INTRODUCTION
Antibiotic growth promoters (AGPs) have traditionally been used in the poultry industry to improve growth, feed efficiency, and gut physiology [1]. However, increasing concerns about antibiotic-resistant microorganisms have led to global efforts to reduce or ban the use of AGPs in livestock production [2]. As a result, regulatory authorities have introduced restrictions and guidelines to promote responsible antibiotic use, which has led to the exploration of alternative strategies to improve poultry health and performance.
To address these challenges, various feed additives are being explored as alternatives to AGPs. Probiotics, prebiotics, organic acids and essential oils have shown promise. Organic acids improve broiler health by supporting immunological function, pancreatic enzyme activity and gut microbiota balance [3–6]. Essential oils containing compounds such as thymol, carvacrol and eugenol provide benefits such as improved immune function and a reduction in pathogenic bacteria [7–9]. The combination of essential oils with organic acids (EOA) can further improve gut health and performance compared to single supplements [10–12].
Alternative feed ingredients such as wheat and corn distillers dried grains with solubles (DDGS) are commonly used in poultry feed. Corn DDGS, a by-product of ethanol production, provides protein and energy, but may have lower protein quality due to high levels of non-starch polysaccharides (NSP) and lower amino acid digestibility [13]. High NSP content in DDGS may promote colonization with Clostridium perfringens, especially under conditions of necrotic enteritis (NE) [14]. Similarly, wheat contains arabinoxylan, an NSP that increases gut viscosity, leading to reduced nutrient absorption and microbial imbalances, e.g. with Escherichia coli and Salmonella spp. [15–19]. In addition, poor protein digestibility associated with high NSP content can lead to microbial fermentation of nitrogen metabolites, which impairs the intestinal barrier, increases tight junction (TJ) permeability and impairs broiler growth [20,21].
Protease (PRO) enzymes contribute significantly to minimizing undigested proteins, maximizing amino acid availability, reducing dietary protein requirements, supporting weight gain and feed efficiency, reducing proteolytic fermentation, reducing biogenic amines, and improving gut integrity [22–24]. Consequently, there is considerable interest in the market to utilize undigested proteins through the use of exogenous enzymes such as PRO. This approach facilitates the formulation of balanced diets with reduced protein levels, which ultimately leads to cost savings in feed production [25,26].
Microencapsulation is an important technique to deliver bioactive compounds into the gastrointestinal tract [27,28]. It ensures the stability and targeted release of these compounds in the hindgut, where pathogenic bacteria are most prevalent [29]. Without microencapsulation, organic acids may dissociate in the upper gastrointestinal tract and essential oils may be absorbed before they reach the hindgut, reducing their efficacy. Microencapsulated organic acids and essential oils show significantly increased bactericidal and bacteriostatic activity compared to unprotected forms [27,28,30].
The hypothesis of this study is that microencapsulated EOA in combination with PRO can improve the growth and gut health of broilers fed a high wheat and corn DDGS diet, which serves as a nutritional model challenging avian gut resilience. The broiler chickens in the current study were raised under AGP-free programs. Chemical coccidiostats, which are not classified as veterinary medicinal products and can be used as feed additives according to the EU regulation [31], were used to control coccidiosis. The microencapsulated organic acids are fumaric, malic, sorbic and citric acids, and the essential oils are vanillin, eugenol and thymol, all encapsulated in hydrogenated vegetable fat. The PRO used is an alkaline serine endopeptidase derived from the fermentation of Streptomyces. The aim is to investigate the effects of this microencapsulated EOA in combination with PRO on the growth and gut health of broiler chickens fed a high wheat-corn DDGS diet. Gut health was assessed by analyzing gut morphology, microbial metabolites in the cecum and mRNA expression of TJ proteins, which are critical for maintaining intestinal integrity.
