INTRODUCTION
Body-thermal homeokinesis (BTH) is defined as a steady state where body temperature of any homeotherm is relatively maintained constant [1]. Therefore, despite the fluctuation of the external environment, ruminants, as a homeotherm, need to maintain a state of BTH within a narrow range of ambient temperatures, named the thermo-neutral zone [2,3]. The homeotherm’s body is described as an open thermodynamic system that continuously exchanges energy with its external environment [4]. In general, the term “external stressor including environment” is broad and may include both biotic and abiotic stressors [5]. Out of all external environmental stressors, heat stress is the most detrimental stressor to different ruminants’ species (such as cattle, buffalo, sheep, goats, and camels) [6–8]. Form a thermodynamic point of view, it can be stated that ruminants under heat stress conditions generally resorted to reducing their body thermogenic mechanisms and recruiting their thermolytic mechanisms [9,10]. Thus, heat stress can disrupt ruminant body homeostasis by evoking several thermophysiological mechanisms that try to maintain BTH. These mechanisms are well documented, where numerous research articles on ruminants have reported that heat stress can lead to a reduction in feed intake, body weight, and energy metabolism, an increase in body water turnover and urination, a redistribution of blood supply far away from internal organs, as well as an acceleration of evaporative cooling via respiration and sweating [6,11,12]. Thereby, noticeable changes can be observed in ruminants’ biophysiological functions under heat stress conditions.
Among ruminants and livestock species, goats are considered the ideal climate-resilient animal model. This is mainly attributable to the fact that they possess superior morphophysiological, thermophysiological, and behavioral advantages over other species to cope with multiple stressors and to survive under demanding environments [13,14]. In fact, being the best-adapted domesticated animals, goats tend to be the primary focus for efficiently countering the adversities associated with climate change. However, despite such exceptional plasticity and adaptation potentials, the production of these animals can be compromised under some conditions. In fact, exposing goats to an elevated environmental temperature accompanied with/without water deprivation might evoke substantial divergences in their homeostatic\homeokinetic physiological responses as a result of employing ample mechanisms to withstand such conditions. Collectively, these responses lead to noticeable impacts at the organ and cellular levels, which negatively influence meat, milk, and wool production and obviously reducing goats’ wellbeing and welfare [15–17]. Ultimately, this can represent an economical problem to goat’s producers, and thus serious measures have to be established and applied to ensure a sustainable production and an appropriate economic return either by the large-scale enterprises or by the marginal small-scale farmers.
The practical management systems during acute heat stress conditions uses physical modification of the environment with/without nutritional modification strategies [18–21]. Of these nutritional strategies comes the chrono-physiological management, which is an emerging bioscience that recently has been incorporated into the practical management systems of livestock reared under heat stress conditions [22–26]. This approach involves management strategies/protocols that can be used to synchronize the internal biological rhythms of animals with some external zeitgebers such as light, ambient temperature, feeding time and frequency [24,27–29] as well as some internal zeitgebers such as animals’ hormones, body fat storage and distribution, cellular hypoxia, energy flow, lactation stage, parity, and fear [30,31], which can subsequently have prominent effects on their health and productivity. As a matter of fact, implementing this approach has interestingly shown positive impacts on feeding behavior, post-prandial metabolism, nutrient partitioning, energy utilization, production, and reproduction performance in several animals [24,25,32–36]. Such information has actually triggered our interest previously [26] in knowing if altering one of the exogenous cues (i.e., shifting feeding time) could affect body thermo-physiology, post-prandial metabolism, and performance in goats reared under hot environmental conditions. We concluded that such protocol had no advantage in these animals under such conditions. Therefore, a question was raised whether using other chrono-physiological management protocols may have positive effects in promoting their production performance under hot climatic conditions.
Consequently, the current experiment was designed to evaluate the biophysiological and performance advantage of reversing the lighting cycle alone and/or the simultaneous shifting of lighting cycle and feeding time in goat kids exposed to experimentally induced thermal stress. It was hypothesized that by optimizing the timing and duration of the external cues, it is possible that it will reflect on the production efficiency of goats leading to more efficient and sustainable production systems.
