INTRODUCTION
Avian flu, also known as avian influenza (AI), is a concern for both the global poultry industry and public health. Identifying the responsible genes and related pathways is of paramount importance to develop resistant chicken strains that can withstand the highly pathogenic avian influenza virus (HPAIV) therefore minimizing the negative impact on poultry production. Certain gene families and alleles associated with disease resilience in avian species are used as markers for selection and breeding [1].
Alleles of the major histocompatibility complex (MHC) genes are often associated with disease resistance and susceptibility in chickens and mammals. The MHC-B region is highly polymorphic and has complex genetic loci that contain clusters of genes responsible for the immune response and immune recognition in chickens [2]. It is responsible for the adaptive and innate immune responses in chickens [3]. Variations in MHC-B affect specific disease resistance to several highly pathogenic viral and bacterial diseases, as well as internal and external parasites in poultry [4,5]. Previous studies have reported that the MHC-B21 haplotype is associated with resistance to H5N1 virus infection, with a 100% survival rate. By contrast, chickens with the MHC-B13 haplotype showed 100% mortality during HPAIV outbreaks in Thailand [6]. The MHC-B21 haplotype is also associated with lower tumor-related mortality due to Marek’s herpes virus infection than other haplotypes [7]. Matsuu et al. [8] reported that despite the presence of significant BF1 and BF2 allele variations in Thai native chickens, none of the alleles, particularly BF1/BF2 alleles that are homologous to the MHC-B21 haplotype, were significantly associated with sensitivity to HPAIV infection.
A set of single nucleotide polymorphisms (SNPs) in the chicken MHC-B region, developed in the study by Fulton et al. [3], has been used to identify haplotypes of the MHC-B region in various chicken breeds around the world [3,9–12]. The panel consisted of 101 SNPs, but the set of 90 SNPs that cover the region between MHCJ06 and MHC178 were utilized for defining haplotype [3]. SNP-based techniques have favorable haplotype discriminating power, which is helpful before conducting a high-resolution haplotyping analysis.
In the present study, MHC-B SNP diversity was carried out with the genetically resistant and susceptible lines of the Vietnamese Ri chicken group. A total of 20 Ri chickens, 10 each line as described in Lee et al. [1], were used to examine the MHC-B haplotype diversity using an MHC-B SNP panel and their distribution in relation to the AI resilience of Ri chickens.
MATERIALS AND METHODS
The Vietnamese Ri chicken population used in the current study is also described in another source [1]. A total of 20 Ri chicken samples (i.e., HPAIV-resistant and susceptible lines) obtained from the Poultry Research Centre of the National Institute of Animal Science (Hanoi, Vietnam) were used. Specifically, 10 individuals were selected for each of the resistant and susceptible groups, designated as R and S groups, respectively. These chickens were chosen based on the genotypes for the BF2 and MX1 alleles to identify genetically resistant or susceptible individuals. Genomic DNA extracted from the chicken population was obtained from the previous research group [1] and diluted to a final DNA concentration of 5 ng/µl before Kompetitive Allele Specific Polymerase Chain Reaction (KASP) genotyping.
To further understand the genetic diversity within MHC-B, we genotyped 20 individuals using an MHC-B SNP panel described in the study by Fulton et al. [3]. The panel consists of 90 previously identified SNPs and is subjected to a fluorescence-based genotyping method called KASP [3].
To test statistically significant differences in allele frequencies for the 89 SNP markers between the R and S groups, we used the chi-square test [13]. Statistical tests were conducted individually for each SNP marker. The applied statistical formula is as follows.
In this context, χ2 stands for the chi-square statistic and c denotes the degrees of freedom, which was consistently set to 1 for all markers. O represents the observed allele frequency values while E stands for the expected values and the variable i refers to each specific SNP marker.
The statistical analysis was carried out using R software version 4.3 and the significance of the results was determined based on a p-value threshold of 0.05 for each SNP marker’s allele frequency calculations and testing.
In contrast to previous studies of BSNP haplotypes in native chicken populations [9,11], we excluded the “MHC065” SNP from BSNP haplotypes therefore utilizing only 89 SNPs. Because the MHC065 was not genotyped in the current population. MHC-B haplotypes were then inferred from the genotypes obtained from the 89 SNPs in all 20 Ri chickens along with homozygous samples with known MHC-B SNP genotypes from the previous study of Manjula et al. [11]. Haplotype analysis was implemented using PHASE 2.1 software using the -MS model with no recombination to iterate all possible haplotypes. The haplotypes were named after their origin within the Ri chicken population followed by haplotype number (e.g., “Ri_S/R_Hap01”).
To distinguish the haplotype diversity among Ri chickens and other native chicken breeds, a phylogenetic tree was constructed using all defined haplotypes based on the 89 SNPs and compared to global chicken populations including the Korean native chicken, Sri Lankan chicken, Bangladesh chicken, and MHC-B standard haplotypes defined using the same set of SNPs in previous studies [3,11,12,14]. The analysis was conducted using the Bayes r option, utilizing sequence differences between haplotypes, in Geneious Prime software v2023.1.2.
