INTRODUCTION
In the modern swine industry, suckling pigs face early weaning stress [1,2], involving dietary and social changes such as switching from sow´s milk to a solid and less palatable plant-based feed, adaptations to a new facility, and establishment of hierarchy between pigs from other litters [3,4]. These sudden events affect normal feed consumption behavior [5]. A reduced feed intake generates morpho-functional modifications of intestinal villus, hyperplasia of crypt depth (CD) [6], reduction in digestive enzyme secretions [7], as well as increased permeability to antigens and toxins [8]. Besides these, the inefficient gastric enzyme activity of pigs during the weaning period, due to a low capacity of hydrochloric acid secretion, allows the flow of a high amount of undigested and contaminated feed to the hindgut [2,9]. As a consequence, it provides ideal conditions for the proliferation of pathogenic bacteria and the onset of post-weaning diarrhea (PWD) [10].
For decades, PWD; one of the most economically relevant diseases in pigs [11], has been efficiently controlled by the therapeutic use of antibiotics [12,13]. However, the continued overuse of antibiotics to combat diseases in both livestock and humans has resulted in the development of bacterial resistance to therapeutic treatments [14,15]. Given the necessity of reducing the use of antibiotics, because of public health concern, it is crucial to develop new feed additive-based nutritional strategies to control gastrointestinal infections related to the weaning transition without adverse effects on human health and the environment [10].
The organic acids, based on their acidifying property and their capacity to control the growth of fungal and enteropathogenic bacteria [16], have been efficiently used for decades as feed hygiene enhancers in animal diets [17,18]. Nursery studies have evidenced that organic acids could be used as a powerful tool in maintaining gut health by suppressing the proliferation of pathogenic bacteria such as Escherichia coli [19,20] Clostridium perfringens [21], and Salmonella [22].
Formic acid has especially been demonstrated to enhance gastric activity [23], gut health [24], immune status [25], and modulate the microbiota [25], leading to improvement of growth performance in nursery pigs. However, formic acid is corrosive [26,27], thus affecting equipment life, creating handling difficulties, and also causing general irritation to workers [28,29]. These disadvantages limit its usage in animal husbandry [16]. Interestingly, formic acid derivatives have been receiving more attention regarding animal feed formulations due to their non-corrosive and non-irritating characteristics [16], without loss of their antimicrobial properties and improvements in growth performance [19,30].
Paraformic acid (PFA), a new formic acid derivative, is a dimer formed from two formic acid molecules and obtained through a polymerization process [22]. Up to now, there is no evidence of whether PFA exhibits beneficial effects on the performance of nursery pigs. Therefore, this study aimed to evaluate the effect of PFA supplementation at different concentrations on growth performance, intestinal morphology, and gut microbiota of nursery pigs.
MATERIAL AND METHODS
The protocol was reviewed and approved by the Animal Care and Use Committee of the South China Agricultural University, Guangzhou, China (approval number 2021f082). The animal experiment was conducted according to the Regulations for the Administration of Affairs Concerning Experimental Animals (Ministry of Science and Technology, China). The maximum dosage of formic acid allowed in all species is 10,000 mg/kg according to European Union (EU) regulations 2017/940. The highest level of PFA used in this study was 10 kg/Ton of formulated feed to follow the regulations established by the EU [31]. PFA is a new molecular ingredient made from formic acid and is broken into formic acid molecules in low pH solutions. The dosages used in this experiment did not show any sign of toxicity in the pigs.
A total of 150 crossbred male pigs (21 ± 1 day old; 8.85 ± 0.15 kg of body weight [BW]) were transferred to the conventional nursery facility of Numega Livestock Research Center, Foshan, China, for a 30-day nursery study. Pigs were randomly assigned to five dietary treatments with five replicates (pen) per treatment and six pigs per replicate. The pigs were raised in a naturally ventilated house and had ad libitum access to feed and water during the entire experiment.
There were five dietary treatments: 1) Negative control (NC): nutrient-adequate control diet, formulated to meet or exceed the nutritional requirement according to the NRC [32]; 2) PFA1: similar to NC plus the addition of 0.30% of PFA (paraformic acid®, Numega Nutrition); 3) PFA2: similar to NC plus the addition of 0.60% of PFA; 4) PFA3: similar to NC plus the addition of 1.0% of PFA; 5) Positive control (PC): similar to NC plus the addition of 0.15% of chlortetracycline (Citifac 20% chlortetracycline; CP BIO). Pigs were fed the same nutritional profile during the two-phase feeding regime ( phase 1 [P1; d 0–14], and phase 2 [P2; d 15–30]; Table 1).
The vitamin premix provided per kilogram diet contain: 11375 IU of vitamin A, 3500 IU of vitamin D3, 26.3 IU of vitamin E, 3.5 mg of vitamin of K3, 3.5 mg of vitamin B1, 8.8 mg of riboflavin, 5.4 mg of vitamin B6, 0.03 mg of vitamin B12, 17.5 mg of pantothenic acid, 35.0 mg of niacin; 1.75 mg of folacin, 0.14 mg of biotin.
