INTRODUCTION
Asthma is a prevalent respiratory condition in the field of animal science, defined by the inflammation of the bronchial tubes, leading to narrowing and increased secretion production, resulting in symptoms like coughing, difficulty breathing, and shortness of breath [1]. It is characterized by difficulty breathing due to a bronchoconstrictor response triggered by a variety of causes, including allergies, air pollution, smoke, dust, and stress [2]. The inflammatory and obstructive response in asthma is regulated by a variety of immune cells, including T cells, eosinophils, dendritic cells, macrophages, neutrophils, and mast cells. These cells trigger the inflammatory response by secreting inflammatory mediators such as cytokines, chemokines, histamine, and leukotrienes. Key cytokines involved in allergic airway inflammation include IL-4, IL-5, IL-13, IL-25, and IL-33. IL-4 and IL-13 are crucial for promoting IgE production and Th2 cell differentiation, while IL-5 is essential for the growth and activation of eosinophils. IL-25 and IL-33 further amplify the Th2 response and enhance the production of other inflammatory mediators. These biochemicals, along with tissue changes in the airways, can lead to airway narrowing, secretion production, and inflammatory symptoms [3–5]. To address these issues, emerging therapies involving exosomes and nutraceuticals are being explored for asthma management [6,7].
Mast cells play a pivotal role as a type of white blood cell, specifically classified as granulocytes, primarily contributing to allergic and inflammatory responses in the body in animal science. These cells are commonly distributed within connective tissues, including the skin, respiratory system, and digestive tract, with notable concentrations near blood vessels and nerves. Upon activation by allergens or pathogens, mast cells unleash a cascade of histamines, cytokines, and other inflammatory mediators, eliciting symptoms such as itching, redness, and swelling [8]. Understanding mast cell function and their involvement in allergic and inflammatory processes is crucial for managing various animal science technologies.
In animal science, macrophages are pivotal immune cells categorized as phagocytes, specialized in engulfing and digesting foreign particles, debris, and infected cells. Widely distributed throughout the body in various tissues and organs, macrophages serve as a frontline defense against invading pathogens. Integral to the innate immune response, which is the body’s immediate defense mechanism against infections, macrophages play a multifaceted role. They phagocytize and eliminate pathogens, present antigens to other immune cells, and orchestrate immune responses [9]. Understanding the functions and behaviors of macrophages is essential in animal science for combating infections and maintaining overall health in animals.
Extracellular vesicles (EVs) are small membrane vesicles synthesized and secreted from most prokaryotic and eukaryotic cells. Such EVs participate in the physiological and immuno-regulatory functions in most types of tissues and cells. EVs display a diverse size range, typically falling between 100 to 1,000 nm in diameter, and are commonly designated as microvesicles, ectosomes, or microparticles in the scientific literature [10]. Exactly, exosomes constitute a distinct class of vesicles that are generated internally within multivesicular endosomes or multivesicular bodies (MVBs) and are subsequently released through the fusion of these compartments with the plasma membrane. Exosomes are vesicles with a diameter of less than 150 nm and contain an abundance of components derived from endosomes.
In animal science, EVs serve as vital mediators of intercellular communication and have been implicated in various physiological and pathological processes, including asthma [11]. Considering the intricate role of exosomes in orchestrating cellular interactions, we postulate that exosomes derived from asthma hold potential significance in modulating immune cell responses, such as mast cells and macrophages. Understanding the involvement of EVs in animal asthma not only illuminates the disease mechanisms but also offers promising avenues for therapeutic interventions. By emphasizing the link between EVs and animal science, we aim to underscore the importance of exploring EVs-mediated pathways in addressing animal health challenges, including asthma management and treatment. Thus, we hypothesized that asthma-derived EVs might affect the recruitment, proliferation, or activation of immune cells such as mast cells and macrophages.
It is important to note that research in this area is ongoing, and while the involvement of EVs in asthma and their potential clinical applications are being actively explored, the mechanisms by which asthma-derived EVs regulate the immune system remain poorly understood. To address this gap, in this study we identified specific constituents within asthma-derived EVs and investigated their potential to activate mast cells and macrophages, thereby contributing to our understanding of asthma pathogenesis in animals.
MATERIALS AND METHODS
Male BALB/c mice (8-week-old) were used for the induction of murine asthma model. Animal studies were performed following the Institutional Animal Care and Use Committee of Jeonbuk National University (Approval No. JBNU 2023-063-002).
