INTRODUCTION
Post-weaning diarrhea (PWD) is a potentially fatal disease in swine production worldwide, causing dehydration, growth delay, and death in severe cases causes of negative impacts on the industry [1]. During the post-weaning period, various stress factors, including dietary, environmental, social, and physiological stressors, affect weaning pigs. These factors reduce feed intake and growth, thus resulting in intestinal dysfunction, and an increased susceptibility to inflammation [2,3]. Furthermore, disruption of the intestinal barrier increases intestinal permeability leading to PWD due to infections by intestinal pathogens such as Escherichia coli [4]. To solve these issues, in-feed antibiotics have been widely utilized in the livestock industry for growth promotion and disease treatment including PWD. However, the incidence of antimicrobial resistance and residual issues remain concerning for animal and public health due to the overuse of in-feed antibiotics [5]. Therefore, various nutritive alternatives such as probiotics, enzymes, minerals, and others, have been utilized to enhance animal health and performance [6].
It is known that copper (Cu) and zinc (Zn) are vital trace elements that act as components of metabolic enzymes and perform biological functions [7]. Both support immunity, reproduction, and growth of animals [8,9]. The most commercial forms in the swine industry are zinc oxide (ZnO) and copper sulfate (CuSO4) because of their relatively low cost compared with other forms [9,10]. These act as alternatives to in-feed antibiotics for promoting growth and have antimicrobial effects such as reducing diarrhea incidence in weaned pigs at concentration levels exceeding normal nutritional requirements according to the National Research Council [11]. Specifically, pharmacological levels (2,000 to 4,000 mg/kg) of ZnO have been commonly supplemented in weanling diets to promote growth performance and reduce diarrhea frequency by enhancing morphological structure and maintaining gut integrity [12,13] . In addition, previous studies reported that a dose of 150 to 250 mg/kg supplementation of CuSO4 stimulates growth rate and feed intake, and sustains fecal consistency via modulating gut microbiota homeostasis in the intestine [14,15].
However, supplementation of high doses of CuSO4 (200 to 250 mg/kg) and ZnO (2,000 to 4,000 mg/kg) in the nursery diet can be excreted through manure without being normally absorbed into the intestine, resulting in environmental pollution [16–18]. Based on these issues, the European Union has legislated new maximum levels to limit Zn and Cu supplementation to 150 mg/kg up to 4 weeks after weaning [19,20]. Thus, new forms of Cu and Zn at lower doses have been proposed to reduce excretions and improve growth performance during the weaning phase. Lipid-coated and concentrated forms of ZnO and CuSO4, protect against the formation of insoluble complexes with other minerals and dissociation in the stomach. They are dissociated by pancreatic lipase, and efficiently absorbed in the small intestine of monogastric animals [21–23]. A previous in vitro study presented the dissociation percentage of coated and uncoated ZnO in the stomach. The results showed that the percentages were 25.03% for coated and 85.26% in uncoated ZnO [24]. Consequently, this reduced the quantity released into the soil through the manure. Despite the growing interest in coated ZnO, there are still limited studies on dietary mixture of coated ZnO and CuSO4 in weaned pigs. Therefore, the objective of this study was to evaluate the effects of low doses of lipid-coated CuSO4 and ZnO on the growth performance, diarrhea, nutrient digestibility, immune responses, and fecal microbiota of weaned pigs.
MATERIALS AND METHODS
All the experimental protocol for this study was reviewed and approved by the Institutional Animal Care and Use Committee of Chungnam National University, Daejeon, Korea (approval# 202103A-CNU-080).
The lipid-coated ZnO and CuSO4 practiced in this study were provided by a commercial company (ACC, Seongnam, Korea). These products are concentrated forms of Zn and Cu from ZnO and copper sulfate pentahydrate (CuSO4ㆍ5H2O) which are microencapsulated in a lipid matrix by fatty acids and hydrogenated palm oil according to the manufacturer’s information.
A total of 96 weaned piglets ([Landrace × Yorkshire] × Duroc; 7.29 ± 0.69 kg of average initial body weight BW]) were assigned to four dietary treatments (4 pigs/pen; 6 replicates/treatment) in a randomized complete block design (block = initial BW). The dietary treatments were (1) a basal weaner diet based on corn-soybean meal (CON), (2) CON supplemented with 2,500 ppm standard ZnO (T1), (3) CON supplemented with 100 mg/kg dietary coated CuSO4 and 100 mg/kg dietary coated ZnO (T2), and (4) CON supplemented with 200 mg/kg dietary coated CuSO4 and 200 mg/kg dietary coated ZnO (T3). The basal diet was mixed to meet or exceed the nutritional requirements of the National Research Council [11] for weaned pigs (Table 1). The study was conducted for 6 weeks, and the pigs were allowed ad libitum access to feeders and water and were housed in the same-sized pen (2 m × 2 m) throughout the experimental period.