MATERIALS AND METHODS
The current experiment was conducted at the experimental station affiliated with the Department of Animal Production, College of Food and Agriculture Sciences, King Saud University, Riyadh, Saudi Arabia, and was conducted in accordance with the ethical standards of the Institutional Research Committee of King Saud University, which ensures the welfare and ethical treatment of animals used in scientific research (process number: KSU-SE-21-26).
Fifteen healthy male goat kids of a native breed (Aardi; black and white coat color) with mean body weight of 22.56 ± 1.13 kg and 6 months of age were randomly allocated into three groups (five kids/group). Kids in the first group (the control group, C) were placed under a normal light : dark (L : D) cycle and fed in the morning. Kids in the second group (treatment group #1, T1) were fed in the morning but placed under a reversed D : L cycle. Kids in the third group (treatment group #2, T2) were placed under a reversed D : L cycle and fed in the evening. All kids were housed individually in pens (1.50 × 1.50 m) inside two insulated climatic chambers and equipped with a software program (Dash control system.) to control the L : D cycle and dry bulb ambient temperature (Ta). Kids had ad libitum water and mineral blocks, and offered a commercial pre-formulated complete Al-Wafi pelleted diet (Arabian Agricultural Services) once a day at 3% of their body weights. Diet composition, according to the manufacturer’s specifications, is enclosed in Table 1. The offered and refused feed was collected daily, and replaced with new ones. It is worth noting that all kids received medical programs (including vaccination and inspection) under veterinarian supervision prior to the commencement of the experiment.
The experiment was divided into two periods. All kids were acclimatized to the experimental conditions, kept under stable conditions (temperature humidity index [THI] ≈ 73-74 Units), and accustomed to the ration and measuring equipment during the preliminary period (~3 weeks). In addition, five kids were intraperitoneally implanted with a wireless-transmitter (CorTemp, HQ) to locate the acrophase value of their body core temperature circadian rhythms measured during this period to ultimately determine the best time for the morning and evening feeding. After analysis, it was determined that the best times were 09:00 h for the morning feeding and 21:00 h for the evening feeding. During the corresponding experimental period (~5 weeks), kids were exposed to a hot condition using a biometeorologically simulated environment with a daily Ta cycle of 25°C to 45°C and a L : D cycle of 12L : 12D for the normal chamber and 12D : 12L for the reversed chamber. Multiple data (i.e., meteorology, biophysiology, and performance) were thereafter collected. However, it is worth mentioning that the heating cycle started at 08:00 h and ended at 15:00 h in the normal chamber (i.e., for C kids), and started at 20:00 h and ended at 03:00 h in the reversed chamber (i.e., for T1 and T2 kids).
Using high accuracy dataloggers (TW-USB-2-LCD+, ThermoWorks) placed above the animal at a height of approximately 2 m from the ground, Ta and relative humidity (RH) were recorded at 30-min intervals. A special software (Box-Car Pro 4, OnsetComp) was utilized for programming these loggers as well as for retrieving data. To determine the environmental intensity on the experimental kids, the obtained Ta and RH data were thereafter used to calculate the THI using a formula adopted from Kelly and Bond [37]. Rectal (Tr), skin (Tsk), and coat (Tct) temperatures were all measured during two consecutive days per week at the maximum values of Ta in each climate controlled chamber (around 14:00 and 02:00 h). A digital rectal thermometer measuring to the nearest 0.10°C was used to determine the Tr, while an infrared thermometer was used to measure the Tsk and Tct at two regions (right shoulder and hip). In addition, a 3M Littmann stethoscope was placed between the 9th and 11th intercostal spaces while counting 10 breaths, and then expressing the recorded time as the number of breathes per minute to determine the respiratory rate (RR) of the kids. Afterwards, blood samples (approximately 10 mL) were withdrawn from the jugular vein, placed into EDTA tubes, and then transferred to the laboratory. After centrifugation (at 1,500×g for 15 min at 5°C), plasma was separated into Eppendorf tubes and then stored at −20°C until spectrophotometrically analyzed for some metabolites (i.e., triacylglycerol [TAG], glucose [GLU], albumin [ALB], and urea [UR]) using the respective commercial kits. Moreover, the amount of offered \ refused feed for each kid was recoded daily using a balance measure to the nearest 10 g to determine their daily feed intake (DFI), while a standard balanced measure to the nearest 0.10 kg was used to measure their average daily body gain (ADG) before experimental diets were introduced. The ratio of feed conversion (FCR) was then calculated as DFI to ADG.