MATERIALS AND METHODS
The experimental protocol was approved by the Animal Care and Use Committee of Kasetsart University (protocol number: ACKU62-AQK-012). A total of 1,400 male Ross 308 broiler chicks (Panus Pokphand) were reared in 56 pens (1.5 × 2.0 m). All birds were randomly assigned to 4 treatments using a completely randomized design. There were 14 replicates with 25 birds per replicate in each treatment. The dietary treatments were: 1) corn and soybean meal-based diet with high levels of wheat and corn DDGS (WD); 2) WD+microencapsulated organic acids and essential oils at 300 mg/kg (EOA); 3) WD+PRO at 125 mg/kg (PRO); and 4) WD+EOA at 300 mg/kg+PRO at 125 mg/kg (EOA+PRO). The EOA contained a combination of fumaric acid, sorbic acid, malic acid and citric acid with vanillin, eugenol, and thymol microencapsulated in hydrogenated vegetable fat. The PRO enzyme was an alkaline serine endopeptidase with PRO activity of 1.10 U/g. Both are commercially available products provided by Jefo Nutrition. During the trial, the birds had unlimited access to water and feed. The ambient temperature was 32°C for the first three days, then steadily dropped to 25°C on day 14. The light settings were 23 hours of light and 1 hour of darkness during the experiment.
The main ingredients of the WD group were corn and soybean meal. In the starter, grower, and finisher diets, 20%, 25%, and 30% wheat replaced corn as the energy source, and 10%, 12.5%, and 15% corn DDGS replaced soybean meal as the protein source. All experimental diets were formulated following the strain recommendations [32]. The diets were mixed with a horizontal mixer and pelleted at 80°C according to the manufacturer’s instructions (Bangkok Animal Research Center). All experimental diets were analyzed for crude protein, ether extract, crude fiber, gross energy, calcium and phosphorus according to AOAC guidelines [33]. The details of the diet composition are listed in Table 1.
Experimental diet: 1) corn-soybean meal basal diet with wheat and corn distillers dried grains with solubles (WD); 2) WD+microencapsulated organic acids-essential oils blend at 300 mg/kg (EOA); 3) WD+PRO at 125 mg/kg (PRO); 4) WD+microencapsulated organic acids-essential oils blend at 300 mg/kg+PRO at 125 mg/kg (EOA+PRO).
Broiler vit/min premix provided per kilograms of diet: vitamin A (all-trans retinol) 1,2000 IU; vitamin D3 (cholecalciferol) 2,400 IU; vitamin E (l-α-tocopherol) 60 mg; vitamin K 240 mg; vitamin B1 300 mg; vitamin B2 800 mg; vitamin B6 400 mg; vitamin B12 2 mg; niacin 5,000 mg; pantothenic acid 1,500 mg; biotin 40 mg; folic 200 mg; Cu (copper sulfate) 1,500 mg; Fe (ferrous sulfate) 4,000 mg; Mn (manganese sulfate) 10,000 mg; Zn (zinc sulfate) 10,000 mg; I (Iodide) 100 mg; Se (Selenate) 100 mg.
The body weight of all birds and the feed intake per pen were recorded on days 1, 7, 14, 28, and 35. Feed intake (FI), feed conversion ratio (FCR), and body weight gain (BWG) were calculated for each bird and each replicate. Mortality was recorded daily, and the weight of dead birds was recorded to calculate the adjusted FCR.
On days 14 and 35, one bird was randomly selected from each replicate (a total of 14 birds per treatment), its body weight (BW) was measured and it was then humanely sacrificed by stunning and bleeding. The mid jejunum was removed for intestinal morphological examination. The intestinal mucosa was scraped with a sterile glass slide. Intestinal mucosa samples were immediately frozen in liquid nitrogen and stored at –80°C for subsequent mRNA expression analysis of TJ proteins. Cecal content samples from 35-day-old birds were collected and stored in a freezer at –20°C to analyze ammonia, biogenic amines and volatile fatty acids (VFA) in the ceca.
After extraction from frozen jejunum mucosal samples using the GenUPTM total RNA kit (Biotechrabbit GmbH), RNA quantity and quality were determined using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific) at 260 and 280 nm. Subsequently, 1 µg of RNA was used to synthesize the first strand of cDNA using a cDNA synthesis kit (Biotechrabbit GmbH), and the resulting cDNA was stored at –20°C for subsequent analysis.
Expression of the claudin-1, Zonula Occludens-1 (ZO-1) and occludin genes was determined by real time PCR using the specific primers listed in Table 2 [34,35]. Rigorous testing ensured primer efficiency and linearity. Each reaction was performed in triplicate for each gene and sample. Gene expression was normalized using glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and TATA-binding protein (TBP) as reference genes, according to the methodology described by Taylor et al. [36].