The percentage of crude protein, crude fat, crude fiber, calcium and phosphorous were determined following the method AOAC 976.05, AOAC 920.39, AOAC 962.09, AOAC 927.02, AOAC 964.06, respectively (Table 2) [33].
Individual BW on d 0, and BW and feed disappearance at the end of each phase were recorded to calculate average daily gain (ADG), average daily feed intake (ADFI), and feed to gain ratio (F:G) per phase.
At the end of P1, rectal stimulation was performed with sterile swabs to obtain fresh feces. Fecal samples were used to evaluate the fecal consistency following the scoring index described by Sherman et al. [34]: 0, normal (feces firm and well-formed); 1, soft consistency (feces soft and formed); 2, mild diarrhea (fluid feces, usually yellowish); and 3, severe diarrhea (feces watery and projectile).
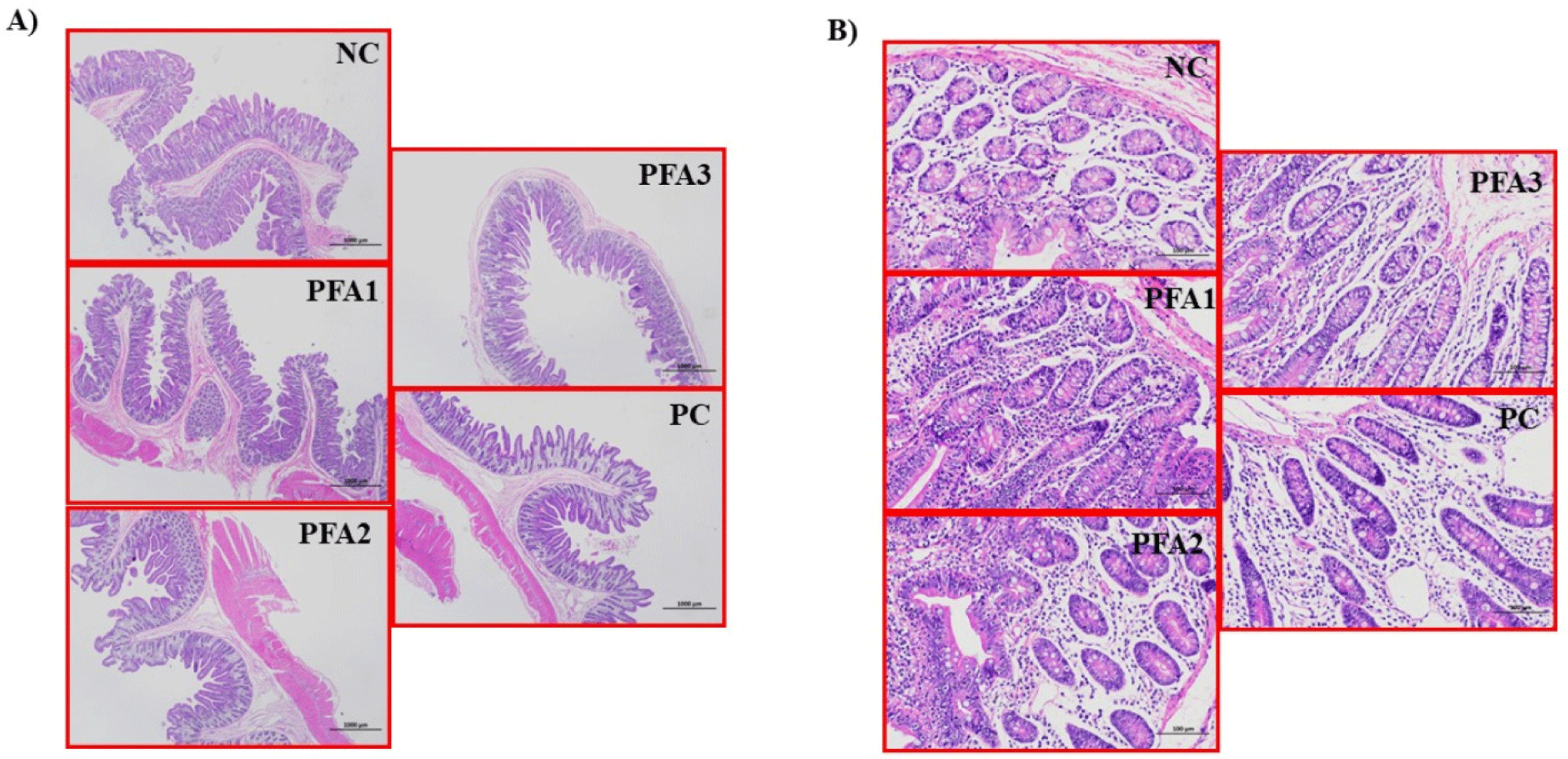
One pig per pen was sacrificed at the end of P2, following the method described by Hu et al. [35]. Per treatment, a total of six subsamples of middle sections of jejunum tissue were collected and used for measuring intestinal morphology according to the procedure described by Núñez et al. [36]. After sampling, tissues were immediately fixed in 10% neutral buffer formalin, dehydrated with normal saline, carefully embedded in paraffin, and then sliced into 6 µm thick sections. Finally, tissues were stained with haematoxylineosin for histological evaluation. The villus height (VH), villus width (VW), CD, and the villus height to crypt depth ratio (VH:CD) conformed to the morphological analysis and were addressed by a computer-assisted system (image-analysis system; Biowizard, Thaitec). The VH was measured from the tip of the villus to the base between individual villi. The VW was determined as the distance of the base width of the duodenal villi, while the CD measurements were taken from the valley between individual villi to the basal membrane. The CH:CD was calculated as the VH divided by CD.