In each pen, pigs’ BW and remaining feed were weighed on days 1, 14, and 42 to figure out the average daily gain (ADG), average daily feed intake (ADFI), and gain to feed ratio (G:F). The fecal score of the pigs was visually monitored in each pen by two independent observers during the first 2 weeks. The score ranged from 1 to 5 (1 = hard and dry feces, 2 = soft feces, 3 = moist feces, 4 = mild diarrhea, and 5 = mild and severe diarrhea) and were calculated by counting the number of pen days with a diarrhea score of 4 or higher as a percentage. Blood samples were collected from the jugular vein of one randomly selected pig per pen using 10 mL tubes with or without ethylenediaminetetraacetic acid (EDTA) on days 1, 7, and 14. Blood samples from tubes without EDTA were left to clot at room temperature for 2 hours and then centrifuged for 15 min at 3,000×g at 4°C to obtain serum. These samples were stored at −80°C for immune response analysis. In the final week of the experiment, 0.2 % chromic oxide (Cr2O3) was fed to all pigs as an indigestible marker. Fecal samples were collected from a randomly one selected pig per pen using rectal palpation for three days after the adaption period and stored at −20°C to measure nutrient digestibility [25,26]. Fecal samples were obtained from three randomly chosen pigs in each dietary treatment on the last day of the experiment and stored at −80°C until metagenomic and fecal microbial analysis
Diets and fecal samples were dried using a forced-air drying oven at 65°C for 72 h and then ground through a grinder (80350, Hamilton Beach, Glen Allen, VA, USA) for apparent total tract digestibility (ATTD) analysis. All ground samples were examined for dry matter (DM), crude protein (CP) by Kjeldahl method, and energy using a bomb calorimeter (Parr 1281 Bomb Calorimeter, Parr Instrument, Moline, IL, USA) following the procedures of the Association of Official Analytical Chemists [27]. The concentration of chromium in the samples was determined using an absorption spectrophotometer (Hitachi Z-5000 Absorption Spectrophotometer, Hitachi High-Technologies, Tokyo, Japan). The ATTD of the DM, CP, and energy for each dietary treatment were calculated according to the previous study [28].
Whole blood samples were collected in EDTA tubes using an automated hematology analyzer (scil Vet abc hematology analyzer; scil animal care company, F-67120 Altorf, France) [29] . The measurements included the numbers of white blood cell (WBC), red blood cell (RBC), and hematocrit (HCT). The serum samples were applied to determine immune responses including tumor necrosis factor-α (TNF-α) and cortisol using porcine enzyme-linked immunosorbent assay kits (ELISA) kits (R&D Systems, Minneapolis, MN, USA). Additionally, levels of serum immunoglobulin A (IgA), serum immunoglobulin G (IgG), and serum immunoglobulin M (IgM) were determined using ELSA kits (Bethyl Laboratories, Waltham, MA, USA). All assays were performed according to the manufacturer’s instructions.
DNA was extracted from fecal samples (200 mg of feces per sample) using the QIAamp Fast DNA Stool Mini Kit (QIAGEN, Hilden, Germany) based on the manufacturer’s protocol. The concentration of DNA was measured using the Colibri Microvolume Spectrometer (Titertek Berthold, Pforzheim, Germany), and the samples with 260/280 ratios between 1.80 and 2.15 were used to additional analysis [30]. The V5 to V6 regions of the 16S rRNA genes were amplified using sets of polymerase chain reaction (PCR) primers consisted of 799F-mod6 and 114R [31]. After PCR amplification, the products were refined using a Wizard® SV Gel and PCR Clean Up System purification kit (Promega, Madison, WI, USA). The sequencing of purified 16S rRNA gene was performed using the Illumina MiSeq platform at BRD Inc (Dongtan, Korea) following the manufacturer’s protocols. Quality control of all raw sequence data was checked utilizing FastQC [32], and then the 16S rRNA gene sequences were analyzed using the Deblur algorithm, which is executed in both QIIME2 software and the Microbiome Helper pipeline [33]. After applying the algorithm, sequences were grouped into operational taxonomic units (OTUs), determined at a similarity cutoff of 97% [34]. Alpha diversity indices such as the observed OTUs, Shannon, Simpson, and Chao1 were measured to compare the diversity of microbial communities within each dietary treatment. In addition, principal coordinated analysis (PCoA) based on unweighted and weighted UniFrac distances was used to visualize differences in microbial communities among the dietary treatments. Taxonomic composition of the dietary treatments was expressed as a percentage at the phylum and genus levels based on their relative abundance.
Data were subjected to the GLM procedure of SAS (SAS Institute, Cary, NC, USA) using a randomized complete block design (block = initial BW). The experimental unit was the pen. The statistical models for growth performance, nutrient digestibility, blood profiles, and immune responses of weaned pigs included dietary treatment as the main effect and initial BW as a covariate. The frequency of diarrhea was analyzed using the Chi-square test. The MicrobiomeAnalyst webtool (https://www.microbiomeanalyst.ca/) was used to analyze alpha and beta diversity. STAMP software v. 2.1.3 [35] was used for taxonomic classification using a two-sided Welch’s t-test. Alpha diversity indices were measured using ANOVA and beta diversity based on unweighted and weighted UniFrac distances was estimated using ANOSIM to determine the differences in microbial diversity among the dietary treatments. Statistical difference and tendency for dietary treatment effects were set at p < 0.05 and 0.05 ≤ p < 0.10, respectively.