Intestinal morphology examinations were performed according to Iji et al. [37]. A 1 cm sample of the jejunum (between the terminal loop of the duodenum and Meckel’s diverticulum) was excised and immediately fixed in 10% formalin. The fixed samples were dehydrated in ethanol, cleared in xylene, and embedded in paraffin. Two sections, each 7 μm thick, were mounted on microscope slides and stained with alcian blue, hematoxylin, and eosin. The stained sections were examined under a light microscope at 40x magnification using an Olympus CX33 microscope equipped with an Olympus DP22 digital camera and DP2-SAL imaging software (Olympus). Villus height (VH), measured from the base transition zone between villus and crypt to the apex, crypt depth (CD), measured from the base of the villi to the bottom of the glands, and villus width (VW), measured from the left villus crypt junction to the right of the villus crypt junction, were quantified. VH/CD ratio was determined by measuring 9 randomly selected villi and their corresponding crypts.
VFA were analyzed by gas chromatography according to Thanh et al. [38]. In brief, 200 mg of cecal content was mixed with distilled water in a 1:1 ratio (w/v) and centrifuged at 16,300×g at 4°C for 20 min. Then, 100 µL of the supernatant was transferred and mixed with 100 µL of 24% metaphosphoric acid in 1.5 M sulfuric acid, stirred for 5 min, and allowed to stand overnight at 4°C. The mixture was then centrifuged at 8,944×g for 5 min at 4°C. The supernatant was mixed with an equal volume of 3 mM crotonic acid and used as an internal standard. Subsequently, 1 µL of the prepared sample was injected and separated by gas chromatography using a CP-Wax 52 CB (50 m × 0.32 mm) column (Agilent Technologies). Helium (2 mL/min) was used as the mobile phase, and the injector and detector temperatures were 250°C and 280°C, respectively. The column temperature was set to 200°C. External standards with 3 mM acetic acid, propionic acid, butyric acid, and 1.5 mM crotonic acid were used to identify the peaks.
The frozen cecal content was analyzed according to Meyer et al. [39]. In brief, 500 mL of 100 mM 3-(N-morpholino) propanesulfonic acid was added to 250 mg of cecal content. The sample was centrifuged at 4°C and 12,888×g for 20 min. Then, 250 µL of the supernatant was mixed with 25 µL of Carrez Clarification Reagent Kit (Sigma-Aldrich) and centrifuged at 4°C and 12,888×g for 10 min. Ammonia was analyzed according to the method described by Weatherburn [40].
Amine analysis of the extracted cecal contents was performed as described by Saarinen [41]. A 500 µL aliquot of 0.4 M perchloric acid was used to deprotonate 250 mg of the frozen sample. The derivatization reaction of the amine in the extracted sample was carried out with dansyl chloride as described by Eerola et al. [42]. The derivative solution was filtered using a nylon membrane filter with a pore size of 0.22 µm. Subsequently, 10 µL of the sample was injected into an ODS2 column (4.0 × 250 m; Waters) using a 717 plus autosampler at 40°C. Peaks were detected at 254 nm using a 2998 Photodiode Array Detector (Waters) and analyzed using Empower Software Build 2154 (Waters). HPLC-grade water was used as mobile phase A and HPLC-grade acetonitrile (Fisher Scientific) was used as mobile phase B. The gradient elution was initially 50%, after 25 min 10%, after 35 min 50%, after 40 min 50% at a flow rate of 1 mL/min. Finally, 1-aminoheptane was used as an internal standard.
Putrescine dihydrochloride and cadaverine dihydrochloride were used as external standards and diluted in water to prepare the stock solution. Subsequently, the external standards were diluted with 0.4 M perchloric acid for serial dilution.
Percentage mortality data were obtained by square root transformation of Y + 0.5 (Y = %mortality). Relative gene expression was Log-transformed (Log2 ∆∆Cq) prior to statistical analysis. All data were tested for normality using the Kolmogorov–Smirnov test before performing statistical analyses. Statistical differences between treatments were analyzed using the GLM procedure from SAS Studio University Edition (SAS Institute). Differences among treatments were determined using Tukey’s test for honestly significant differences. Significant values were determined based on a p-value ≤ 0.05, and trends were reported at 0.05 < p ≤ 0.1.