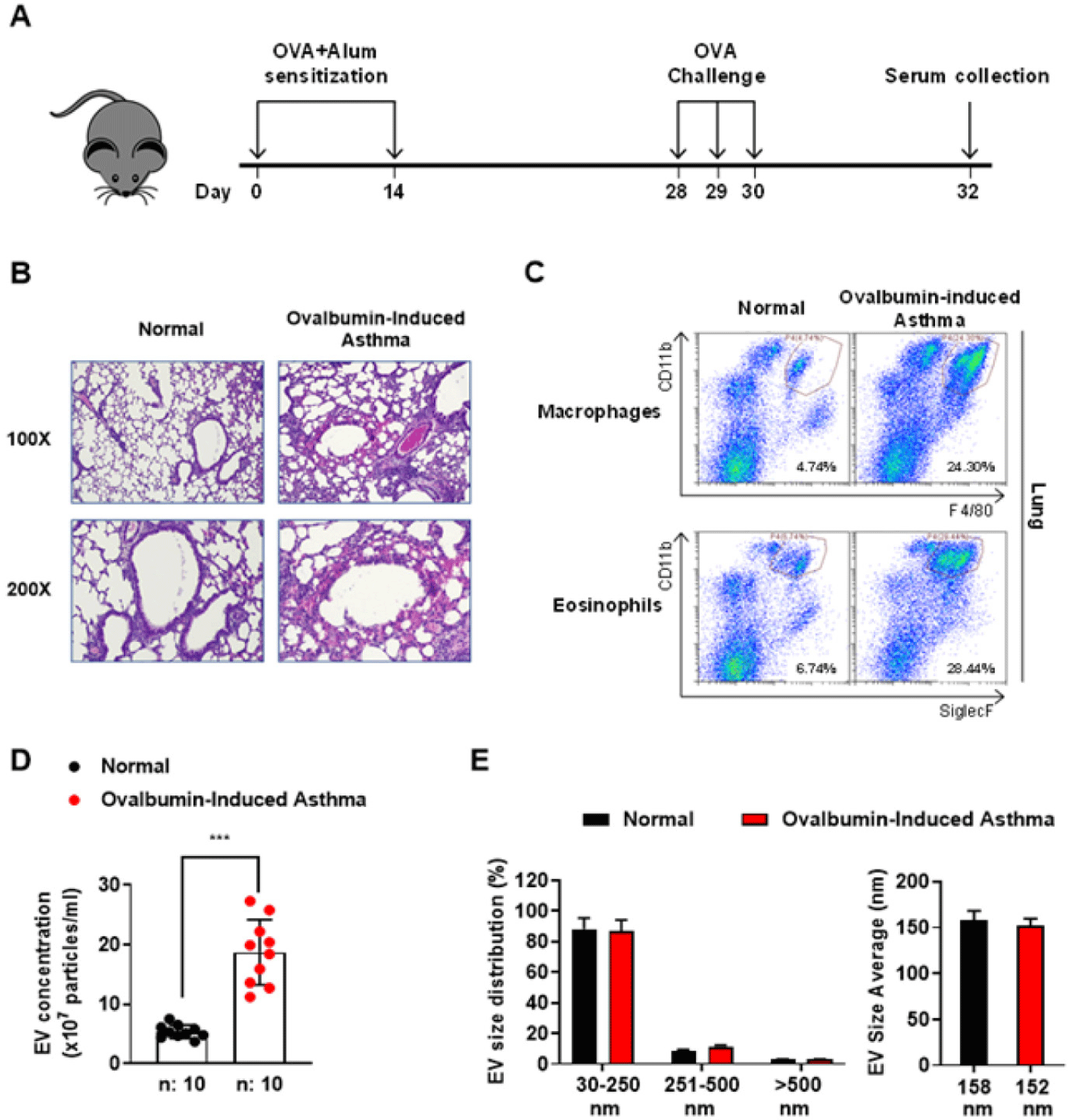
The OVA-induced murine asthma model was conducted as shown in Fig. 1A, mice were immunized with 20 mg OVA (Cat No. A5503, Sigma-Aldrich, St. Louis, Mo, USA) and 2.25 mg aluminum hydroxide (Cat No. 77161, Thermo Fisher Scientific, Rockford, IL, USA) adjuvant on day 0 and 14. On day 28, 29, and 30, the immunized mice were subjected to aerosolized OVA (3%) using an ultrasonic nebulizer (Cat No. NE-U17, Omron, Kyoto, Japan) for 20 minutes in a Plexiglass exposure chamber (24.5 × 40.5 × 15.0 cm). The mice induced with OVA were euthanized on day 32 for serum collection. Collected serum was mixed gently with cold phosphate-buffered saline (PBS) in a 1:1 ratio, and centrifuged at 13,000×g for 40 min at 4°C. The clear serum was transferred to 5 mL ultracentrifuge tubes containing 3 mL cold PBS, and subsequently centrifuged at 120,000×g for 80 min at 4°C in a Type 90Ti Fixed-Angle Rotor (Cat No. 355530, Beckman Coulter, Brea, CA, USA). EV pellets were washed with PBS and centrifuged at 120,000×g for 80 min at 4°C. The final EV pellets were resuspended with gentle shaking in 100 µL PBS and stored at −80°C before utilizing in experiments. EVs’ protein concentration was quantified by using BCA assay.
The concentration and size of asthma-derived EVs were evaluated using a Nanosight NS300. (Malvern Panalysis, Malvern, UK) as previously described [12].
Normal/asthma-EV samples were fixed with 4% paraformaldehyde (PFA) and dried on a glow-discharged copper grid and washed 7 times with distilled water. Finally, the EVs were stained with 2% uranyl acetate. The grids were examined and analyzed using Krios G4 Cryo-TEM (Thermo Fisher Scientific), and the images were represented at 22,000× magnification.
Bone marrow cells were acquired through the process of flushing the bone marrow from the femurs and tibias of mice. For the BMMs culture system, cells were incubated in growth media (α-MEM containing 2 mM L-glutamine, 10% FBS, 100 U/mL penicillin, and 100 mg/mL streptomycin) with 30 ng/mL M-CSF treatment for 3–5 days. Subsequently, the cells were stimulated with or without normal/asthma-EVs, with lipopolysaccharide (LPS) (100 ng/mL) and IFN-γ (50 ng/mL) for M1 macrophage activation, and with IL-4 (20 ng/mL) for M2 activation. For BMMCs, cells were cultured in BMMC media (RPMI 1640 media contained 2 mM L-glutamine, 10% FBS, 1 mM sodium pyruvate, 0.1 mM nonessential amino acids, 100 U/mL penicillin, 100 mg/mL streptomycin, 0.05 mM 2-mercaptoethanol, 25 mM HEPES, and 10 ng/mL IL-3) for 4–8 weeks. Cells with purity > 95% were used for subsequent experiments.
