INTRODUCTION
While antibiotics have been continuously used to improve animal productivity, the overuse of antibiotics in animal, the environment, and human may continue [1,2]. This can lead to antibiotic resistance and side effects that make the proper treatment of disease impossible [3]. A feed additive needs to be developed that has an effect on animal growth that can replace that of antibiotics [4]. Interest in safe animal products and demand for antibiotic-free animal production is increasing [4,5]. With the expectation of improved growth performance, high quality and safe animal products, and disease prevention, the demand is rapidly increasing among researchers and consumers for multifunctional feed additives that combine animal food by-products, phytogenics, and probiotics [5–7].
Eggshell waste is considered a potential calcium alternative in livestock production, and is produced in the order of 50,000 tonnes [8]. However, improper disposal of eggshell waste leads to the formation of ammonia, hydrogen sulfate, foul odor, and environmental pollution [9]. During eggshell calcification, approximately (5−6) g of calcium carbonate are deposited in the shell. The mineral composition of the eggshell includes Ca2+, P−, Na+, K+, HCO3−, and Mg2+, all of which are essential minerals that meet the nutritional requirements for the growth and development of both laying hens and broiler [10,11]. The use of eggshell wastes in feed could contribute to the environmental safety, economic efficiency, productivity, and egg quality of laying hens.
Probiotics are live microorganisms, and have been used extensively as feed additives in the livestock industry [5,12]. The most common probiotics are Lactobacillus, Bacillus, Bifidobacterium, Saccharomyces, and Enterococcus; these improve the balance of gut microbes and prevent pathogen colonization, thereby improving growth, FCR, and health. These bacteria produce antimicrobial substances, such as organic acids and bacteriocins, to inhibit pathogenic microorganisms [12,13]. Numerous studies have been reported on the effect of the combination of probiotics and phytogenics on growth performance, immune response, and gut microbiota in chickens [14–16]. Hidayat et al. [6] observed that the combination of probiotic Lactobacillus acidophilus (1.2 mL/day) and 4% phytobiotics (bay leaves, onion peels, and garlic peels) improved ileal histomorphology, ileal protein digestibility, and FCR. Lee et al. [17] reported that the Artemisia Annua fermented with Lactobacillus plantarum improved the Haugh unit value and prevented lipid oxidation of egg for 3 weeks storage, compared to the control and non-fermentation group, which suggesting higher antioxidation activity in the FA group.
Phytogenic substances are derived from plants, such as herbs, spices, and oleoresins, and are rich in bioactive compounds. They have been used as feed additives to improve animal productivity [18,19]. Among the various phytogenics, Schisandra chinensis is well known as a high polyphenolic compound that has antioxidant, antimicrobial, antiviral, anticancer, and anti-inflammatory effects, and produces a substantial amount of the S. chinensis pomace [20]. Schisandra chinensis pomace contains higher levels of fiber, polyphenols, lignans, vitamins, and minerals than S. chinensis fruit, due to the concentration of these compounds during processing of the S. chinensis [21,22]. In addition, several studies have reported the effect of S. chinensis and pomace supplementation on improving the antioxidant activity, immunity in laying hens and physicochemical properties, and meat color stability in broilers [23–26].
Many studies and applications have explored the use of probiotics and phytogenics as feed additives to improve productivity. However, few studies have investigated the effects of eggshells, S. chinensis by-products, and multi-probiotics on the laying performance and health of laying hens. Each of the feed additives—eggshell, S. chinensis by-products, and probiotics—has a distinctive nutritional value and a range of metabolites with beneficial physiological activities. The combined nutritional and functional benefits of these additives are hypothesized to positively influence the productivity, blood profile, and gut health of laying hens. Therefore, this study aims to investigate the synergistic effects of these novel additives to enhance the productivity, egg quality, blood characteristics, visceral organs, tibia properties, and gut microbiota.