RESULTS
This experiment was conducted to investigate the effects of EOA in combination with PRO on the growth and gut health of broilers raised without AGPs. It is crucial to challenge intestinal homeostasis, as in the absence of such challenges, gut-acting growth promoters may have limited effects on performance [43,44]. Therefore, this study employed a nutritional model that challenged avian gut resilience using a diet high in wheat and corn DDGS. The EOA blend was supplemented in a microencapsulated form to ensure the stability and targeted release of these compounds in the hindgut, where pathogenic bacteria are most prevalent. Additionally, PRO was included to assess its potential in improving nutrient utilization, particularly in overcoming the poor digestibility associated with the high NSP content in corn DDGS and wheat. The results of this study are presented below.
In the current study, no effects of the dietary treatments (p > 0.05) were observed on BWG, FI and mortality rate (Table 3). On day 8–14, the PRO group had a higher FCR than the EOA+PRO group (p < 0.01), while the FCR of the WD and EOA groups did not differ from the others (p > 0.05). On day 1-35, the WD group had a higher FCR than the EOA+PRO group (p < 0.05), while the FCR of the EOA and PRO groups did not differ significantly from the other groups (p > 0.05).
Dietary treatments: WD = corn-soybean meal basal diet with wheat and corn distillers dried grains with solubles; EOA = WD + microencapsulated organic acids-essential oils blend at 300 mg/kg; PRO = WD + PRO at 125 mg/kg; and EOA + PRO = WD + microencapsulated organic acids-essential oils blend at 300 mg/kg + PRO at 125 mg/kg.
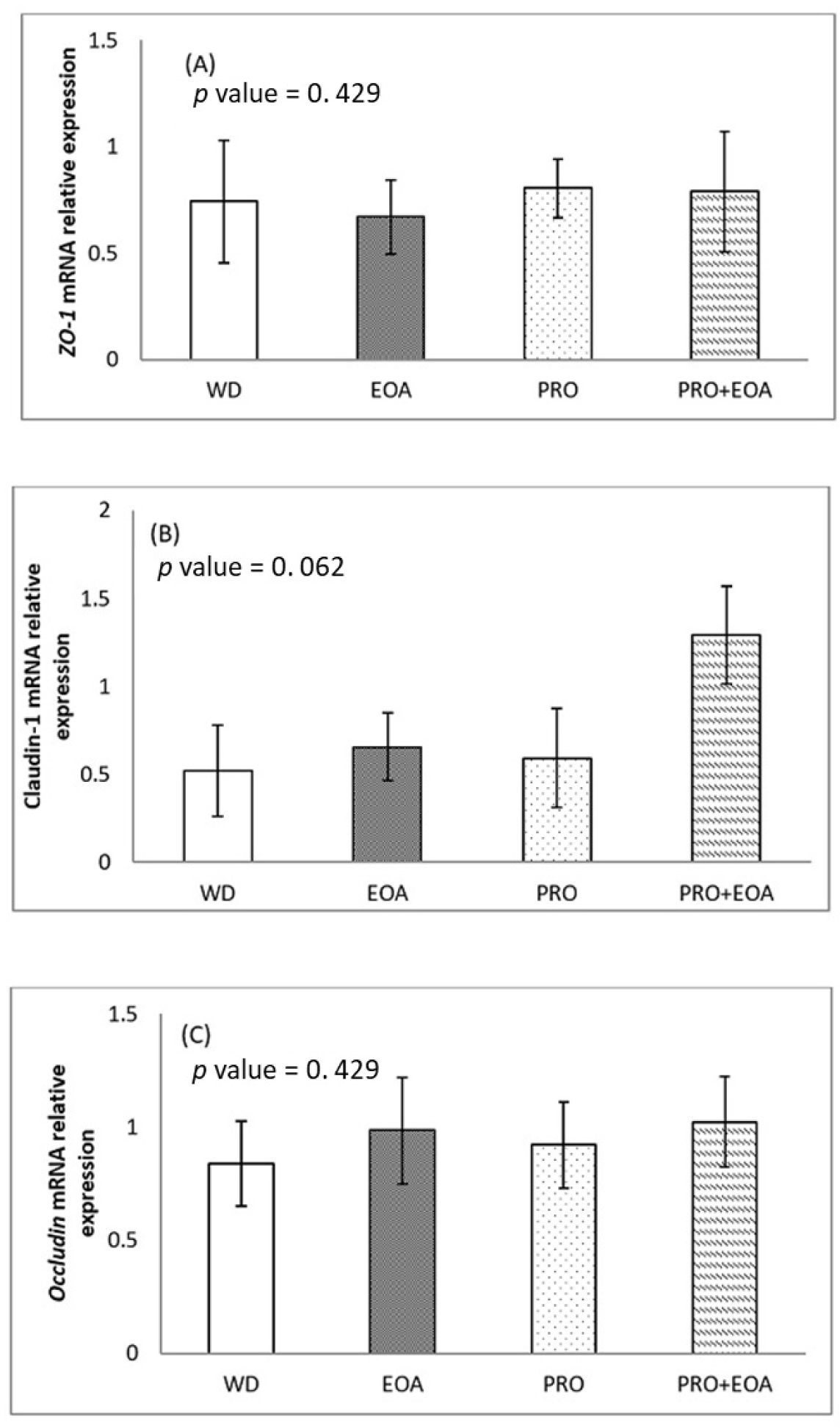
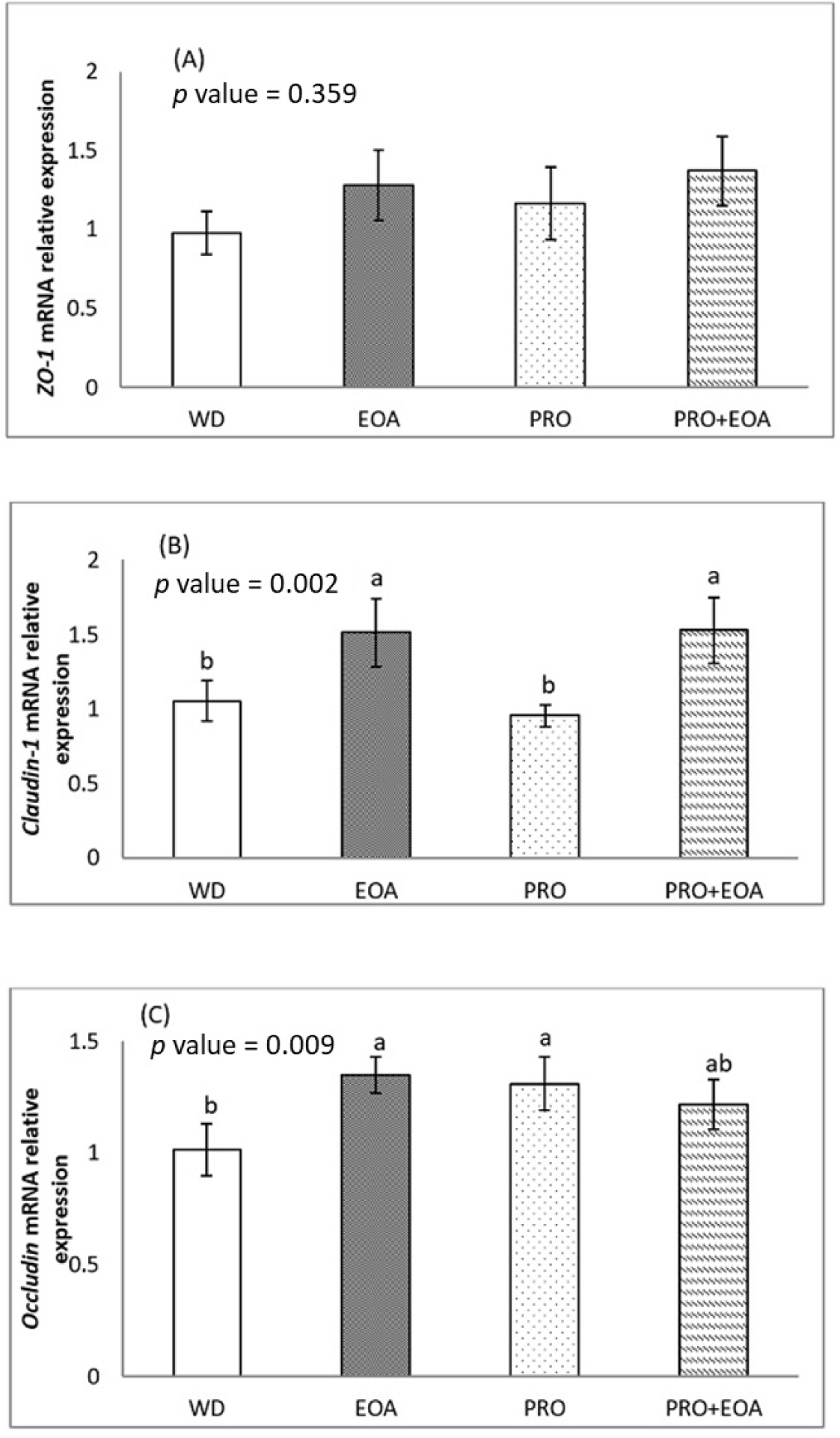