2.0 × 105 BMMCs were washed twice with Tyrode buffer (20 mM HEPES [pH 7.4], 135 mM NaCl, 5 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, 5.6 mM glucose, and 0.05% bovine serum albumin [BSA]) and pre-warmed at 37°C. The cells were then stimulated with or without 100 μg/mL of normal/asthma-EVs for 15 min at 37°C. Subsequently the cells were chilled on ice and centrifuged at 400×g for 80 min at 4°C. Culture supernatant was transferred to the new tube, and cell pellet was lysed in 0.1% triton x-100. The β-hexosaminidase in the supernatant and pellet was reacted with p-nitrophenyl-N-acetyl-β-D-glucosaminide for 1 h at 37°C. β-hexosaminidase was measured at 405 nm using microplate reader.
The lung tissues were fixed with 4% PFA and embedded in paraffin. The paraffinized tissues were cut into 5-μm sections and stained with hematoxylin-eosin (H&E). Stained samples were analyzed by iSolution DT36 software (Carl Zeiss, Oberkochen, Germany) at the Center for University-wide Research Facilities (Jeonbuk National University, Jeonju, Korea).
The amounts of Ig kappa and lambda light chain, IL-6 (Cat No. 88-7064, Thermo Fisher Scientific), IL-8, and TNF-α (Cat No. 88-7324, Thermo Fisher Scientific) in mice serum-derived EVs were measured using commercial ELISA kits following the manufacturer’s instruction.
Purified EVs were subjected to analysis using reversed-phase liquid chromatography (LC) coupled to a Q Exactive mass spectrometer. The LC system utilized columns packed with ReproSil-Pur 130 C18-AQ3 μm particles. Peptides were separated using a gradient over 78 min with buffer A: 0.1% formic acid and buffer B: acetonitrile plus formic acid. Separated peptides were introduced into the mass spectrometer. The mass spectrometer operated in a data-dependent mode, acquiring survey scans at a resolution of 70,000. The top 20 most abundant peaks were selected for fragmentation using higher energy collisional dissociation. Fragmentation spectra were acquired at a resolution of 17,500. Dynamic exclusion was employed to minimize peptide re-sequencing within a 50-second time window.
To determine the cell population in spleen, splenocytes were isolated and washed twice with PBS. The cells were blocked by CD16/32 monoclonal antibody (14-0161-86, Invitrogen, Carlsbad, CA, USA) stained with FITC-conjugated anti-FcεRI (11-5898-82, Invitrogen), PE-conjugated anti-CD117 (12-1171-82, Invitrogen), FITC-conjugated anti-F4/80 (11-4801-82, Invitrogen), PE-conjugated anti-SiglecF (12-1702-82, Invitrogen), APC-conjugated anti-CD11b (17-0112-82, Invitrogen), PE-Cyanine7-conjugated anti-CD4 (25-0041-82, Invitrogen), PerCP-Cyanine5.5-conjugated anti-CD8a (45-0081-82, Invitrogen), and APC-conjugated anti-CD19 (17-0193-82, Invitrogen) in a FACS buffer. Subsequently, the cells were fixed with 4% PFA and analyzed with a CytoFlex (Beckman Coulter) and CytExpert software. Macrophage: F4/80+CD11b+, Eosinophil: SiglecF+CD11b+, Th cell: CD3+CD4+, Tc cell: CD3+CD8+, B cell: CD19+.
Total RNA was extracted from bone marrow-derived macrophages using the AccuPrep® Universal RNA Extraction Kit (Bioneer, Daejon, Korea), subsequently RNA concentration was determined by Nanodrop spectrophotometer. AccuPower® RT Premix & Master mix kit (Bioneer) was used for cDNA amplification. mRNA expression was determined using SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA). The primer sequences used were as follows: mouse IL-1β forward, 5’-GAAATGCCACCTTTTGACAGTG-3’; mouse IL-1β reverse, 5’-TGGATGCTCTCATCAGGACAG-3’; mouse iNOS forward, 5’-ACATCGACCCGTCCACAGTAT-3’; mouse iNOS reverse, 5’-CAGAGGGGTAGGCTTGTCTC-3’; mouse Arginase 1 forward, 5’-TGTCCCTAATGACAGCTCCTT-3’; mouse Arginase 1 reverse, 5’-GCATCCACCCAAATGACACAT-3’; mouse Retnla forward, 5’-CCAATCCAGCTAACTATCCCTCC-3’; mouse Retnla reverse, 5’-CCAGTCAACGAGTAAGCACAG-3’.
