] reported that plasma AMH profiles correlated with AFC after random-cycle OPU in Hanwoo cows, as well as with the retrieval of oocytes, suggesting that AMH could serve as a useful indicator of donor selection. Therefore, in this study, we aimed to validate the hypothesis of predicting donor selection using AMH testing under conditions that induce superstimulation of follicles. Consistent with previous studies, the precise synchronization of follicle waves and induction of superstimulation, as conducted in this study, are the most effective production methods [19,38,39]. Controlled internal drug release (CIDR) was used in all donors to maintain stable P4 concentrations, with 12 AU FSH administered over four treatments within two days. Overall, the cattle responded well to superstimulation induction with six rounds of OPU per cow. The average rate of COCs recovery was 11.8 ± 1.7, the average quantity of transferable embryos was 5.1 ± 1.0, and the ratio of transferable embryos was 40.9 ± 4.2 per cow. These efficient rates of oocyte production were consistent with those reported in previous studies, making a comparison between superstimulation and AMH feasible.
Previous studies have shown that repeated AMH tests provide information that is very similar to that provided by a single test [40]. Our study involved conducting a single AMH test, in line with the understanding that a single measurement of AMH concentration offers adequate information to estimate ovarian reproductive capacity. While previous studies have indicated a pattern of increasing and decreasing AFC up to the age of five years in both beef and dairy cows, our study used donor averaging 4.1 ± 0.6 years old. This suggests that the use of a single threshold value for AMH level is practical for selecting donors, making it possible to predict ovarian reserves and overproduction capacity using AMH testing, which is a significant advantage in real-world applications. AMH concentrations vary widely between animal breeds. A comparison between dairy and beef breeds showed that among dairy breeds, Bos indicus (Nelore breed), which is known for its high genetic AFC, exhibits higher AMH levels than other dairy breeds, including Holsteins, Jerseys, and crossbred cattle following in ascending order [30,41–43]. Thus, depending on the species, characteristics, and age of the animal, AMH is an important indicator of AFC, oocyte recovery, and follicular production capacity [44,45]. Hanwoo cattle are commonly used for beef production in Republic of Korea. This study measured AMH levels in Hanwoo donors and categorized them into high, medium, and low groups according to their concentration. These concentrations varied within the range of 276.5 to 2212.5 pg/mL, and the average AMH concentrations for each group were as follows: H group, 1,484.9 ± 129.2 pg/mL; MH group, 775.5 ± 39.0 pg/mL; L group, 371.6 ± 17.8 pg/mL. This indicates a higher tendency for AMH concentrations in Hanwoo cows aimed at beef production than in breeds with reduced breeding capacity, and similar levels were observed in dairy breeds [42,43]. In conclusion, Hanwoo cattle belong to the Bos taurus lineage and exhibit AMH levels more in line with those of Bos indicus breeds, supporting previous research that Hanwoo cows, even though they are beef breeds, display similar tendencies as Holstein dairy cows [44–46].
The strong association between the AMH concentration in the plasma, the AFC, the number of COCs and the capacity to produce embryos has been confirmed in several animal species [27–29,47,48]. According to Widodo et al. [49], Holstein AMH concentration positively correlates with number of COCs and embryos from individual OPU donors. Consistent with these findings, our study revealed a positive correlation between AMH concentrations and the quantity of superstimulated antral follicles (R2 = 0.58) and the number of retrieved COCs (R2 = 0.69), indicating that higher numbers are associated with higher AMH levels. Furthermore, although a positive correlation was observed between AMH levels and the quantity of transferable embryos (R2 = 0.40), there was a weak positive correlation between high AMH levels and the proportion of transferable embryos in each group, albeit statistically insignificant (R2 = 0.15). Recently, Ghanem et al. [30] reported a high correlation between AMH profiles and AFC, quantity of retrieved COCs, and number of embryos produced by each donor in Hanwoo with random estrus cycle. Similarly, Batista et al. [43] reported that high concentrations of AMH in Nelore and Holstein cows showed a strong correlation with AFC and retrieved COCs in donors of these breeds.
In line with other results, AMH concentration proved to be very accurate in predicting ovarian production associated with standardized superstimulation protocols [40,50–53]. Thus, strong correlations between AMH concentration and AFC, COC count, and the number of transferable embryos suggest that increasing the superstimulation response enhances these correlations. In this study, we divided the donors into three groups according to AMH concentration. Comparing the AFC, COCs and number of transferable embryos between each group, we found that group H had a 105.26% increase in AFC, 139.24% increase in COCs and 155.17% increase in transferable embryos compared to group L. Compared to group M, group H had a 10.37% increase in AFC, 43.13% increase in COCs and 7.2% increase in transferable embryos. The M group also showed an 85.96% increase in AFC, 67.08% increase in COCs and 137.93% increase in transferable embryos compared to the L group. Therefore, selecting high-AMH donors using the same housing, feeding, FSH, and labor costs would result in nearly half the cost of embryo production compared to cows that do not consider AMH levels. Consequently, considering AMH levels in donor selection can be a strategic approach for reliable donor selection by embryo production and transfer specialists, offering a significant advantage in reducing embryo production costs.
In summary, AMH shows a strong correlation with the response to superstimulation and the potential for embryo production in each donor. Categorizing AMH concentrations into different groups revealed a strong correlation between high AMH levels and AFC, the number of retrieved COCs, and the number of embryos produced by individual donor cows. Therefore, the results of this study provide a valuable practical method for enhancing the efficiency of Hanwoo donor cow selection and embryo transfer programs during the superstimulation response protocol and OPU procedures, indicating that AMH testing could serve as a reliable indicator for predicting the IVEP capacity of Hanwoo donors.