Figs. 1 and 2 show the effects of the dietary treatments on the mRNA expression of selected intestinal barrier TJ proteins in the jejunum mucosa on days 14 and 35. On day 14, the mRNA expression of ZO-1 and occludin did not differ between the four dietary treatments (p > 0.05). There was a trend towards higher expression of claudin-1 mRNA in the EOA+PRO group compared to the others (p = 0.062). On day 35, the expression of ZO-1 mRNA did not differ significantly between treatments (p > 0.05). The EOA and EOA+PRO groups had higher claudin-1 mRNA expression than the WD and PRO groups (p < 0.01). Occludin mRNA expression was higher in the EOA and PRO groups than in the WD group (p < 0.01), while the EOA+PRO group had similar expression to the other groups (p > 0.05).
On day 14, no significant differences in gut morphology were observed among the four dietary treatments (p > 0.05), as indicated in Table 4. On day 35, there were no differences in jejunal VH and VW between treatments (p > 0.05). However, the EOA+PRO group had a lower jejunal crypt depth and a higher VH/CD ratio compared to the other treatment groups (p < 0.01).
Dietary treatments: WD = corn-soybean meal basal diet with wheat and corn distillers dried grains with solubles; EOA = WD + microencapsulated organic acids-essential oils blend at 300 mg/kg; PRO = WD + PRO at 125 mg/kg; and EOA + PRO = WD + microencapsulated organic acids-essential oils blend at 300 mg/kg + PRO at 125 mg/kg.
Table 5 illustrates the effects of the dietary treatments on the microbial metabolites in the cecal content on day 35. No significant differences in the ammonia and VFA content were found among the dietary treatments (p > 0.05). In terms of biogenic amines, the WD group had a higher putrescine content than the other dietary treatments (p < 0.05). In addition, the WD group tended to have a higher cadaverine content than the other dietary treatments (p < 0.1).