Cells were stimulated with or without 100 μg/ml of normal/asthma-EVs for 10 min and chilled on ice to stop the stimulation. Cells were lysed in 100 μl of NP-40 lysis buffer with 1 mM phenylmethylsulfonyl fluoride (Cat No. 36978, Thermo Fisher Scientific) and a protease inhibitor cocktail tablet (Cat No. 11836153001, Sigma-Aldrich) on ice. The protein content of cellular extracts was quantified using Bradford assay (Abs at 595 nm). The lysates were heated at 100°C for 5 min in Laemmli’s 5× sample buffer (Cat No. EBA-1052, ELPIS-BIOTECH, Daejon, Korea). Samples were introduced to sodium dodecyl sulfate-polyacrylamide gel (10%) and transferred to polyvinylidene fluoride (PVDF) membranes. The protein-transferred membranes were blocked in 5% BSA for 1 h and incubated with specific primary antibodies overnight. The membranes were incubated with HRP-conjugated secondary antibodies. Protein band images were analyzed using Fusion SOLO X (Vilber, Marne-la-Vallée, France).
The data are presented as the mean ± SD. Statistical analysis was conducted using student t-test and one-way analysis of variance (ANOVA), followed by Turkey’s post hoc analysis. Significance level below 0.05 was considered as statistically significant. Statistical comparisons were analyzed using Graphpad Prism version 9.
RESULTS
OVA-induced murine asthma models effectively replicate numerous features of human allergic asthma. In the overall mechanisms of the OVA-induced murine asthma model, there are three main categories: 1) sensitization phase, 2) Challenge phase, 3) pathophysiological outcome. This process includes antigen sensitization, T-cell activation, IgE production, and mast cell activation, leading to airway inflammatory response, airway hyperresponsiveness (AHR). These models are crucial for understanding asthma pathophysiology [13,14]. To obtain the EVs from the asthma-induced mice serum, asthma was induced by sensitization and challenge of OVA (Fig. 1A). H&E staining of the lung tissues showed that the inflammatory cells were infiltrated into the lung tissues in the ovalbumin (OVA)-induced asthma group compared to the normal group (Fig. 1B). In the lung tissue, more F4/80+CD11b+ macrophages and SiglecF+CD11b+ eosinophils were detected (Fig. 1C). We found that the EV concentration obtained from the OVA-induced asthma group was higher by about 4 times (Fig. 1D). The EV sizes derived from the normal and OVA-induced asthma groups were distributed in the range of 30–250 nm, with a mean size of 158 and 152 nm for the two groups, respectively (Fig. 1E). We also observed the shape and roundness of normal/asthma-derived EVs (Fig. 2A). These results indicated that EVs in normal and asthma-derived serum showed no differences in morphology, size but quantitative difference.