RESULTS
Pigs fed dietary T2 and T3 had higher (p < 0.05) ADG and G:F on day 1 to 14 than those fed CON (Table 2). Additionally, the groups supplemented with Cu and Zn tended to have a lower (p < 0.10) frequency of diarrhea than those fed CON. However, no differences were found in overall average ADFI for the first 14 days after weaning among the dietary treatments. In addition, dietary T2 and T3 increased (p < 0.05) ADG over the entire experimental period compared with the CON. As shown in Table 3, no differences were found ATTD of DM, CP and energy among dietary treatments.
CON, a basal weaner diet based on corn and soybean meal; T1, CON + 2,500 ppm standard ZnO; T2, CON + 100 mg/kg dietary coated CuSO4 and 100 mg/kg dietary coated ZnO; T3, CON + 200 mg/kg dietary coated CuSO4 and 200 mg/kg dietary coated ZnO; BW, body weight; ADG, average daily gain; ADFI, average daily feed intake; G:F, gain to feed ratio.
| Item2) | CON | T1 | T2 | T3 | SEM | p-value |
|---|---|---|---|---|---|---|
| DM (%) | 70.68 | 75.27 | 80.17 | 81.15 | 5.67 | 0.324 |
| Energy (%) | 75.34 | 77.83 | 79.74 | 84.33 | 5.21 | 0.414 |
| CP (%) | 69.84 | 73.33 | 76.40 | 82.59 | 6.48 | 0.542 |
Pigs fed dietary T2 tended to have lower (p < 0.10) the number of WBC on day 7 and HCT on day 14 than those fed CON (Table 4). However, there was no difference in RBC among dietary treatments. Regarding immune responses (Table 4), dietary T1 tended to have a lower (p < 0.10) concentration of serum cortisol on day 7 and a higher (p < 0.10) serum immunoglobulin M (IgM) than CON. In addition, dietary T2 tended to have decreased (p < 0.10) serum concentrations of TNF-α on day 7 and increased (p < 0.10) serum IgG on day 14 compared with CON. However, no differences were found in serum IgA levels among the dietary treatments.
CON, basal weaner diet based on corn and soybean meal; T1,= CON + 2,500 ppm standard ZnO; T2,= CON + 100 mg/kg dietary coated CuSO4 and 100 mg/kg dietary coated ZnO; T3, CON + 200 mg/kg dietary coated CuSO4 and 200 mg/kg dietary coated ZnO; WBC, white blood cell; RBC, red blood cell; HCT, hematocrit; TNF-α, tumor necrosis factor-alpha; IgG, immunoglobulin G; IgM, immunoglobulin M; IgA, immunoglobulin A.
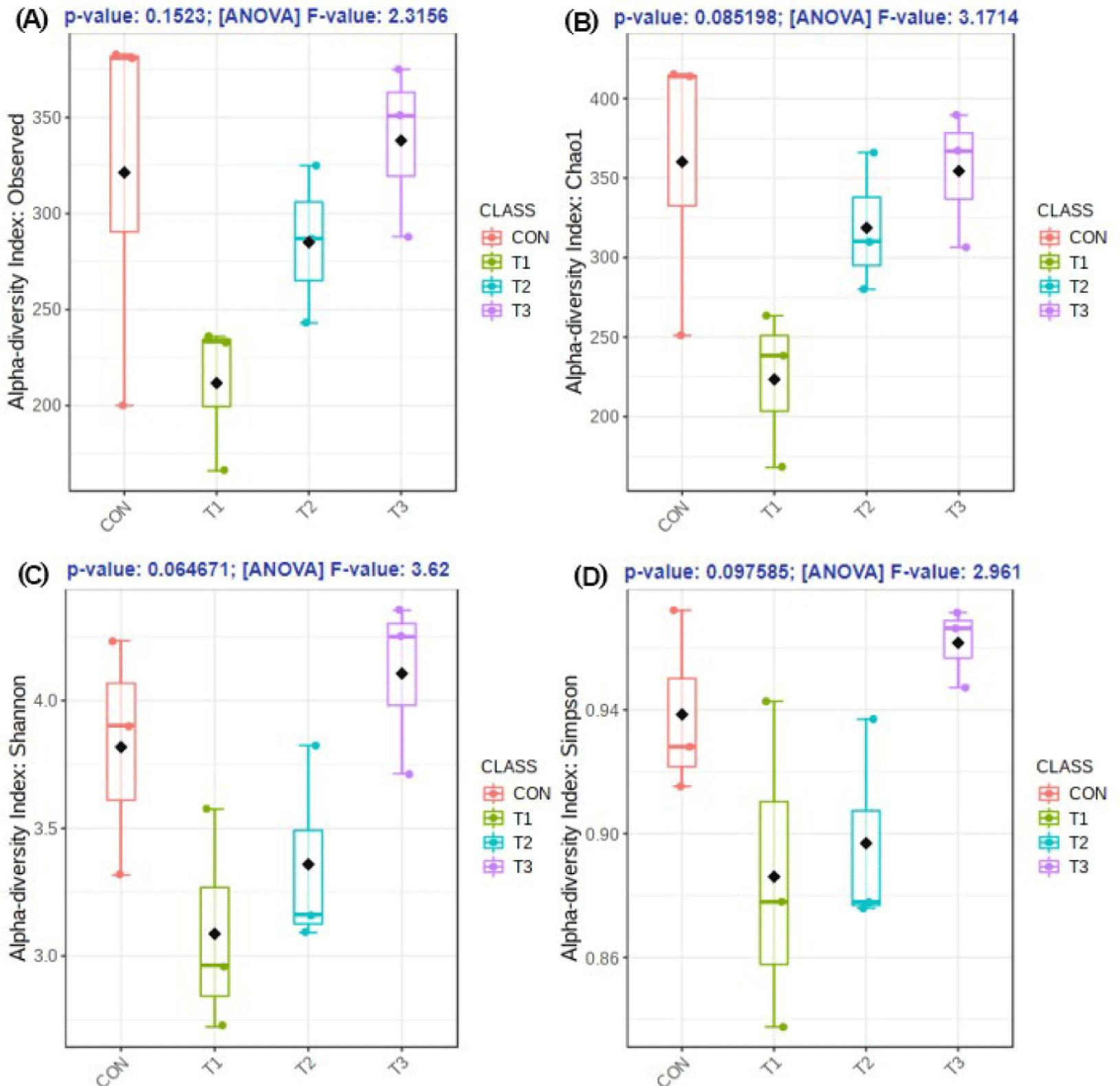
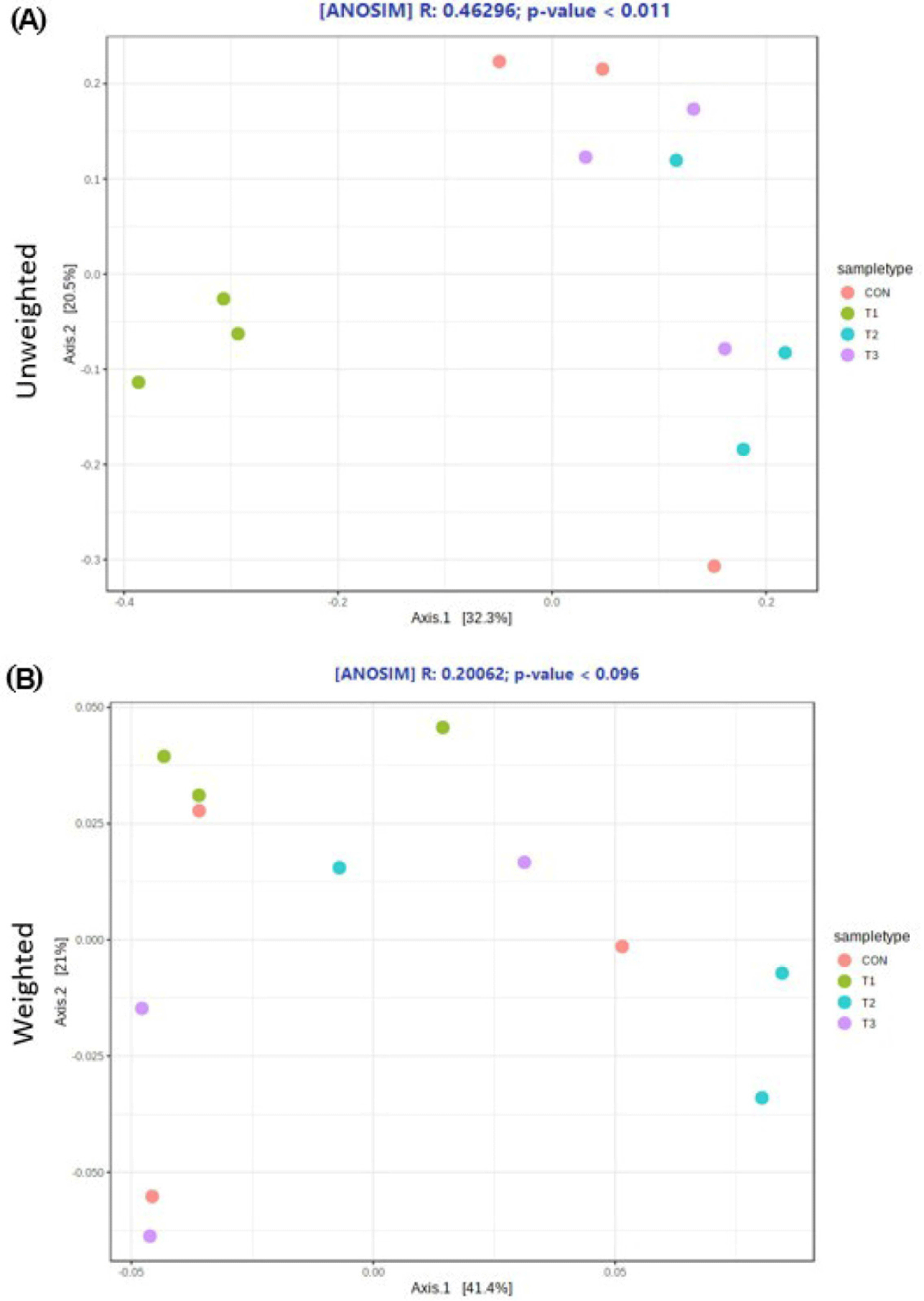
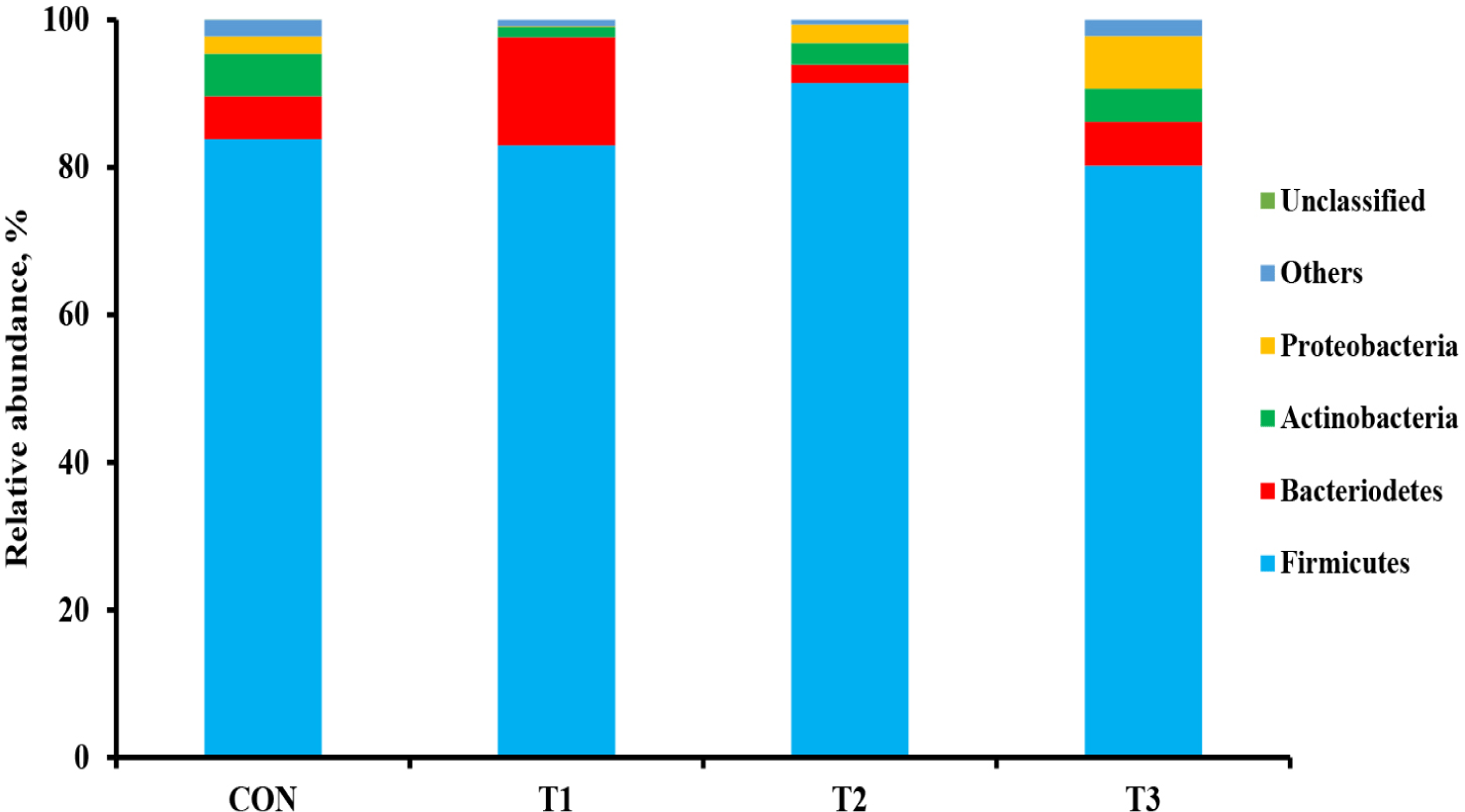
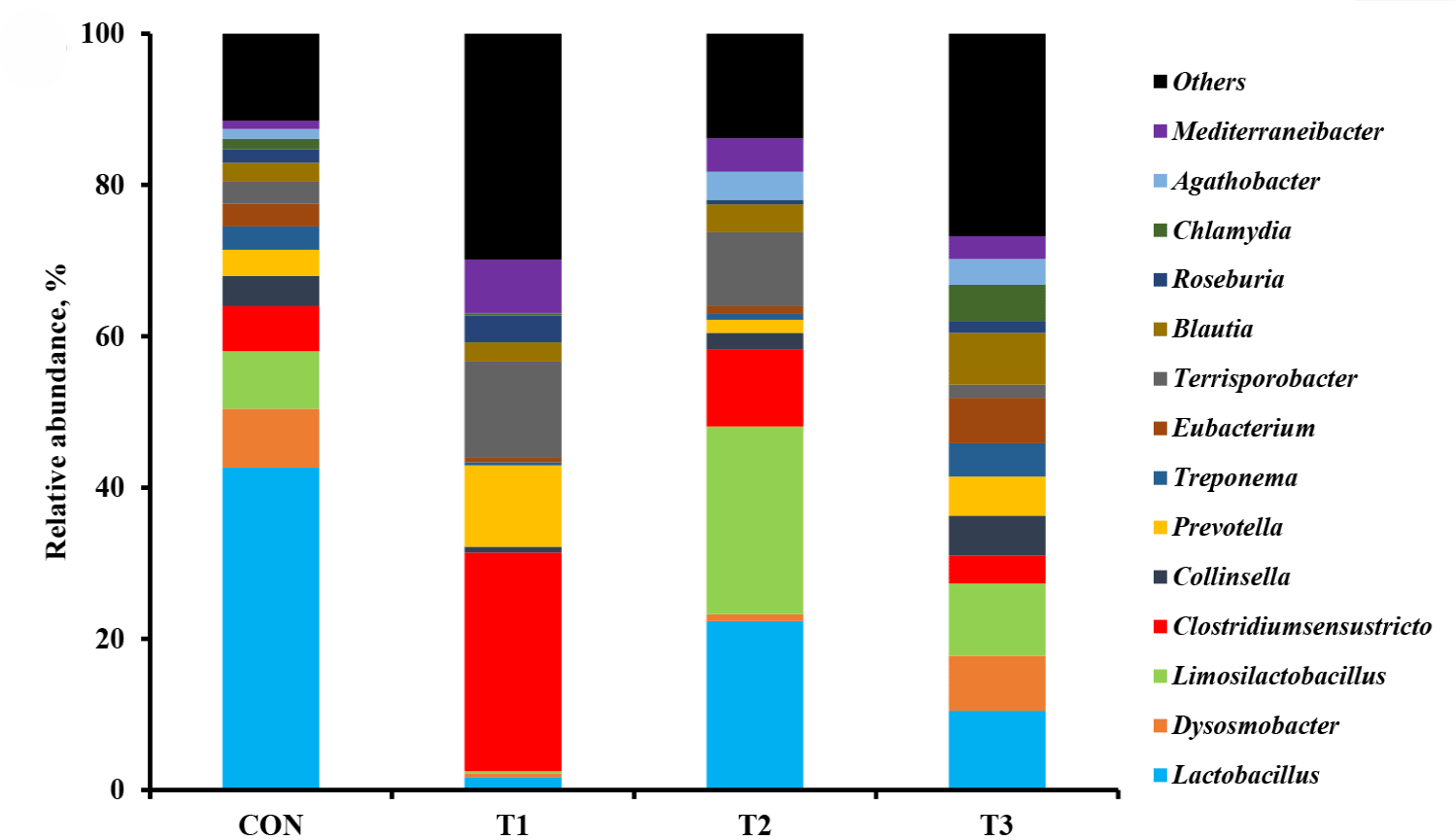
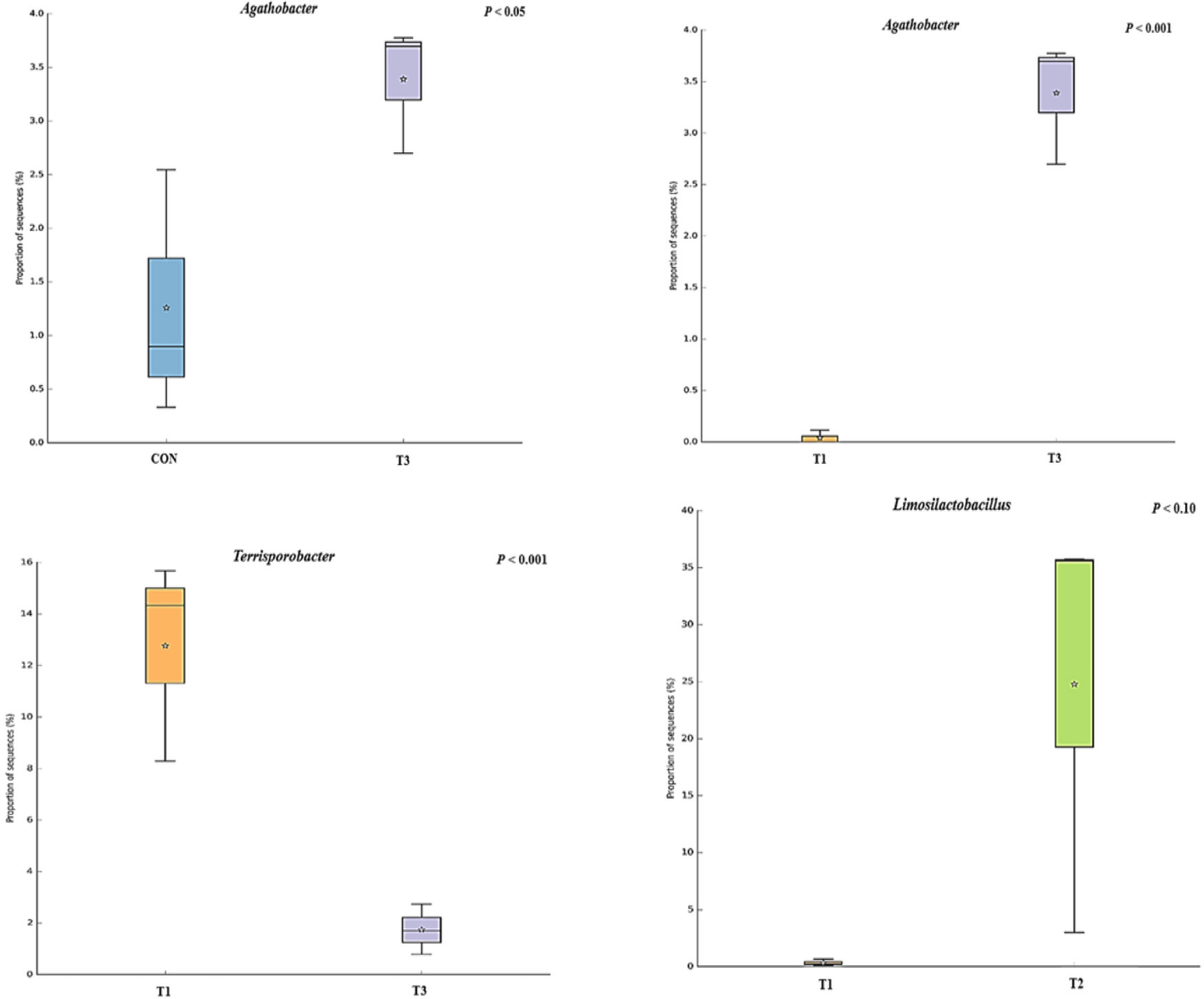
The microbial alpha diversity indices are shown in Fig. 1. Dietary T1 tended to have lower (p < 0.10) Chao1, Simpson, and Shannon indices than CON and T3. The beta diversity of the fecal microbiota determined using PCoA plots is presented in Fig. 2. PCoA plots based on the unweighted UniFrac distance, confirmed that T1 had distinct clustering from other groups, but overlapped