Each value represents the mean of 14 replicates except volatile fatty acid represents 11 replicates.
Dietary treatments: WD = corn-soybean meal basal diet with wheat and corn distillers dried grains with solubles; EOA = WD + microencapsulated organic acids-essential oils blend at 300 mg/kg; PRO = WD + PRO at 125 mg/kg; and EOA + PRO = WD + microencapsulated organic acids-essential oils blend at 300 mg/kg + PRO at 125 mg/kg.
DISCUSSION
In this study, body weight gain, feed intake, and mortality rate did not differ significantly among the dietary treatments, all of which were based on a basal diet containing a high proportion of wheat and corn DDGS. However, the FCR for the EOA+PRO group was lower than that of the WD group from day 1 to 35, whereas the EOA and PRO groups did not differ substantially from the others. The possible explanation could be the combined effect of EOA and PRO, which could improve the FCR of the birds by stimulating digestive enzyme activity and improving nutrient utilization under the challenging conditions of high wheat and corn DDGS in the diet. Several studies have shown that EOA can stimulate the activity of digestive enzymes and improve feed efficiency in broiler chickens [45,46]. In addition, administration of a single-component enzyme (serine alkaline endopeptidase) in broilers also improved ADG and FCR in a rye-wheat–soybean meal [18] and corn–soybean meal-canola-based diets [47]. Chowdhury et al. [30] partially confirm the results of this study, showing that broilers fed a diet supplemented with microencapsulated EOA and PRO achieved better FCR than those fed EOA alone. In addition, they found that higher EOA content (300 mg/kg diet) increased FCR regardless of whether PRO was included in the diet or not.