Data was analyzed using the PROC MIXED procedure of the SAS program (SAS Inst.) as a completely randomized design. The model included the influence of treatment, animal, time, and interactions. Moreover, the descriptive analysis was acquired using the PROC MEANS procedure. Means showing significant differences in ANOVA were tested using the PDIFF option, and the probability value was set at p < 0.05.
RESULTS
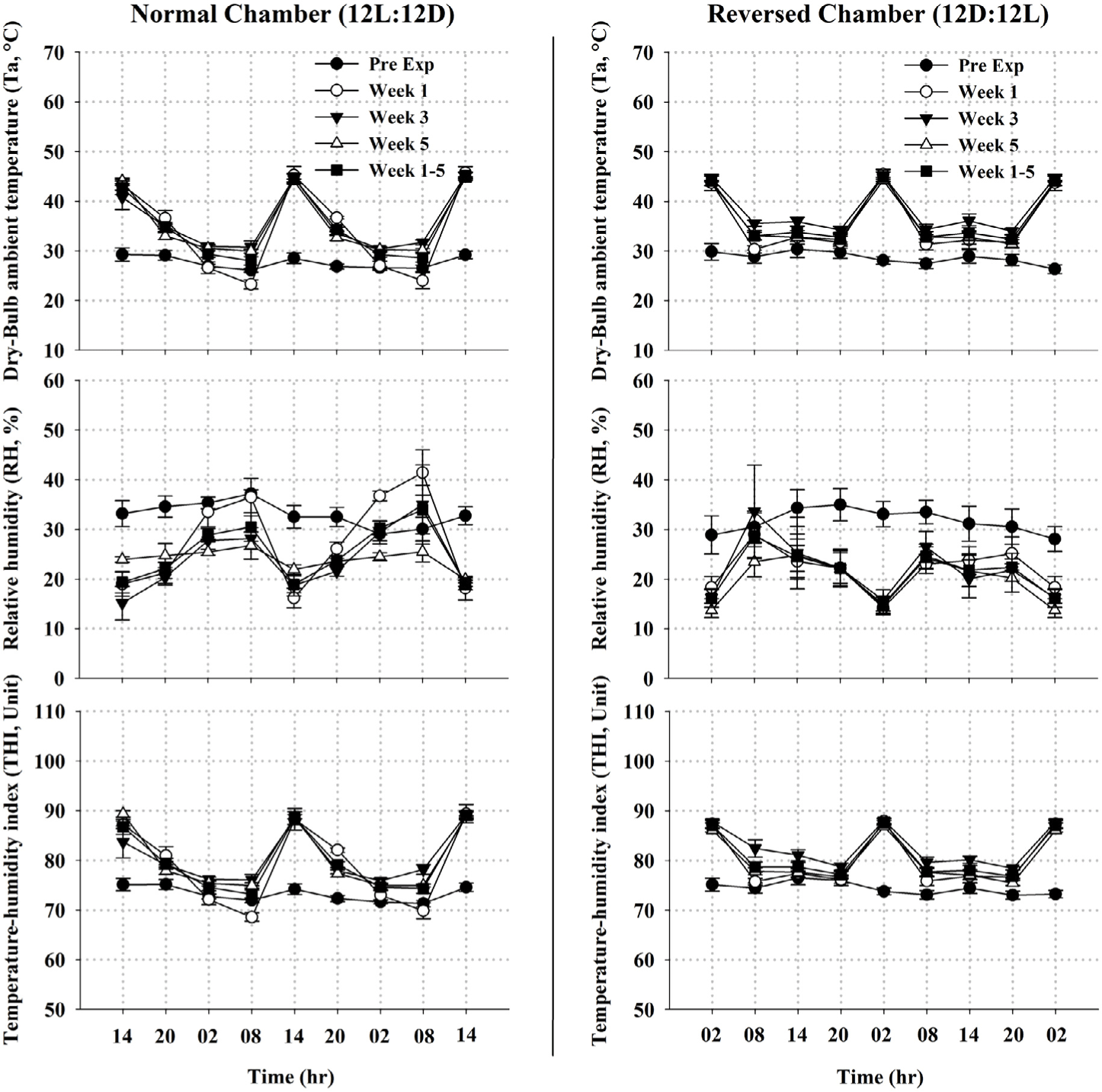
Metrological data measured during the experimental period is presented in Fig. 1. The recorded Ta, RH, and THI data inside both chambers exhibited a monophasic circadian rhythm from Weeks 1 to 5. For the 12L : 12D chamber (where kids of the control group were placed), the overall means of Ta, RH and THI were 34.72 ± 0.89°C, 27.57 ± 2.15% and 79.42 ± 1.02 units, respectively. The minimum values of Ta and THI were recorded in the early morning (05:00 to 07:00 h) and differ (p < 0.05) from the maximum values recorded in the afternoon (13:00 to 15:00 h). Meanwhile, RH showed the reverse trend (Fig. 1). On the other hand, the overall means of Ta, RH and THI were respectively 34.83 ± 0.37°C, 22.46 ± 0.66% and 79.17 ± 0.34 units for the 12D : 12L chambers (where kids of the treated groups were placed). The minimum values of Ta and THI were recorded in the evening (17:00 to 19:00 h) and differ (p < 0.05) from the maximum values recorded in the early morning (01:00 to 03:00 h). The RH showed the reverse trend as well (Fig. 1). Comparing the Ta, RH, and THI data recorded at their acrophase (14:00 vs 02:00 h) and trough (06:00 vs 18:00 hr) shown no difference between both chambers, therefore attesting a reasonable approximation of the uniform distribution of the environmental condition on all experimental animals.