clustering with CON, T2, and T3 (r = 0.463, p < 0.05). However, there was some distinct separation of fecal microbial communities based on the weighted UniFrac distance among the dietary treatments (r = 0.201, p < 0.10). The relative abundances of the fecal microbes at the phylum and genus level among the dietary treatments are shown in Figs. 3 and 4, respectively. At the phylum level (Fig. 3), Firmicutes were the most predominant bacteria in all dietary treatments (CON, 83.82%; T1, 82.98%; T2, 91.44%; T3, 80.19%), followed by Bacteroidetes in CON (5.83%) and T1 (14.67%), and Actinobacteria and Proteobacteria in T2 (2.9%) and T3 (7.16%). At the genus level (Fig. 4) and based on statistical differences among dietary treatments (Fig. 5), dietary T2 tended to increase (p < 0.10) relative abundance of Limosilactobacilus (24.77%) compared to those fed dietary T1 (0.33%). In addition, pigs fed dietary T3 had higher (p < 0.05) relative abundance of Agathobacter (3.39%) than those fed CON (1.26%) and dietary T1 (0.04%). Additionally, dietary T3 had a lower relative abundance of Terrisporobacter (1.74%) compared to those fed dietary T1 (12.77%).
DISCUSSION
Minerals are inorganic elements that improve growth and reproduction in pigs [11]. In the livestock industry, micro minerals such as Cu and Zn, typically in the form of CuSO4 and ZnO are widely supplemented into the diet in amounts that exceed