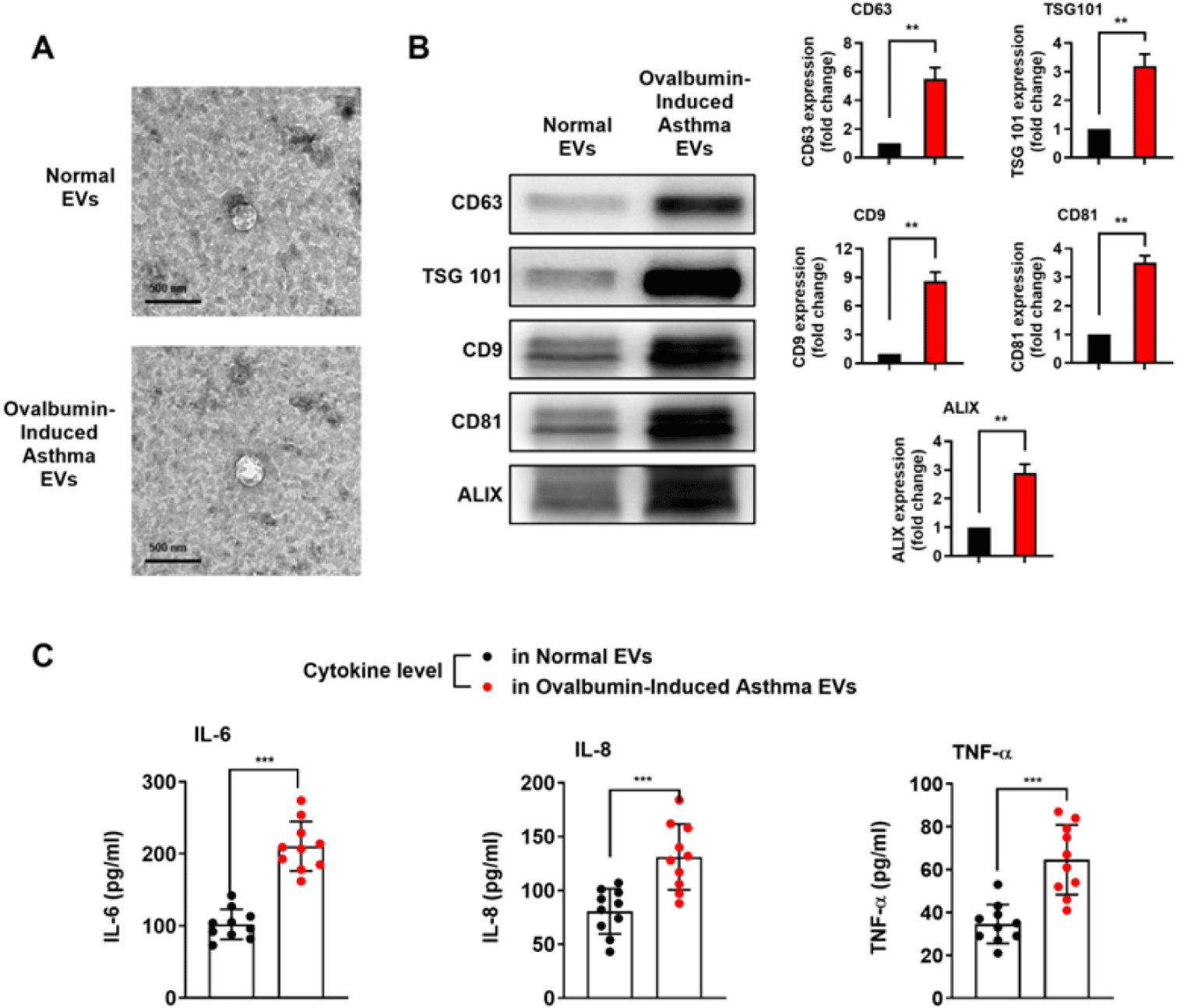
To examine whether exosome marker proteins are increased in OVA-induced asthmatic serum-derived EVs, we conducted western blotting. The expressions of CD9, CD63, CD81, ALIX and Tumor susceptibility gene 101 (TSG101) were increased in the OVA-induced asthma group (Fig. 2B). During an asthma exacerbation, there is often an upregulation of various inflammatory mediators, including cytokines such as IL-6, IL-8, and TNF-α. These cytokines play a critical role in the inflammatory response, resulting in the recruitment and activation of immune cells [15–18]. Given these studies, the levels of pro-inflammatory cytokines were investigated. The cytokine levels of IL-6, 8, and TNF-α were significantly elevated in the OVA-induced asthma group compared to the normal group (Fig. 2C). In these results, we could hypothesize that in the fast-paced environment of asthma, asthma-derived EVs may serve as a tool for stimulating immune cells.
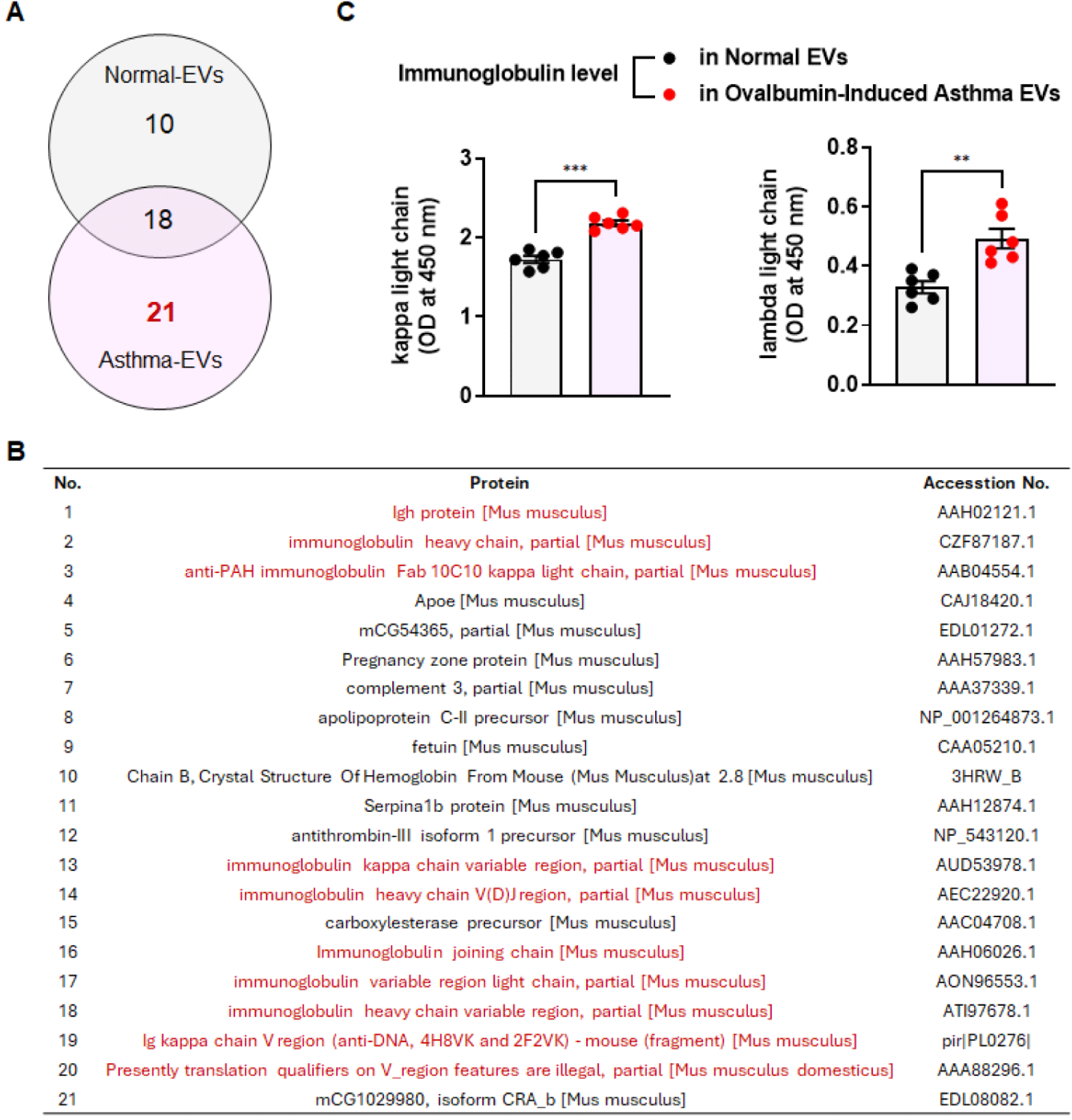
To investigate the profile of asthma-derived EV components, we performed proteomic analysis. Following analysis of the top 50 proteins, we identified 21 novel proteins in the asthma EVs (Fig. 3A). Furthermore, there was a broad distribution of immunoglobulins within the asthma EVs (Fig. 3B). Elevated levels of immunoglobulins can be associated with various conditions, including certain immune and inflammatory disorders [19]. It also appears to be correlated with allergic asthma, Kraneveld et al. showed a significant increase in the concentration of free kappa light chains in the blood of adults with asthma compared to nonasthmatic individuals [20]. As asthma severity increased, there was a tendency for Ig kappa and lambda light chain detection to increase as well [21]. Thus, we conducted ELISA to determine the levels of immunoglobulins (kappa and lambda light chain) in normal and OVA-induced asthma serum-derived EVs. The results of ELISA showed that kappa and lambda light chains were increased in the OVA-induced asthma EVs group compared to the normal EVs group (Fig. 3C).