Changes of Tr, Tsk, Tct, RR, TAG, GLU, ALB, and UR, measured at the maximum values of Ta in each climate-controlled chamber (14:00 and 02:00 h), as a response to different chrono-physiological management protocols are presented in Table 2. Starting from the first week of the experiment, elevation of Ta had increased Tr, Tsk, Tct, and RR in all experimental groups (Table 2). However, with the exception of Tr and Tsk, differences (p < 0.05) were observed in Tct and RR where it was lower in kids of the T1 and T2 groups compared to their counterparts in the C group (Table 2). Moreover, reversing the lighting cycle alone (T1) and/or the simultaneous shifting of both the lighting cycle and feeding time programs (T2) under hot climatic conditions had no influence on the plasma concentrations of ALB and GLU throughout the whole period (from the first to the fifth week) (Table 2). However, kids in both treatments showed (p < 0.05) higher plasma concentrations of TAG compared to the C group kids. Plasma UR was additionally affected but only in T2 kids, where it was (p < 0.05) higher compared to other groups (Table 2).
Elevation of Ta started from the 1st week of the experiment. Measurements were recorded during two consecutive days per week at the maximum values of Ta in each climatic-controlled chamber (14:00 and 02:00 h); however, data of week 1, 5, and all weeks (1–5) were merely presented herein.
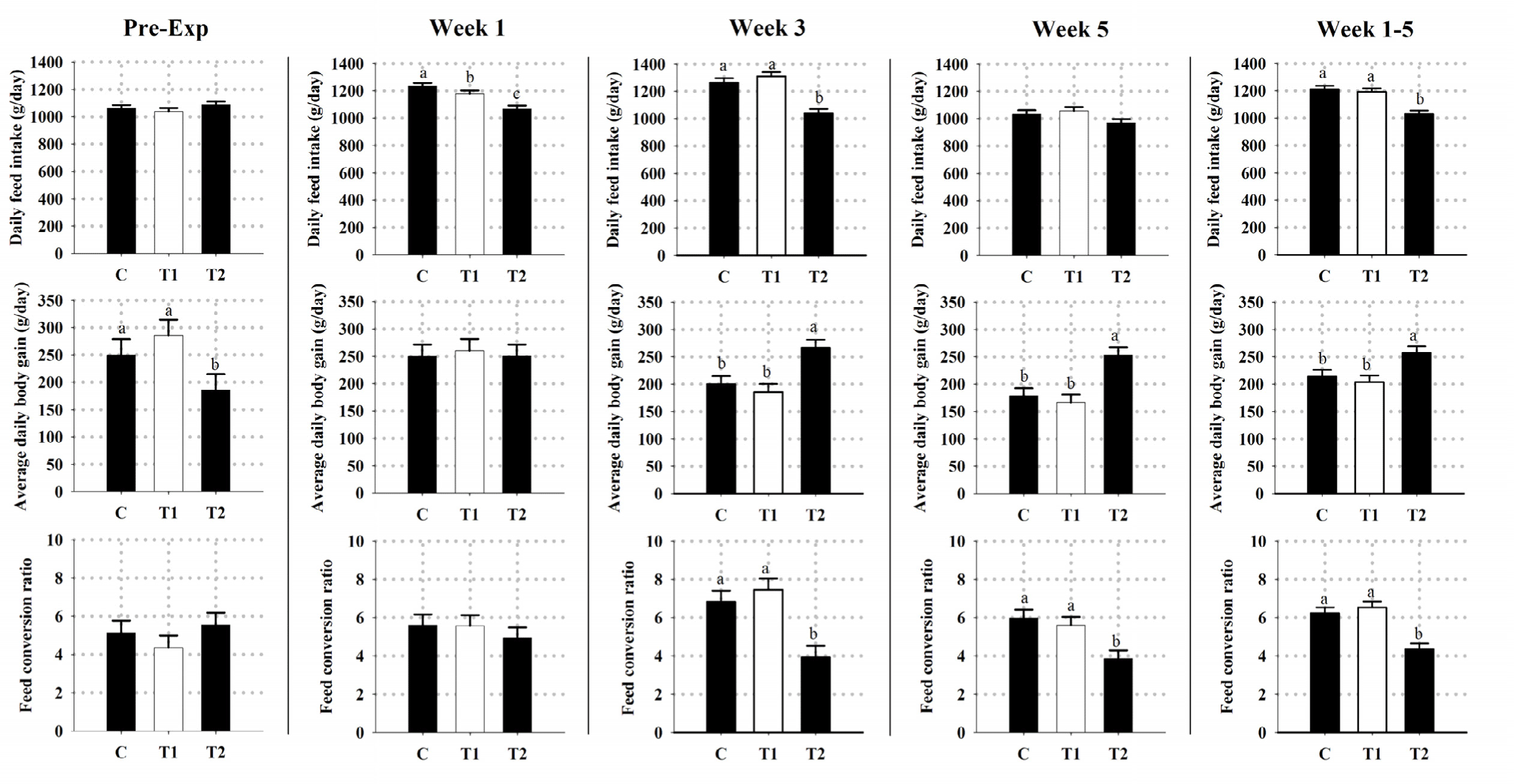
Furthermore, the obtained results of DFI, ADG and FCR in all groups are shown in Fig. 2. Throughout the whole experimental period, simultaneous shifting of both the lighting cycle and feeding time (T2) had (p < 0.05) reduced the overall mean of DFI and increased the overall mean of ADG. This was subsequently reflected on having (p < 0.05) a lower (better) overall mean of FCR in T2 kids compared to other kids (Fig. 2).
DISCUSSION
Heat stress is widely recognized as one of the potent environmental stressors, which can impair the production performance of small ruminants [38–42]; therefore, understanding and mitigating these stressors is crucial. One approach to address such issue could be through manipulating the L : D cycle or by selecting an appropriate feeding time, which may help in