the nutritional requirements of weanling pigs [36–38]. However, when these compounds reach the stomach of piglets, large amounts of ZnO and CuSO4 are dissociated into Zn and Cu ions, respectively, and great quantities are lost in the digestive tract. Because only a small amount of these complexes can reach the intestinal tract, high doses are required. However, the inclusion level in feed must be lowered owing to global regulations on environmental pollution through excretion and/or malabsorption by overnutrition. Therefore, modified forms such as organic, nanoparticles and lipid-coated forms have been researched and utilized into swine feed [38–41]. Results from this study demonstrated that the dietary T2 and T3 groups enhanced the ADG and G:F for the first two weeks and the overall period compared with that of the CON group, which is consistent with previous studies where supplementation in coated or nano-sized forms [42,43]. An improvement in growth performance was observed with the supplementation of a lower dosage of dietary-coated CuSO4 and ZnO in the present study due to the relatively high bioavailability and absorption of Cu and Zn in the small intestine compared with standard forms of CuSO4 and ZnO. In general, no additive effects were observed when excess Zn was added to Cu [44]. Specifically, metallothionein in the intestinal mucosa is induced by high concentrations of Zn, resulting in Cu binding, and causing Cu deficiency by disturbing its absorption [45,46]. Therefore, the results suggested that balanced doses of coated CuSO4 and ZnO were better absorbed and enhanced growth rate compare with CON as same as supplementation of pharmacological levels of ZnO.
In this study, supplementation with dietary coated CuSO4 and ZnO tended to have decreased the frequency of diarrhea. In addition, HCT in the blood profiles, which increases with dehydration and is used as an indicator of increases in diarrhea [47], did not differ among the dietary treatments in the first week after weaning. However, supplementation with low dose of dietary coated microminerals tended to decrease HCT on day 14, and we demonstrated that this supplementation positively affected the fecal score. Furthermore, no differences in the ATTD of DM, CP, and energy among the dietary treatments. However, several previous studies have reported that ZnO and CuSO4 supplementation in the form of lipid-coated or nano-type positively affected nutrient and energy digestibility, which were attributed to digestive enzymes and morphological changes in the small intestine [42,43,48]. Therefore, additional research on digestive enzyme activity and nutrient digestibility should be conducted because they may differ depending on the processing type, method, or concentrations of the microminerals.