TJ proteins, including claudins, occludins, ZO-1, and the actin-myosin cytoskeleton, establish connections between layers of epithelial cells in the intestine and form a barrier that separates the lumen contents from the underlying tissue [48,49]. These tight junctions are essential elements of the intestinal epithelial barrier and play a crucial role in maintaining the integrity of the gastrointestinal tract. When this barrier is compromised, luminal antigens such as microbes and toxins can disrupt homeostasis and increase the risk of systemic infection, chronic inflammation, and malabsorption [48,50]. The breakdown of the intestinal barrier has been associated with the pathogenicity of specific gut bacteria, including Campylobacter jejuni, Salmonella enterica and Clostridium perfringens [51]. In this study, the additives EOA, PRO, and EOA+PRO had no effect on ZO-1 mRNA expression. The higher expression of claudin-1 mRNA in the EOA and EOA+PRO groups compared to the WD control group suggests an improvement in gut integrity when the diet is supplemented with these additives. There was no discernible difference between the PRO and the WD control groups, suggesting that the increased claudin-1 mRNA expression in the EOA+PRO group may be due to the effect of EOA rather than PRO. It is possible that claudin-1 mRNA expression was upregulated due to the antibacterial properties of EOA. In addition, Yang et al. [28] observed that the EOA group expressed more claudin-1 mRNA than the antibiotic group or the control group, but there was no significant change in the mRNA expression of occludin or ZO-1. McKnight et al. [52] observed comparable levels of claudin-1 mRNA expression in both the EOA and antibiotic groups, which were higher than those in the control group.
In this study, the EOA and PRO groups showed a higher level of occludin mRNA expression than the WD group, while EOA+PRO was not significantly different from the others. This result suggests that either the mixture of organic acids and essential oils or the PRO can stimulate occludin by upregulating occludin mRNA expression without a combination effect of EOA and PRO in the EOA+PRO group. The combination of essential oils and organic acids has been shown to be beneficial, e.g. in terms of improved feed efficiency or upregulated mRNA expression of TJ proteins such as claudin-1 and occludin when added to broiler diets [28,43,45,52].
Morphological indicators of intestinal health, such as VH, CD and the VH/CD ratio, provide information about the ability of the intestine to digest and absorb nutrients [53,54]. Higher villi generally indicate a healthier gut, as they provide a larger surface area for nutrient absorption, while shallower crypts are typically associated with a healthier gut, as deeper crypts may indicate increased cell turnover or pathological conditions [54,55]. A higher VH/CD ratio usually reflects a well-functioning and healthy gut, while a lower ratio may indicate problems such as inflammation or impaired nutrient absorption [55]. In addition, a lower VH/CD ratio indicates a reduced number of absorptive cells and an increased number of goblet cells, leading to increased mucin secretion [55,56]. Changes in mucin quantity or composition may impair nutrient uptake or increase energy requirements to maintain homeostasis [55,57]. The addition of EOA to broiler diets has been shown to be an effective strategy to improve gut morphology [45,46,58]. These results could not be confirmed in this study, as supplementation with EOA did not produce any significant effects on intestinal morphology. However, the EOA+PRO group showed increased jejunal VH/CD ratio and decreased CD, suggesting a combination effect of PRO supplementation in combination with EOA on intestinal morphology. The discrepancies between the present study and previous research may be due to differences in dietary formulations, microbial and environmental conditions, methodological approaches, and the synergistic effects of the supplements used.