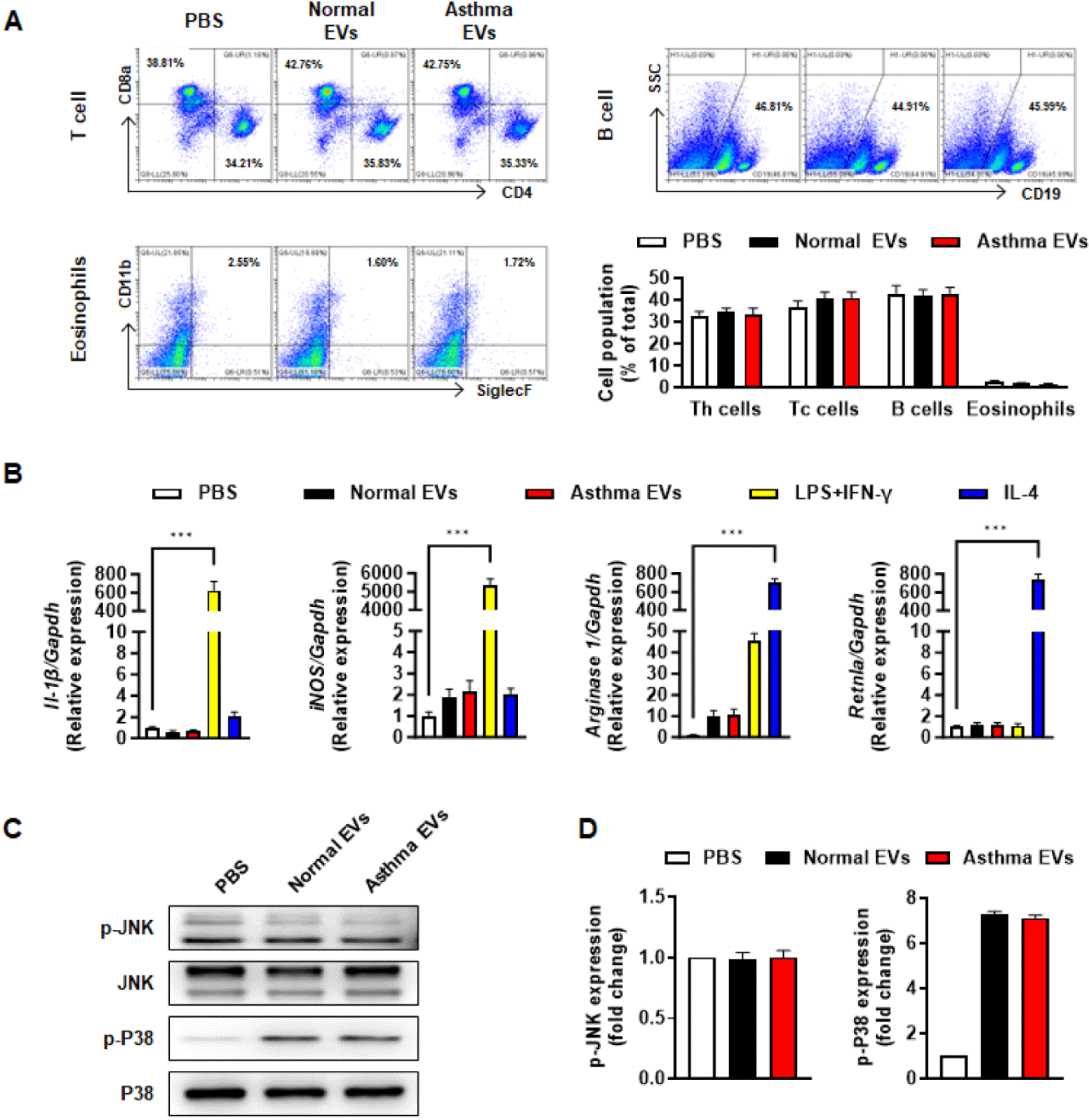
Many studies have shown that when asthma is triggered, immune cells such as T/B lymphocytes, eosinophils, macrophages and mast cells, infiltrate the lung tissue and cause an inflammatory response [22]. Based on these studies, we hypothesized that there might be a component in asthma-derived EVs that triggers immune cell activation. First, we tested whether asthma serum EVs could increase proliferation of T/B cell and eosinophils. No differences were found between normal EVs and asthma EVs on proliferation of T/B cells and eosinophils in spleen (Fig. 4A). We also examined M1/M2 macrophage activation via representative marker gene expression by using RT-qPCR analysis. However, contrary to expectations, neither normal EVs nor asthma EVs induced M1/M2 macrophage activation (Fig. 4B). Additionally, asthma-derived EVs had little effect on macrophage activation via phosphorylation of MAP kinase (Figs. 4C and 4D).