RESULTS AND DISCUSSION
Table 1 summarizes the differences in allele frequency in the Ri chicken population, confirmed by KASP genotyping, presenting the top 10 SNPs with chi-square test statistics. No significant differences in allele frequencies were observed between the R and S groups. The SNP that had the most notable frequency difference between the groups was MHC127, with a fixed frequency of allele 1 at 1.00 in the R group and an observed frequency of 0.50 in the S group. The chi-square statistic for this SNP was 0.667 and the calculated p-value was 0.414.
A total of 32 BSNP haplotypes (shown in Fig. 1) were identified from the 20 Ri chickens tested. The number of haplotypes present in each group is summarized in Table 2. Haplotypes prefixed with “Ri_R or Ri_S” were exclusive to the R and S groups, respectively. These haplotypes had varying frequencies, with some occurring only once (e.g., Ri_R_Hap01) and others being more prevalent.
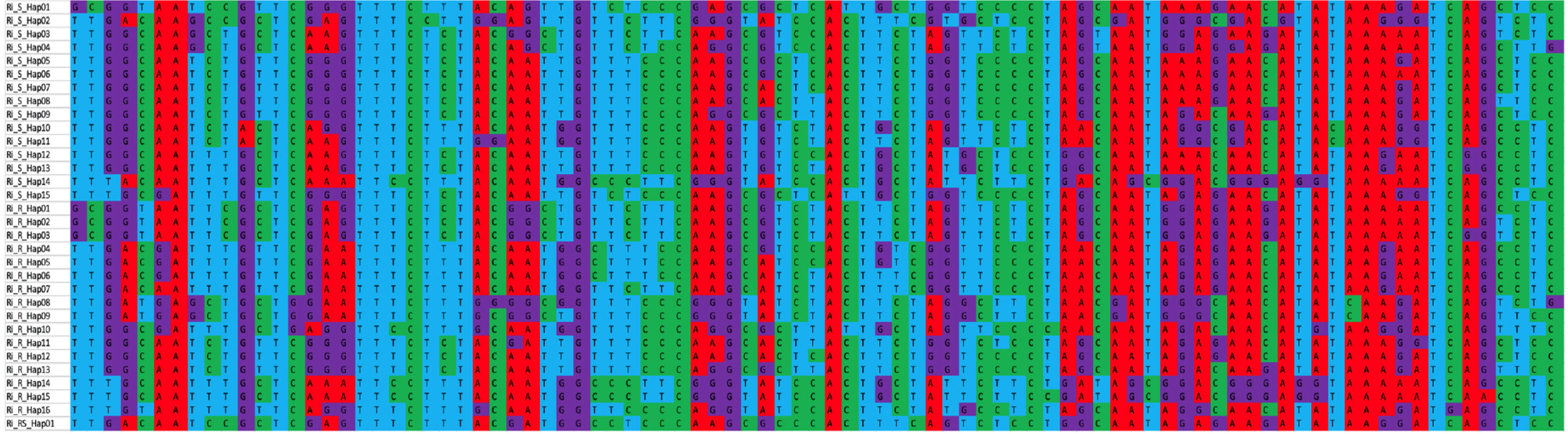
Of the 32 haplotypes, Ri_RS_Hap32 was present in both the R and S groups with a frequency of 1 in both groups indicating a shared genetic characteristic. Within the R group, the most prevalent haplotype was Ri_R_Hap09, observed in three individuals, followed by Ri_R_Hap13 and Ri_R_Hap14, each present in two individuals. In the S group, haplotype Ri_S_Hap21 was the most frequent, followed by haplotype Ri_S_Hap17.
However, the relatively low frequency of group-specific haplotypes makes it challenging to determine the presence or absence of haplotype sharing at the group level as each group-specific haplotype was found only in a few individuals. Similar results were also observed in the native Bangladesh chicken and red jungle fowl with many alleles appearing only once or twice in the population [14]. Some of the new haplotypes differed from each other at only one or two loci. The identification of recombinant haplotypes is unlikely because of the limited population size.
In comparison to other similar studies [11,12], the limited sample size in the current study was not necessarily due to a shortage of individuals but rather a reflection of the substantial diversity in the MHC-B region within the Ri chicken population. Given that all the animals are heterozygous, it is evident that the Ri chicken population is very diverse in the MHC-B region. Additional research on a larger number of Ri chickens is necessary to establish representative group-specific haplotypes.
Fig. 2 shows the constructed phylogenetic tree of the 32 haplotypes in the Ri chicken population. The tree is broadly divided into two major clades, with intermixed group specificities within these clades. Ri chicken clustered with the Bangladesh chicken regardless of the sub-group but separated from the other global chicken haplotypes. Although no study has been conducted on the genetic distance between Ri chicken and Bangladesh chicken breeds, it can be inferred from these results that the characteristics of the MHC-B region are somehow similar between two populations. Consequently, the phylogenetic tree does not reveal distinct similarities in BSNP haplotypes specific to the R and S groups. This concurs with the finding that no SNPs had significant differences in allele frequencies between the groups.