synchronizing both exogenous and endogenous cues with the internal biological rhythms and attaining the best economic nutrients use [22,23,35]. This experiment was consequently conducted to explore the influence of these two external zeitgebers and their interaction on the biophysiological and productive performance of goat kids exposed to experimentally induced heat stress.
According to the calculated average THI in both chambers, kids appeared to be under heat stress conditions during the experimental period [43,44]. However, the obtained findings revealed that reversing the lighting cycle alone (T1) and/or simultaneously shifting both lighting cycle and feeding time (T2) had no general subsequent impact on the thermal status (as expressed mainly by Tr and Tsk) of heat-stressed kids, despite the observed elevations in Tct and RR. Under thermoneutral conditions, endothermic animals (such as goats) try to maintain a state of thermal homeokinesis, but when they were exposed to supraneural conditions it forced them to recruit their thermolytic mechanisms and reduce their thermogenic mechanisms [45,46]. As a matter of fact, the noticed increase between Pre Exp and Week #1 in these variables clearly indicated that kids were trying to recruit their thermolytic mechanisms (Tsk and RR) under an abrupt increase of the surrounding Ta. Nonetheless, the observed absence of alteration in body thermal status throughout the experiment might be attributed to the fact that the goats used are well adapted to cope with such conditions compared to dairy cows in term of the heat produced by the metabolism and how the thermoregulation system acts at the hypothalamic level [35,38,42,47–49]. Additionally, this might return to the combined impact of both of shifting the program of lighting cycle and feeding with the daily Ta cycle applied herein, which differs from those performed by other researchers on different species. Further research is obviously required to exclude such a combined effect on these animals.