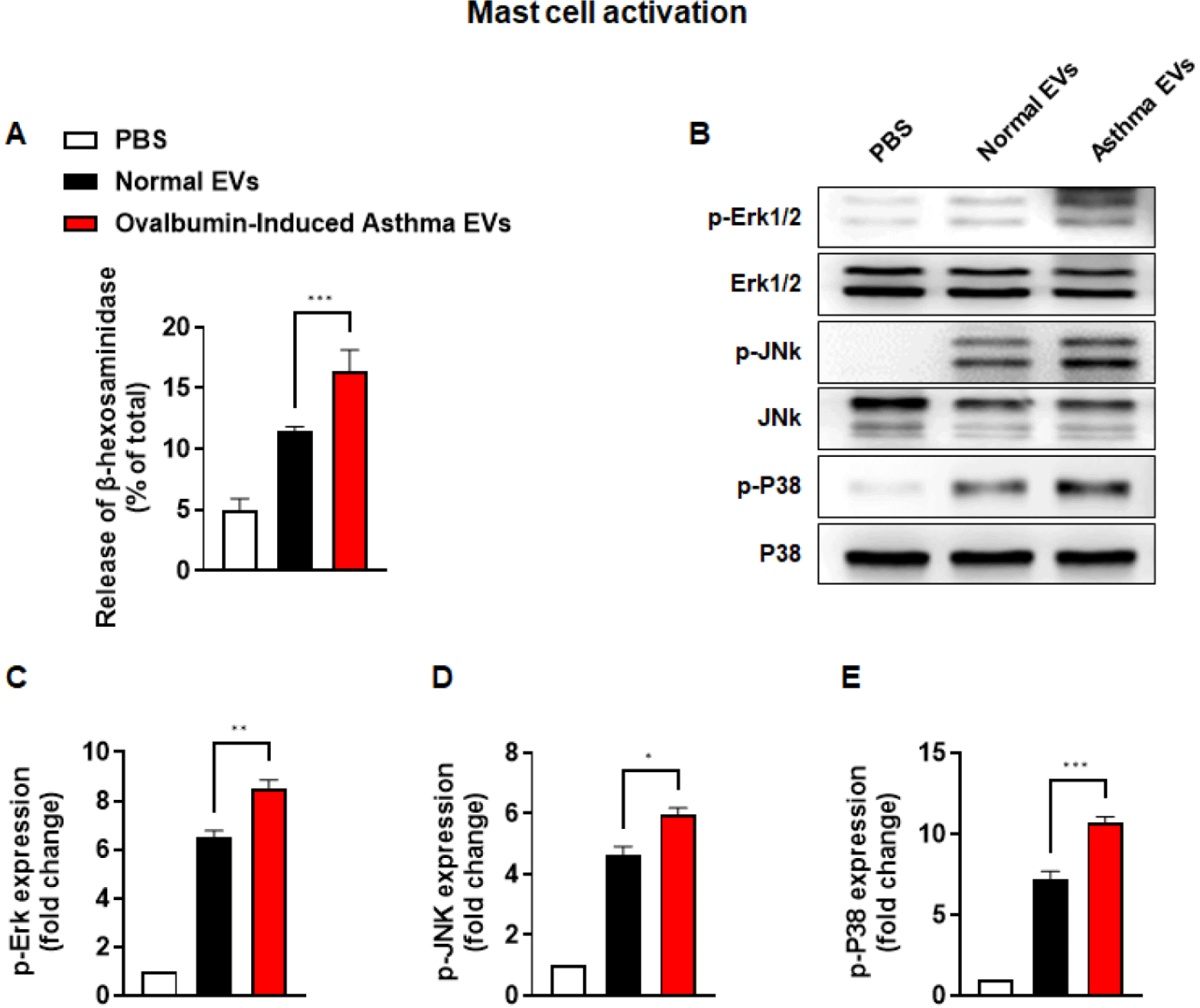
Next, we checked the release of β-hexosaminidase as a representative indicator to confirm mast cell degranulation. β-hexosaminidase release was significantly higher in the asthma EVs group than in the normal EVs group (Fig. 5A). We also observed that phosphorylation of Erk1/2, JNK, and p38 was increased by asthma EVs more than by normal EVs (Figs. 5B, 5C, 5D, and 5E). Collectively, these findings show that asthma-derived EVs can stimulate mast cell activation but not activation of T/B lymphocyte, eosinophil and macrophage.