As intestinal permeability increases due to weaning stress, potentially pathogenic bacteria can penetrate and cause not only intestinal inflammation but also systemic inflammatory responses [47]. Changes in the WBC count, indicative of systemic inflammation, are associated with alterations in the levels of cytokines involved in maintaining immunity and homeostasis [49,50]. Serum TNF-α is one of the pro-inflammatory cytokines, used as a potential indicator of inflammatory reactions and damaging the mucosal barrier system [51]. Changes in these parameters regulate the systemic immune responses against infections or diseases caused by weaning stress. Previous studies reported that supplementation of CuSO4 and ZnO reduced and downregulated the concentrations and mRNA levels of inflammatory cytokines including TNF-α in the intestinal mucosa of weaned pigs [24,37]. The current study further demonstrated that dietary coated CuSO4 and ZnO alleviated systemic immune responses caused by weaning stress through the reduction of WBC and serum TNF-α levels. Serum IgA, IgM, and IgG levels, which are the major components of humoral immunity, are reduced by weaning stress and immature immunity of piglets [52]. In this study, dietary coated CuSO4 and ZnO improved serum IgG levels of weaned pigs, consistent with the results of previous studies [17,53]. IgG is a type of antibody that leads to control the infection via binding many pathogens such as bacteria and viruses by immune cells such as macrophages. Furthermore, it has been reported that free Cu and Zn ions possess antimicrobial properties against E.coli in the small intestine [54]. Although the specific mechanisms of microminerals activities are not entirely elucidated yet, it is widely accepted that these metallic ions degrade bacterial cell membranes by disrupting the integrity of bacterial cell membranes, inducing cell death. In addition, ions increase the release of reactive oxygen species within microorganisms, leading to pathogen destruction [55]. Collectively, dietary supplementation with coated Cu and Zn modulates immune responses by preventing the breakdown of the intestinal barrier and overproduction of pro-inflammatory cytokines.
Many studies have reported that dietary supplementation of pharmacological concentration of Cu and Zn improves intestinal microorganisms by increasing the number of beneficial bacteria and reducing pathogenic microbial composition [56,57]. The diversity and composition of the intestinal microbiota in pigs are considerably affected by health condition and the digestion of nutrients compositions through physiological functions [58]. In our study, the Shannon index tended to increase with coated CuSO4 and ZnO compared to that with the standard dosage of ZnO, indicating greater diversity in the fecal microbiota of piglets. Shen et al. [24] showed that a pharmacological dosage of ZnO reduced the richness of microbial populations in the jejunum and feces of weaning pigs, which was consistent with the results of our study. In general, diversity is often associated with the presence of beneficial bacteria that can counteract pathogens [59]. However, the relative abundance of bacteria among dietary treatments was compared through taxonomic classification for a more precise interpretation. At the phylum level, the Firmicutes and Bacteroidetes accounted for approximately 90% of all treatments in the fecal microbiomes of weaned pigs. Among the dietary treatments, dietary T2 had the highest proportion of Firmicutes. Since Lactobacillus and Clostridium were dominant genera within Firmicutes, we determined that the overall portion of Lactobacillus (22.4%), Limosilactobacilus (24.78%), and Clostridium sensu stricto (10.23%) in the T2 was higher than in the other treatments. At the genus level, the present study showed that pigs fed dietary T2 had a higher relative abundance of genus Limosilactobacilus compared with T1. Limosilactobacilus is a genus of lactic acid bacteria that recently split from Lactobacillus and includes the species Limosilactobacilus reuteri , which is a microorganism with properties that promotes intestinal health and is widely used as a probiotic strain [57,60]. Furthermore, the relative abundance of fecal microbiota increased Agathobacter and decreased Terrisporobacter in dietary T3 compared with that in CON and dietary T1. Agathobacter is a beneficial bacterium that contributes to short-chain fatty acid production, particularly butyrate, and is positively correlated with overall gut health in humans through metabolic interactions of the gut microbiota [61]. Furthermore, Terrisporobacter shows a positive correlation with increased serum markers such as endotoxin and TNF-α, which promote oxidative stress, inflammation, and malnutrition of gut microbiota in weaned pigs [62,63]. Therefore, the higher relative abundance of Limosilactobacilus and Agathobacter may have contributed to the suppression of Terrisporobacter and stabilization of the intestinal environment, thereby enhancing the growth performance of pigs fed dietary coated CuSO4 and ZnO than those fed CON and standard ZnO diets.
CONCLUSION
Our study demonstrated that dietary coated CuSO4 and ZnO supplementation in a nursery diet improved growth performance and modulated the immune responses and gut microbiota in weaned pigs. These results indicate that improvement in growth performance and immune responses may be associated with changes in the fecal microbiota composition compared with CON group. In conclusion, dietary coated CuSO4 and ZnO have positive effects in weaned pigs and represent potential alternatives to high levels of ZnO diets.