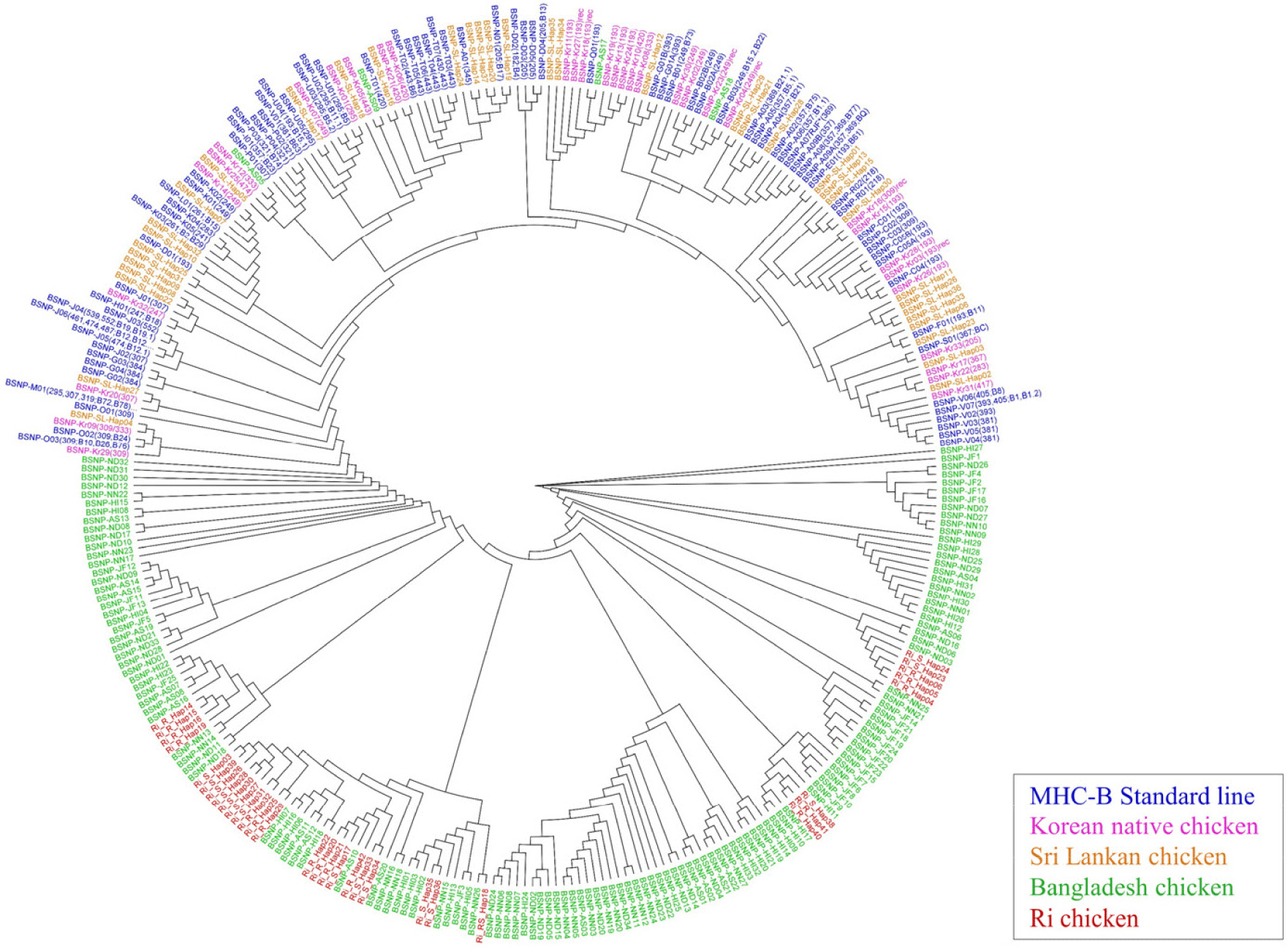
This study analyzed chicken populations using SNP haplotypes that excluded the MHC065 marker. One of the reasons why SNP genotyping failed may be due to the highly polymorphic nature of the MHC region. In the future, when it is possible to confirm the genotype, updated research in the region could be reported.
CONCLUSION
The present study showed that none of the MHC-B SNP alleles were significantly associated with AI resistance in the Ri chicken population. Unique MHC-B haplotypes were also discovered wherein phylogenetic analysis of these haplotypes showed a closer relationship between the Ri and Bangladesh chickens than other chicken populations. However, further investigations are still needed to evaluate the relationships of the Ri chicken with other chicken populations.