MATERIALS AND METHODS
The eggshell (ES) was produced and supplied by Poonglim Food. Briefly, ES membranes were removed by washing with water, followed by heating at 150°C for 12 h, and then the ES were crushed to a particle size of 1−5 mm using a hammer mill (SM−D3, Wilhelm Siefer GmbH). Schisandra chinensis by-products (SC) were obtained from a juice factory, Omija Valley, sun-dried for 24−48 h, and stored at 4°C, until use. Table 1 shows the source and composition of the feed additive, Biocalcium® (Hanong). The dietary supplement, combination of eggshell waste, Schisandra chinensis by-product, and multi-probiotics (ESM) consisted of 40% Eggshell, 5% by-products of S. chinensis, and 109–1011 CFU/g of Multi-probiotic strains, including Bacillus subtilis, Bacillus licheniformis, Saccharomyces cerevisiae, Lactobacillus plantarum (isolated from ES and SC), and supplemental nutrient premix with phytase.
A total of 216 Hy-line Brown hens at 70 weeks of age were assigned to four dietary treatment groups: basal diet (control); basal diet+0.1% ESM; basal diet+0.2% ESM, basal diet+0.4% ESM. Each treatment consisted of nine replicates, with six birds each. All hens were housed in three-tier battery cage with two birds in each cage (43 × 45 × 42 cm, length × width × height). The basal diet used in this experiment was formulated with nutrient levels that meet the requirements of the 2017 Korean Poultry Feeding Standard (Table 2). The appropriate amount of ESM was added to the basal diet, and mixed for 5 min using a feed mixer (DKM 350SU, Daekwang). After a 2-week adaptation period to the basal diet, the experimental diets were fed for 4 weeks of the experimental period. Food and drinking water were provided ad libitum throughout the entire experimental period. An automatic lighting controller was used to maintain a 16 h of light and 8 h dark period, and the temperature was maintained at (22 ± 3)°C. At the end of the experiment, hens were fasted for 18 h, prior to sampling. One bird per replicate was randomly selected and euthanized with carbon dioxide for evaluation of the blood, organ, and tibia characteristics.
Vitamin mixture provided the following nutrients per kg of diet: vitamin A, 20,000 IU; vitamin D3, 4,600 IU; vitamin E, 40 mg; vitamin K3, 4 mg; vitamin B1, 3.6 mg; vitamin B2, 8 mg; vitamin B6, 5.8 mg; vitamin B12, 0.04 mg.
The number of eggs laid by birds in each replicate was recorded daily at 10 am, and expressed as the percentage of egg production. The hen–day egg production rate (EPR) is calculated by dividing the total number of eggs collected by the number of live hens daily in each replicate [27]. The total number of eggs produced in a day was weighed collectively for each replicate, and used to estimate the average egg weight (AEW). Daily egg mass was calculated by multiplying the EPR by the AEW. Feed intake (FI) was measured weekly once per replicate, weighing the amount of feed distributed and that of residual and scattered feed. The Feed conversion ratio (FCR) was calculated from the FI and daily egg mass [28].
Twenty-seven eggs per treatment (3 eggs per replicate) were randomly selected after each week, and analyzed for their quality on the same day of collection. Egg quality characteristics, including Haugh unit, albumen height, yolk color, eggshell weight, eggshell strength, and eggshell thickness were determined using an automatic egg analyzer (Digital egg tester DET6000, NABEL). The Haugh unit (HU) was calculated using the following equation: HU = 100 × Log [H + 7.57 – (1.7 × W0.37)], where H is the albumen height (mm), and W is the egg weight (g) [29].
At the end of the experiment, one bird (75 weeks of age) per group of replicates was randomly selected, and euthanized by CO2 injection. After euthanasia, approximately 8 mL of blood was collected by cardiac puncture. The collected blood was kept refrigerated in a clot activator tube (CAT). Serum was separated from the blood sample in the CAT tube by centrifugation at 1,500 for 10 min using a centrifuge (HA-1000-3, Hanil Science Medical). The separated serum was stored at −20°C for observation of the biochemical properties. Serum concentrations of aspartate aminotransferase (AST), alanine aminotransferase (ALT), blood urea nitrogen (BUN), triglycerides (TG), lactate dehydrogenase (LDH), total cholesterol (TC), high-density lipoprotein (HDL; mg/dL), HDL (% total), low-density lipoprotein (LDL)+very low-density lipoprotein (VLDL), glucose, total protein (TP), albumin, creatinine, and calcium were determined by automated clinical chemistry analyzer (FUJI DRICHEM 7000i, Fujifilm). HDL (%) was expressed as the ratio of HDL to TC content, and LDL+VLDL was calculated by subtracting HDL from TC [30].