Reversing the lighting cycle alone and/or simultaneous shifting of both lighting cycle and feeding time exhibited some influence on blood biochemistry. It is well known that body metabolites can be controlled by both endogenous and exogenous cues, but they are mostly controlled by external cues according to many investigators [24,50–53], thereby suggesting that some metabolites could be responsive to the applied experimental treatments. In fact, evidence from the present experiment has indicated that plasma concentrations of TAG and UR (but not plasma ALB and GLU concentrations) were both affected in kids of the T1 and T2 groups compared to their twins in C group. The observed increases in plasma UR concentrations in T2 kids is largely a response to the reversed 12D : 12L cycle and shift in the feeding time, and thus could be exogenously regulated as previously reported [45]. In contrast, blood GLU is merely controlled by internal cues [54]; that is why plasma GLU did not show any entrainment by the applied external zeitgebers herein, while plasma TAG was affected by these zeitgebers. In fact, the shift in feeding time alone had no effect on plasma TAG as mentioned in our previous experiment, which would basically indicate that this variable is mostly controlled by photic zeitgeber [26,55]. These findings are consistent with some reports on goats, dairy cows, and Syrian hamsters [22–25,50,56]. However, further experiments are warranted to adequately examine such protocols without manipulating the normal daily heating process especially when reversing both lighting cycle and feeding time, as applied here in T2.
Notably, our findings pointed out that shifting light and feeding time clearly succeeded in demonstrating some consequences on promoting the growth performance of heat-stressed goat kids, thereby attesting that such protocol could have reduced or desynchronized the heat load emerging from the combined effects of both thermal stress and post-prandial metabolism. These findings contradict previous reports on dairy cattle, beef steers, sheep, and turkeys [25,35,57–60]. Growth is a productive characteristic ordinarily controlled by the genetic factors, the environmental factors (such as light, temperature, and feeding time), and the interaction between those factors [26,32,56,61,62]. Actually, environmental photic and nonphotic cues can positively and/or negatively alter the behavioral activity, ingestion, digestion, post-feeding rumen fermentation, endocrine secretion, and consequently post-prandial metabolism in ruminants [24,32,63,64]. Based on the obtained findings, it was clearly evident that simultaneous shifting of both the lighting cycle and feeding time, T2 protocol, had reduced kids DFI, increased their ADG, and subsequently had better FCR compared to kids in other groups. Despite the exact cellular mechanism of how such protocol had manipulated body chemicals and hormones to influence the brain, gut, and muscle tissues is not known, these findings emphasizes the role of light as the primary zeitgeber for entrainment of circadian clocks and productivity in animals [32,56,61,62]. However, further examinations are necessary to pinpoint the exact reason for such findings.
In conclusion, reversing the lighting cycle alone (T1) and/or the simultaneous shifting of both the lighting cycle and feeding time protocol (T2) under hot climatic conditions had no influence on body Tr and Tsk temperatures as well as plasma concentrations of ALB and GLU. Kids in both treatments showed higher Tct and RR as well as plasma concentrations of TAG compared to the C group kids. Moreover, it was clearly evident that kids in T2 had reduced kids DFI, increased their ADG and plasma concentrations of UR, and had better FCR compared to kids in other groups. Collectively, this would suggest that using such protocol had desynchronized the heat load emerging from the combined effects of both thermal stress and post-prandial metabolism. Compared to other protocols, our findings point out that simultaneous shifting of both lighting cycle and feeding time protocol has proven to be suitable in enhancing the production performance of growing heat-stressed goats. However, further experiments that reduce the respective time of blood sampling in relation to feeding time, increase the feeding frequency, assess stress-related and behavioral indicators, and using other protocols (such as simultaneous shifting of light and feeding without manipulating the normal daily fluctuations of Ta) may be of interest.