Sterile swabs were used to collect jejunum and cecum digesta samples. Samples were preserved in Puritan® Liquid Amies and transported to lab on ice, then stored at −80°C until DNA extraction. The genomic DNA was extracted using the Omega Bio-tek E.Z.N.A. TM stool DNA kit, followed by agarose gel electrophoresis and Nanodrop to detect the purity and concentration of the DNA. The V4 region of 16S rRNA was amplified using the 515F and 806R primer. The TIANSeq Rapid DNA Library kit (TIANGEN Biotech) was used to build a sequencing library, and then sequencing was performed through the Illumina Miseq System (illumine).
Data were analyzed using the PROC GLM procedure of SAS (SAS Institute) as a Randomized Complete Design. Pen was the experimental unit for ANOVA. Orthogonal contrasts were used to determine the linear and quadratic effect of increased levels of PFA in diets (PFA1, PFA2, and PFA3). Probability (p) value < 0.05 were considered significant, and p values between 0.05 and 0.10 as trends. Raw sequencing data were analyzed via QIIME2 (2019. 10 release). Alpha diversity and beta diversity were used to analyze the complexity of species diversity based on different indexes (Shannon index, and Chao1 index).
RESULTS
All piglets were healthy throughout the experimental period. In P1, there were statistical differences in ADG, with the highest gain in pigs fed PFA2 (p < 0.05), while there were no differences in ADG during P2 (Table 3). However, a quadratic response was observed (p < 0.05) in the overall phase 1 and phase 2 (P1&2) with the highest ADG in pigs fed PFA2. The results of ADG were consistent with the BW per phase, where there was a significant difference in the BW at the end of P2 (p < 0.05) and a quadratic tendency on the final BW, being those fed PFA2 the heaviest pigs. No differences were observed in the ADFI during P1. Pigs fed PC showed the highest ADFI (p < 0.05) in P2 and P1&2, with NC, PFA2, and PFA3 as intermediate, and the PFA1 group with the lowest ADFI. Furthermore, there was a positive linear response (p < 0.05) in ADFI in pigs fed increasing levels of PFA (PFA1, PFA2, PFA3) in P2 and P1&2. Regarding to F: G ratio, pigs fed any PFA level showed lower F: G than NC and PC treatments in P1, P2, and P1&2 (p < 0.05). Additionally, a quadratic response was observed in the P1&2 (p < 0.05) with the lowest ratio in pigs fed PFA2.
NC: nutrient adequate control diet; PFA1, similar to NC plus 0.3% of PFA; PFA2, similar to NC plus 0.6% of PFA; PFA3, similar to NC plus 1.0 % of PFA; PC, similar to NC plus 0.15% of chlortetracycline.
Ortogonal contrast to determine linear and quadratic response effects of increased levels of PFA in diets (PFA1, PFA2, and PFA3).
Pigs fed any level of PFA (0.3%, 0.6%, and 1.0%) or PC had better fecal scores than pigs fed the NC diet (p < 0.05; Table 4). Furthermore, increasing the level of PFA led to a linear reduction in the fecal score at the end of P1 (p < 0.05).
| Parameter | Treatments1) | SEM | p-value2) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| NC | PFA1 | PFA2 | PFA3 | PC | Treatment | Linear | Quad | ||
| Fecal score | 2.0a | 1.05b | 1.03b | 0.95b | 0.92b | 0.14 | 0.05 | 0.02 | 0.93 |
NC, nutrient adequate control diet; PFA1, similar to NC plus 0.3% of PFA; PFA2, similar to NC plus 0.6% of PFA; PFA3, similar to NC plus 1.0 % of PFA; PC, similar to NC plus 0.15% of chlortetracycline.