The weight of the visceral organs was determined from the weight of the liver and spleen. This was expressed as a weight ratio per 100 g of live body weight using an electronic balance (EL4002, Mettler Toledo). The intestine was divided into four sections (duodenum, jejunum, ileum, and cecum). The duodenum was measured from the pancreatic loop, the jejunum from the end of the pancreatic loop to the Meckel’s diverticulum, the ileum from the Meckel’s diverticulum to the ileocecal junction, and the cecum as the average of the right and left cecal lengths. The lengths of the four intestinal segments were measured, and expressed as the ratio of the length per 100 g of live body weight.
At the end of the experiment, one bird (75 weeks old) per group of replicates was randomly selected to collect the left tibia, after the removal of non-bone tissues (fat, tendon, and muscle). The tibiae were individually sealed in plastic bags to minimize moisture loss, and stored at 4°C for one day. Tibia length and width were measured using a micrometer caliper, and the weight was recorded. Tibia strength was determined from a 3-point flexural test (ASAE Standards S459, 2001) using an Instron Universal Testing Machine (Model 3342) with a 50 kg load range and a crosshead speed of 200 mm/min; the tibia was supported on a 4 cm span [31].
Bone mineral density (BMD) of all the collected tibiae was analyzed by quantitative computed tomography (QCT) at the College of Veterinary Medicine, Konkuk University (Korea, Seoul). Three positions of each tibia including the neck (section of the mastoid arthrodesis), 1/3 of the proximal portion, and 2/3 of the distal portion, were scanned using a CT scanner (LightSpeed Plus, GE HealthCare).
Three birds were randomly selected per treatment, and for each bird, approximately 1 g of the chicken ceca contents was collected, and quenched with liquid nitrogen. PCR conditions, DNA extraction, bioinformatics, and NGS sequencing analysis were performed according to a previously described method [32]. Briefly, a PowerSoil DNA Isolation Kit (Mobio Laboratories) was first used to isolate genomic DNA. The V3−V4 region of the bacterial 16S rRNA gene was then amplified using 341F and 785R primers. Sequencing was performed on the Illumina Miseq platform using the commercial service of Macrogen (Seoul, South Korea). Amplicon sequence variants (ASVs), Chao1, Shannon, and Gini–Simpson indices were checked to compare alpha diversity. Principal coordinate analysis (PCoA) and unweighted pair–group mean average (UPGMA) analysis based on the UniFrac distance matrix were used.
Data was analyzed in a completely randomized design with 4 treatments using the PROC GLM procedures of SAS 9.4 (SAS Institute). The replicate group (9 hens each) was the experimental unit for the analysis of performance data. Egg quality traits were statistically analyzed each week, using the number of eggs as the experimental unit. For blood parameters, organ weight, intestinal length, bone quality measurements, and cecal microbiota, the individual bird was used as the experimental unit. Significant differences between the treatments were determined using Duncan’s multiple range test at p < 0.05. Significance level 0.05 ≤ p < 0.10 was indicated as a trend. Data are presented as the least squares mean and SEM.
RESULTS
Table 3 shows the effect of ESM on laying performance. Laying performance tended to increase in the ESM 0.2% group, compared to the control group (p = 0.08). ESM 0.1% supplementation had a higher egg weight, compared to the control (p < 0.05). ESM 0.1% and ESM 0.2% tended to increase egg mass, compared to control (p = 0.051). There were no differences between the treatments in feed intake and FCR during the experimental period.
Table 4 presents the egg quality characteristics. The Laying hens fed ESM 0.4% group had the highest value for egg yolk color, while the ESM 0.2% group had the lowest value for egg yolk color (p < 0.05). There were no differences in the Haugh units, albumen height, and eggshell characteristics between the treatments during the experimental period.
Table 5 shows the effect of ESM on the blood biochemical parameters of the layers. The TC content was significantly higher in the ESM 0.4% group than in the control group (p < 0.05). There was a tendency for ESM 0.4% to have a higher calcium content, compared to the control (p = 0.059).