DISCUSSION
EVs are small membrane vesicles synthesized and secreted from almost prokaryotic and eukaryotic cells [23]. Such EVs participate in the physiological and immuno-regulatory functions in most types of tissues and cells [24]. These EVs display a diverse range of sizes (100–1,000 nm in diameter) and are generally known in the literature as microvesicles, ectosomes, or microparticles [25]. Other types of vesicles, the exosomes, are generated inside multivesicular endosomes or MVBs and are secreted when these compartments fuse with the plasma membrane. Exosomes are vesicles smaller than 150 nm in diameter and are enriched in endosome-derived components. The discovery of EVs has provided a new perspective on cell-to-cell interactions, having the ability to deliver several types of components, including proteins, lipids, nucleic acids, and RNA. Interactions between EVs and recipient cells are not random but are influenced by factors that lead to preferential binding to certain cell types [25]. Research on EVs has expanded rapidly in recent years, and they have been implicated in the pathophysiology of several diseases, such as cancer [26,27], neurodegenerative diseases [28,29], cardiovascular diseases [30,31], infectious diseases [32], and asthma [33–35]. Recent studies investigate the role of EVs in understanding the pathogenesis of asthma, a chronic respiratory disease characterized by inflammation and airway narrowing, suggesting the potential utility of EVs as both diagnostic tools and therapeutic interventions. The study highlights the importance of further research to fully comprehend the extent of EVs’ involvement in asthma. Various immune cells, including T lymphocytes, B lymphocytes, eosinophils, macrophages, and mast cells are involved in the inflammatory processes that contribute to asthma symptoms [36–38].
Mast cells are primarily located in the airway mucosa, especially near blood vessels and nerves. When triggered by various stimuli, mast cells release a variety of substances, including histamine, leukotrienes, and prostaglandins [39]. These substances contribute to airway inflammation and can cause bronchoconstriction, leading to asthma symptoms [8,39]. Additionally, mast cell activation can occur through both IgE-dependent and IgE-independent mechanisms. In IgE-dependent activation, mast cells become sensitized to specific antigens through the binding of antigen-specific IgE antibodies to their surface receptors, called FcεRI. When the antigen encounters the sensitized mast cell, it binds to the IgE antibodies, leading to cross-linking of the FcεRI. This cross-linking triggers a signaling cascade within the mast cell, ultimately leading to the release of preformed mediators, including histamine, as well as the synthesis and release of other inflammatory mediators, such as leukotrienes and cytokines [40,41]. IgE-independent activation can occur through various mechanisms such as direct stimulation by pathogens, certain drugs, toxins, or physical factors like trauma or temperature changes [42]. These stimuli can directly activate mast cells through receptors other than FcεRI, such as toll-like receptors (TLRs) or receptors for cytokines and chemokines. This pathway may lead to the release of inflammatory mediators similar to those released in IgE-dependent activation, contributing to immune responses and inflammation. In our results, asthma serum-derived EVs could stimulate mast cell activation without IgE sensitization (Fig. 5).
Macrophages are immune cells that play a role in engulfing and digesting cellular debris and pathogens. In asthma, macrophages can contribute to inflammation by releasing pro-inflammatory cytokines [9,43]. Understanding the complex interplay between these immune cells and the inflammatory process in the airways is crucial for developing effective treatments for asthma. Medications used in asthma management often target these immune responses to reduce inflammation and bronchoconstriction. Inhaled corticosteroids, bronchodilators, leukotriene modifiers, and biologics targeting specific immune pathways are examples of medications used to control asthma symptoms. In a previous report, we documented heightened levels of small EVs in the circulation of individuals with systemic mastocytosis (SM). These EVs bear distinctive mast cell markers, indicating their potential to impact the phenotype of non-hematopoietic cells [44]. In the current study, we proved that OVA-induced asthma serum-derived EVs possess greater amounts of pro-inflammatory cytokines and immunoglobulins, further contributing to the activation of mast cells (Fig. 6). And the reason asthma-derived EVs may exclusively stimulate mast cells, not other immune cells, could be that mast cells have specific receptors particularly responsive to the cargo carried by these EVs. These receptors could interact with EV components in a way that triggers mast cell activation, while other immune cells lack these receptors or express them at lower levels. These observations align with the broader understanding of mast cells’ roles in asthma and underscores the intricate relationship between immune cell activity, inflammation, and the potential systemic effects of immune-related conditions.
This study demonstrates the roles of asthma serum-derived EVs on mast cell activation. In a future study, however, it is crucial to comprehend the precise impact of asthma-derived EVs on other immune and epithelial cells beyond the mast cells we have identified, since those cells collaborate in the asthmatic environment. Furthermore, recent studies have reported about relationship between miRNAs in EVs and asthma [45–49]. EV miRNAs show potential for investigating the molecular mechanisms of asthma and may provide new insights into disease pathogenesis, as well as potential therapeutic targets and diagnostic biomarkers. However, additional research is necessary to clarify the precise functions of individual miRNAs transported by EVs in asthma and to confirm their clinical usefulness.
Of note, the investigation of exosomes in asthma not only sheds light on the underlying mechanisms of the disease but also holds great potential for translating these insights into clinical applications. Further research in this area may lead to innovative diagnostic tools, more effective treatments, and a better understanding of the heterogeneity within the asthma patient population.
CONCLUSION
In this study, we identified the EV profiles in murine asthmatic serum through concentration, size distribution and TEM analysis. The asthma serum-derived EVs included higher amounts of cytokines, and immunoglobulins. Also, EVs from OVA-induced murine asthma serum could activate mast cells but not other immune cells, such as T lymphocytes, B lymphocytes, eosinophils, and macrophages. Altogether, we propose that asthma-derived EVs could be a potential therapeutic target for allergic and mast cell-related diseases, as well as asthma disease.