There were no statistical differences in the VH, VW, CD, and VH:CD. Pigs fed PFA2 had the best numerical response regarding the morphological parameters evaluated in this study (Table 5; Fig. 1).
NC, nutrient adequate control diet; PFA1, similar to NC plus 0.3% of PFA; PFA2, similar to NC plus 0.6% of PFA; PFA3, similar to NC plus 1.0 % of PFA; PC, similar to NC plus 0.15% of chlortetracycline.
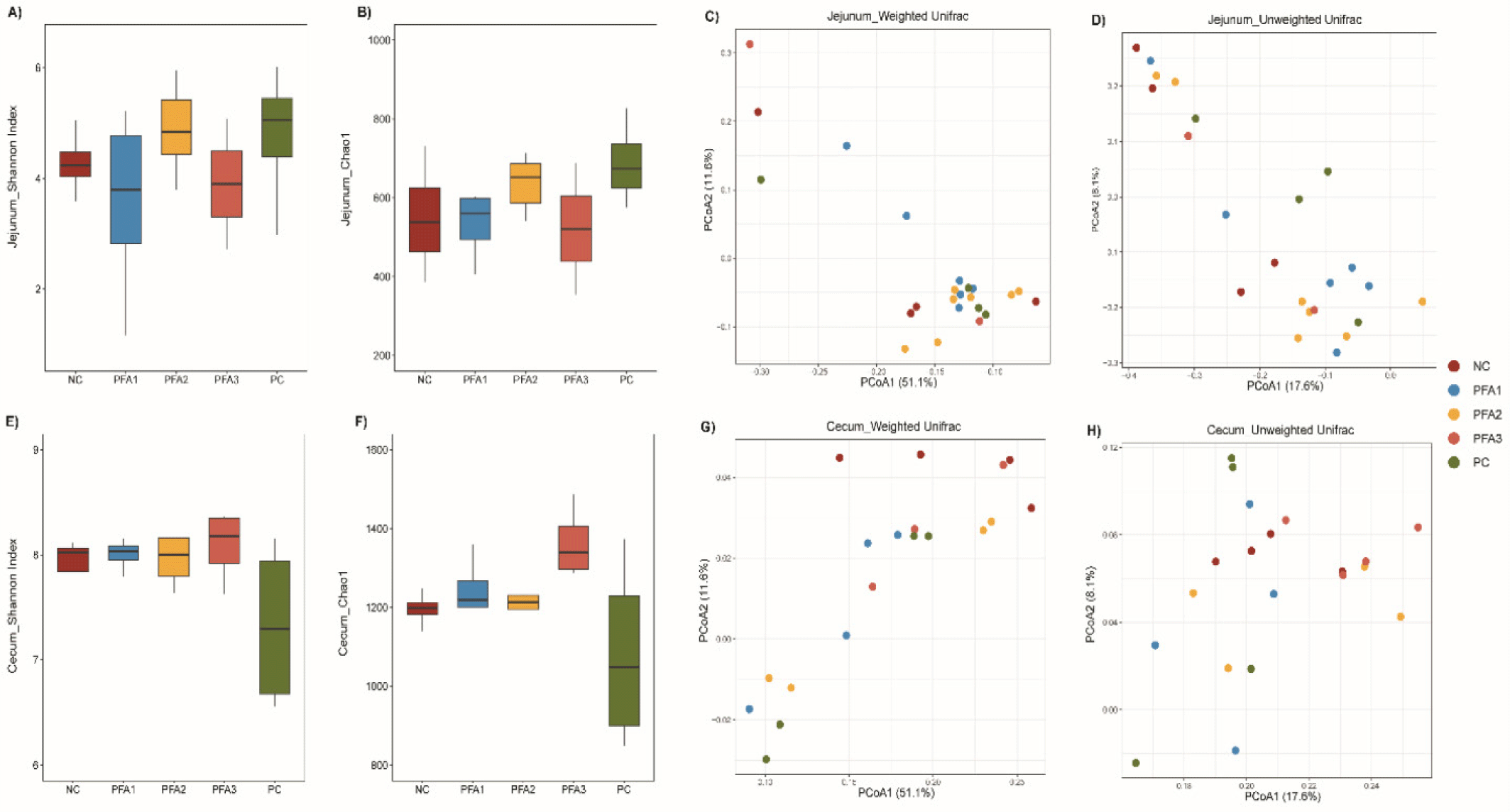
The bacterial diversity and richness were not significantly influenced by the different dietary treatments in the jejunum (Shannon index and Chao1 index: Figs. 2A and 2B, respectively), while the weighted and unweighted Unifrac based on principal coordinate analysis show differences in community structures based on treatment groups (Figs. 2C and 2D). In the cecum, no differences were observed in the Shannon index between treatments (Fig. 2E), while a tendency to differ was observed in the Chao1 index, with higher diversity in the PFA3 group (Fig. 2F). In addition, differences in community structures among treatments were observed in the weighted and unweighted Unifrac based on principal coordinate analysis (Fig. 2G and 2H, respectively).