The possible mechanisms of EOA and PRO that improved the expression of TJ proteins and intestinal morphology under nutritional challenge in this study might be related to toll-like receptors (TLRs), which are part of the innate immune system, recognize pathogens and trigger inflammatory reactions [59]. Excessive activation of TLRs can lead to chronic intestinal inflammation, which damages the intestinal mucosa, disrupts tight junctions and increases intestinal permeability [50,60]. EOA, which contain antimicrobial and anti-inflammatory compounds such as thymol and carvacrol, influence signaling through TLRs by reducing exposure to pathogens and attenuating excessive inflammatory responses. This in turn contributes to the maintenance or improvement of tight junction protein expression and intestinal morphology [45]. While PRO enzymes support gut health by improving protein digestion, which helps maintain tight junction integrity and enhance gut morphology [26]. Efficient protein breakdown prevents excessive stress on TJ proteins and reduces gut inflammation, leading to better gut barrier function and healthier intestinal structure [26,50].
In this study, the lower putrescine levels in the EOA, PRO and EOA+PRO groups compared to the WD group may be due to the suppression of putrefactive proteins and microbes in the gut. Previous studies have shown that the combination of essential oils and organic acids reduces the prevalence of pathogenic bacteria such as Clostridium perfringens, Escherichia coli and Salmonella spp., while beneficial bacteria such as Lactobacilli increase [10–12]. This change in microbial composition could explain the lower putrescine levels observed. In addition, the improved protein and amino acid digestibility in birds fed PRO-containing diets may have limited the nutrients available for microbial growth, thereby reducing microbial metabolites [61, 62]. Several studies have also found a decrease pathogenic microbial populations such as in Clostridium perfringens, Escherichia coli and Salmonella spp. in the ileum of broilers fed diets containing PRO [62–64]. Park and Kim [65] found that the combined effect of essential oils and PRO on reducing ammonia emissions may be due to their role in enhancing nitrogen retention, although this combination did not show a synergistic effect on growth performance or bacterial counts.
Volatile fatty acids are associated with microbial fermentation in the hindgut [66]. Low quality dietary proteins can increase the content of VFA in the cecum. For example, Meyer et al. [39] reported that the addition of feather meal at 5% increased the propionic acid concentration in the ceca of laying hens. The use of corn gluten [67] or DDGS [14] as a protein source in broiler feed increased propionic acid and butyric acid level in the ceca. Yang et al. [28] showed a significant increase in butyric acid with a tendency to increase acetic acid and total short-chain fatty acids in the ileal contents of the EOA-supplemented group compared to the antibiotic group, with no significant difference observed compared to the control group. It was anticipated that dietary treatments or feed additives would modify the microbial substrate, thereby altering VFA levels in the ceca. However, in the present study, no significant effects of dietary treatments on cecal VFA levels were observed. This lack of effect may be attributed to the low inclusion level of the essential oils blend at 300 mg/kg, which might not have been sufficient to induce detectable differences in cecal VFA concentrations. In contrast, a study by Ceylan et al. [68] demonstrated that higher levels of essential oils, at 700 or 1,200 mg/kg, significantly increased cecal acetate, propionate, butyrate, and total short-chain fatty acid concentrations in broilers.
The non-significant differences in VFA levels observed in our study could also be related to the high absorption rate of VFAs in the lower intestinal tract. VFA absorption in the ceca occurs rapidly, reducing existing VFA concentrations and facilitating the renewal of cecal contents [69]. Over 95% of VFAs produced from fermentation are ionized at the prevailing pH of the large intestine and are actively absorbed by Na+-coupled monocarboxylate transport proteins (SMCT1). Meanwhile, the non-dissociated form is transported by the H+-coupled low-affinity monocarboxylate transporter protein (MCT1) [69,70]. Both transporters function concurrently in poultry to maximize VFA absorption across a wide range of lumen pH levels [71,72].
CONCLUSIONS
In summary, dietary supplementation with a combination of EOA and PRO improved growth performance by improving feed efficiency in broiler chickens fed a high wheat and corn DDGS diet. This improvement was accompanied by better gut health as evidenced by reduced jejunal crypt depth, increased VH/CD ratio and increased mRNA expression of the tight junction protein claudin-1. In addition, both the combined treatment with EOA and PRO and the individual EOA and PRO supplements significantly reduced putrescine levels in the hindgut. Further studies are recommended to better understand the actual mechanism of action of these changes in the gut of broiler chickens.