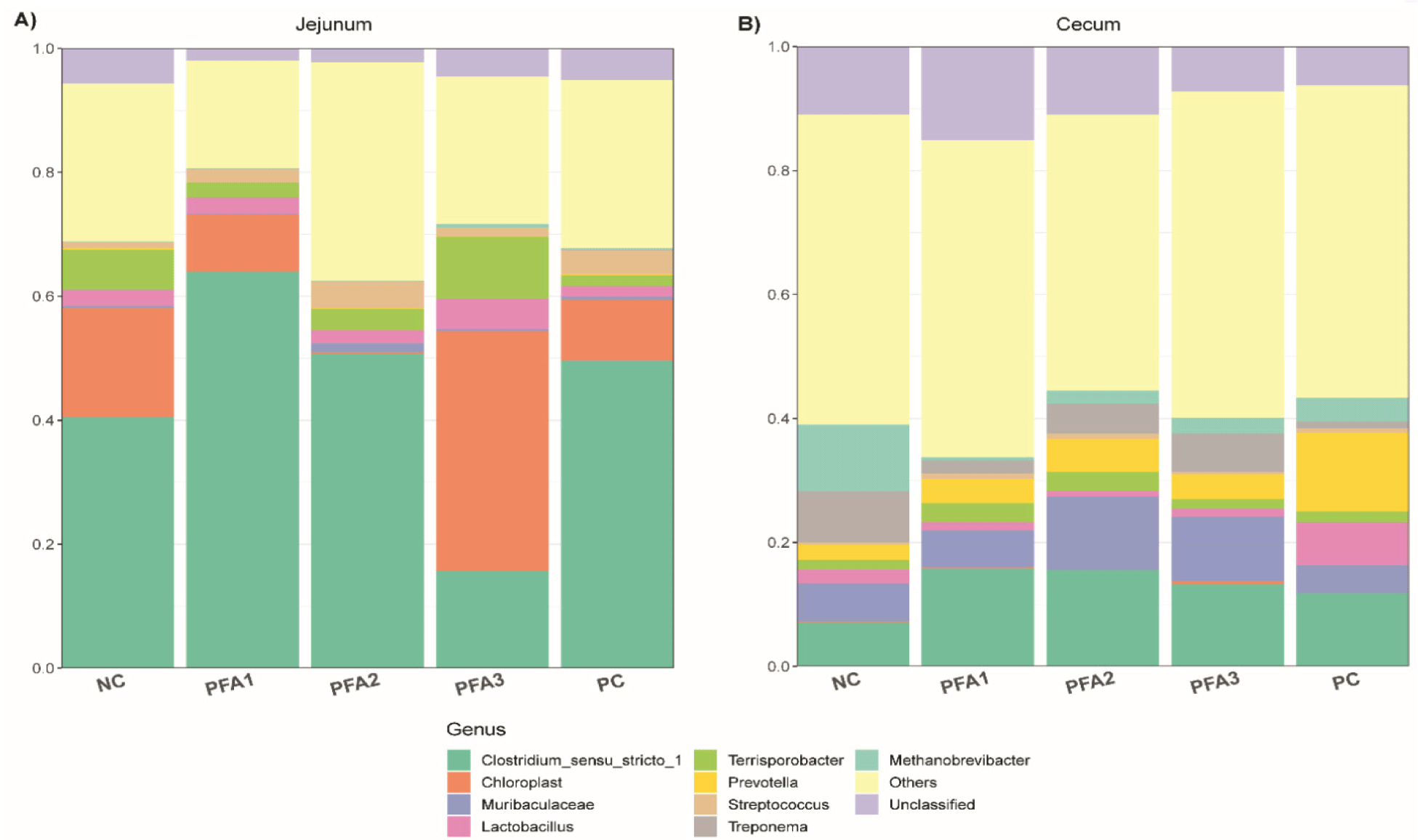
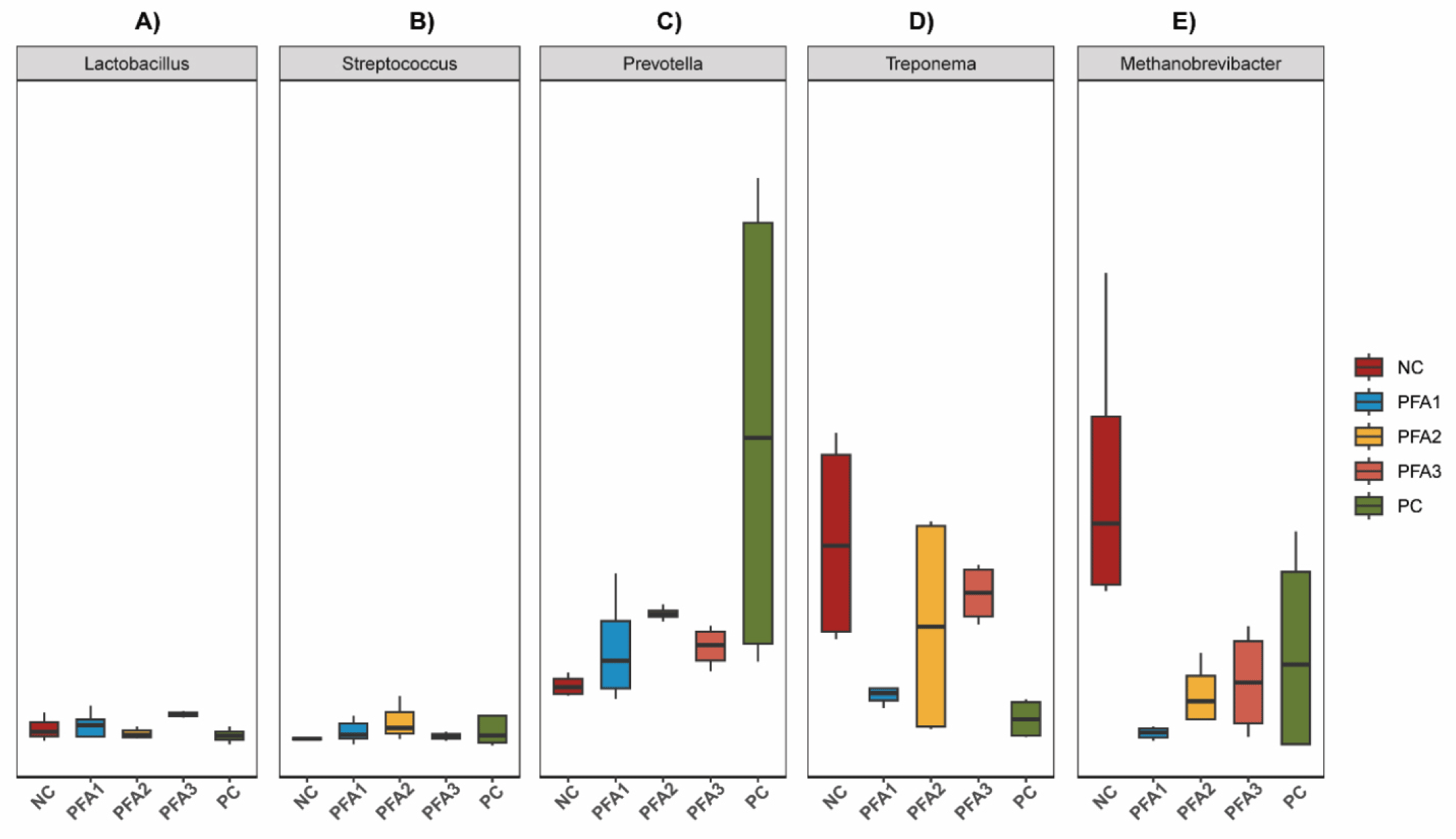
The relative abundance of the most dominant jejunal and cecal microbiota is shown in Fig. 3. Lactobacillus and Streptococcus showed higher relative abundance in pigs fed PFA3 and PFA2, respectively, (Fig. 4A and 4B). On the other hand, the most notable changes in the relative abundance, at the genus level, in cecum samples were Prevotella, Treponema, and Methanobrevibacter (Fig. 4C, 4D, and 4E, respectively). Pigs fed PC had the highest relative abundance of Prevotella among treatments, followed by PFA groups (PFA1, PFA2, and PFA3) as intermediate, and NC as the lowest group. Furthermore, PFA1, PFA2, PFA3, and PC treatment had a lower relative abundance of Treponema and Methanobrevibacter than the NC group.
DISCUSSION
Organic acids have gained attention in the last few years due to their antimicrobial effects on gut microbiota and improvements in the general performance of pigs [24,37,38]. Several studies summarized by Luise et al. [16] suggested that incorporating formic acid as a feed supplement might improve the general performance of nursery pigs. Among them, the main evidence indicates that formic acid modifies the acidic condition of the feed, hindering the growth of pathogenic bacteria and improving the hygiene of the feed [39]. Furthermore, formic acid reduces stomach pH, offering the ideal condition for more efficient activity of digestive enzymes [23] as well as acting as an antimicrobial agent, suppressing the survival and colonization of low pH intolerant pathogenic bacteria [40].
In the current study, the ADG of pigs that received PFA-supplemented nursery feed highlighted the health benefits that eased weaning transition stress. The supplementation of PFA2 evidenced a better daily gain of 66.63 g and 65.48 g over pigs fed NC in P1 and P1&2, respectively, and 18.46 g and 38.08 g over pigs fed PC diet in P1 and P1&2, respectively. Similar results were reported by Dahmer et al. [25] where nursery pigs fed 0.70% of formic acid showed higher ADG than those supplemented with the basal diet. Interestingly, pigs had an ADG of 470 g, similar to the ADG found in this study (466 g) with 0.60% of PFA inclusion. Additionally, Luise et al. [41] reported overall improvements in ADG with nursery pigs supplemented with 0.64% of formic acid on day 21 after weaning. The growth performance improvements found in this study with pigs fed PFA might be due to the reduction of pathogenic bacteria in the feed attributed to the acid´s presence before consumption, as well as the enhancement of pepsin enzyme activity by lowering the stomach pH, which in turn improved the nutrient utilization, and a lower amount of undigested feed available in the gut for pathogenic bacteria growth. This assumption might be supported by the results of the fecal score, where the pigs under PFA supplementation or PC had a similar fecal consistency, classified between normal and soft and well-formed feces, while those fed NC showed an incidence of mild diarrhea. The incidence of diarrhea in nursery pigs is a consequence of a complex interaction of several infectious agents that colonize the intestines and secrete their endotoxins [11], which in turn generate a cascade of inflammatory responses, intestinal tissue damage as well as secretion of fluids [1]. As a result of these complex interactions, PWD is generated leading to a reduction in nutrient utilization, and reductions on the general growth performance of nursery pigs.
Some studies have reported no positive effects on ADFI and F:G ratio in nursery pigs fed 0.2 % [42] or 0.5% [43] of formic acid. Such results are contradictory to the findings of this study, where increasing the level of PFA stimulated the ADFI and showed a lower F:G ratio, mainly in those fed intermediate levels of PFA (0.6%), when compared to those fed NC or PC diets. Based on the physicochemical properties of organic acids, a normal formic acid molecule has a pungent odor plus irritating and corrosive characteristics [28,44]. Eisemann and van Heugten [45] evaluated three different levels of formic acid (0.8%, 1.0% y 1.2%) in combination with ammonium formate, and reported a reduction in feed intake as the inclusion level of formic acid was increased during the nursery phase 2 and grower phases. However, feed intake tended to increase in those pigs fed diets devoid of formic acid plus ammonium formate. Furthermore, Ettle et al. [46] studied the self-selection of feed with or without acidifier and its impact on feed intake behavior. Pigs under the feed self-selection study had preferences for unacidified diets versus acidified diets with 1.2% or 2.4% of K-diformate. However, in the second part of the experiment, pigs were given the choice between a 1.2% formic acid diet or 1.2% sorbic acid diet, and they showed a preference for the sorbic acid-based diet over the formic acid-based diet, reducing feed intake due to possible low palatability. Based on the above-mentioned, it is possible to speculate that the supplementation of PFA might not exert negative effects on feed palatability, allowing the supplementation with a higher inclusion level of formic acid without reductions on ADFI as evidenced by the positive linear response as increased the PFA inclusion on the overall ADFI. Additionally, the supplementation of PFA2 showed to exert the highest benefit on feed efficiency, supported by the reduction in the F:G ratio as well as the obtained quadratic response.
Overall, pigs fed NC and PC consumed 11.74 g and 54.36 g, respectively, more than pigs fed PFA2. Interestingly, pigs fed PFA2 gained 66.21 g and 38.08 g more than the NC and PC groups, respectively. The highest daily gain obtained in the PFA2 group supports the BW of PFA2 pigs with 1.89 kg over NC group and 0.85 kg over PC group. These results show that the PFA practical inclusion of 0.6 % in nursery diets is feasible as a potential substitute for antibiotics, during the early nursery period. Further studies should be conducted to evaluate PFA supplementation from the nursery and follow-up on pig performance through the finisher period to determine the potential impact of PFA supplementation compared with antibiotics at the end of the fattening period.
It has been well evidenced that weaning is a stressful period that affects intestinal morphology and health through a reduction in intestinal cell renewal and increments of apoptosis or cell death [47,48]. However, healthy intestinal morphological structures such as VH, and CD are important morpho-functional characteristics for nutrient digestion and absorption that exert pronounced effects on performance [49]. In the current study, the supplementation of PFA at different concentrations, or PC did not show differences in VH, CD, VW, and VH:CD ratio. However, the PFA2 group showed a remarkable numerical increase in VH, VW, VH:CD ratio, and lower CD than pigs under the PC diet or NC. Long et al. [50] evaluated a synergistic blend of free and buffered short-chain fatty acids composed of formic acid, acetic acid, and propionic acid at a 0.30% inclusion level in nursery pigs. They found a lack of notable changes in VH and CD in the duodenum, jejunum, or ileum compared to the antibiotic or control group. Furthermore, Manzanilla et al. [43] reported no differences in VH and CD with pigs fed 0.5% formic acid versus 0.30% of a plant extract containing carvacrol, cinnamaldehyde, and capsicum oleoresin. Similarly, a chicken study reported no changes in morphological structures of the intestine when the birds were fed 0.05% or 0.10% of formic acid, plant extract mixture, or antibiotic as growth promoters [51]. VH reflects a balance between the mitotic activity of the crypt enteric cells and the desquamation produced principally by external aggressors [43]. Additionally, antimicrobial compounds such as organic acids have been evidenced to control the pathogenic load in the intestines, which in turn decreases the presence of toxins and reduces the damage on intestinal morphology, mainly on the VH, thus offering conditions for nutrient utilization [52]. PFA at a concentration of 0.6% might potentially maintain better gut health based on the slight increase in VH reported in this study. Furthermore, the positive effects on F:G ratio of pigs fed PFA2 might be due to the slight improvements in VH, VW, and VH:CD ratio, offering a better absorptive area for nutrient utilization.
A balanced microbiota has been correlated with gut health and is responsible for different functions in the host such as nutrient absorption, metabolism, gastrointestinal development, and immune function [53]. Additionally, a good healthy condition has been linked with a high alpha diversity in humans [54,55] and pigs [56,57]. The Chao1 index is an indicator of microbial richness [58]. In this study, pigs fed PFA3 showed to stimulate the cecal microbial diversity, as reported by the Chao index. An organic acid-based study by Wei et al. [59] reported a higher diversity of microbial species in nursery pigs fed 0.10% of a blend of organic acids than those fed the control diet. Likewise, Li et al. [22] evaluated the supplementation of 0.1% of PFA in 42-day broiler chickens and evidenced a greater microbial richness. Nursery pigs are predisposed to face gut dysbiosis during the first weeks of weaning, and this imbalance of microbiota dramatically affects the microbial richness and predisposes the pigs to gastrointestinal disorders [10]. Based on these results, the use of PFA might help minimize dysbiosis and maximize the proliferation of beneficial bacteria, leading to improved bacterial richness.
It has been well reported that the genera Lactobacillus [59] and Streptococcus are two of the most dominant groups of lactic acid bacteria in the proximal small intestine [60]. Lactobacillus and Streptococcus produce lactic acid, which benefits the control of some harmful bacteria in the gut. However, some potential pathogenic bacteria can multiply and colonize the main site of nutrient absorption and generate significant damage to intestinal morphology [61]. Because organic acids have demonstrated to reduce pH of stomach and small intestine due to their acidifying properties, the supplementation with PFA2 and PFA3 seems to modulate the proliferation of these bacteria, possibly, by adequations of the intestinal pH, thus offering the ideal condition for their proliferation. The improvements in growth performance might also be influenced by the proliferation of healthy microbiota and reduction of the development of potential pathogenic bacteria in the site of nutrient utilization.
Mathanobrevibacter, a genus belonging to the order Methanobacteriales, is H2-oxidizing methanogens [62]. Approximately, 1.2% of ingested energy is lost by methane production in pigs, thus contributing to the greenhouse effect [63]. Recently, Li et al. [22] evaluated the feed supplementation of 0.1 % PFA for broiler chickens and reported a significant reduction in the relative abundance of methanogenic bacteria. Our results are similar to those evidenced by Li, where the supplementation of PFA reduced the abundance of Methanobrevibacter. Together, these results suggest that the supplementation of PFA reduces methane emissions, thus providing for a more environmentally friendly swine industry.
Several species of treponemes are swine pathogens [64]. The genus Treponema causes ear necrosis and ulcers in pigs [65]. Interestingly, organic acids have been shown to efficiently reduce the Treponema abundance, specifically, the Brachyspira hyodysenteriae isolated from pigs [66]. The supplementation of PFA might help to maintain a healthier microbial population one month post-weaning by reducing the Treponema abundance in the gut. In addition, the abundance of Prevotella, a group of fiber-fermenting bacteria, gradually increases during the transition period from a milk-based diet to a solid plant-based diet [67], and has been positively correlated with the growth performance of nursery pigs [68]. The supplementation of PFA groups or PC increases the relative abundance of Prevotella. Similar results were reported by Pluske et al. [69] where a blend of organic acids, including formic acid, modulates the prevotella abundance similarly to an amoxicillin-supplemented diet, demonstrating that organic acid derivatives can help to maintain healthy gut microbiota.
CONCLUSION
This study demonstrated that the supplementation of 0.6% PFA in nursery pig diets can efficiently replace the use of antibiotics, as a growth promoter, through beneficial modulation of the gut microbiota, enhancement of intestinal morphology, control of diarrhea incidence, and improvements in growth performance. This finding supports the benefits of using PFA as a feed additive in nursery pig diets. Further studies have to be conducted to evaluate PFA supplementation during nursery and follow-up on pig performance through the fattening period to determine the potential practical implication of PFA supplementation compared to antibiotics.