CON, control, basal diet; ESM 0.1%, basal diet + 0.1% ESM; ESM 0.2%, basal diet + 0.2% ESM; ESM 0.4%, basal diet + 0.4% ESM.
ESM, combination of eggshell waste, Schisandra chinensis by-product, and multi-probiotics; AST, aspartate aminotransferase; ALT, alanine aminotransferase; BUN, blood urea nitrogen; TG, triglyceride; LDH, lactate dehydrogenase; TC, total cholesterol; HDL, high-density lipoprotein; LDL, low-density lipoprotein; VLDL, very-low-density lipoprotein; TP, total protein.
Table 6 shows the organ weight and intestinal length of laying hens. There were no significant differences between treatments in the relative organ weight and intestinal length.
Table 7 shows the tibia characteristics and BMD of laying hens. There was no significant effect of ESM treatment on the bone weight, length, width, and bone breaking strength. However, there were significant differences in the tibia BMD between treatments. The proximal tibia of laying hens fed ESM 0.4% had higher BMD, compared to the control group and the ESM 0.2% group (p < 0.01). In addition, the ESM 0.4% group had higher total tibia BMD, compared to the control group (p < 0.05).
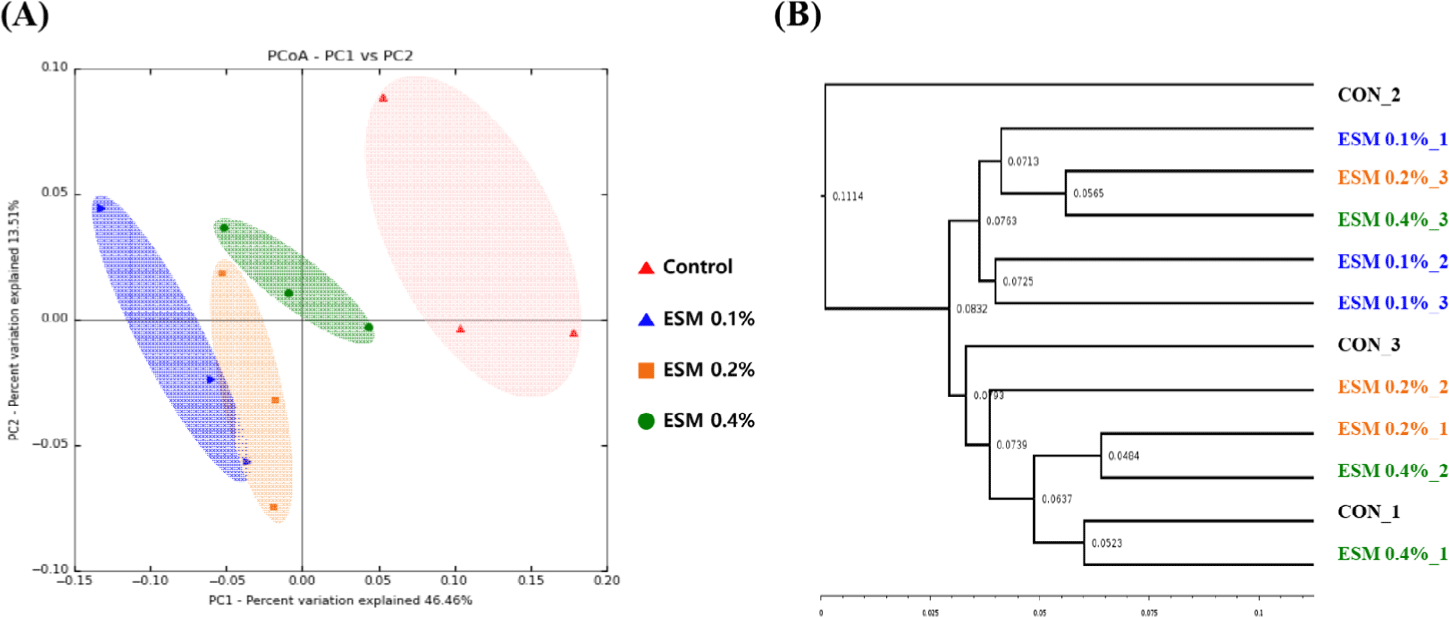
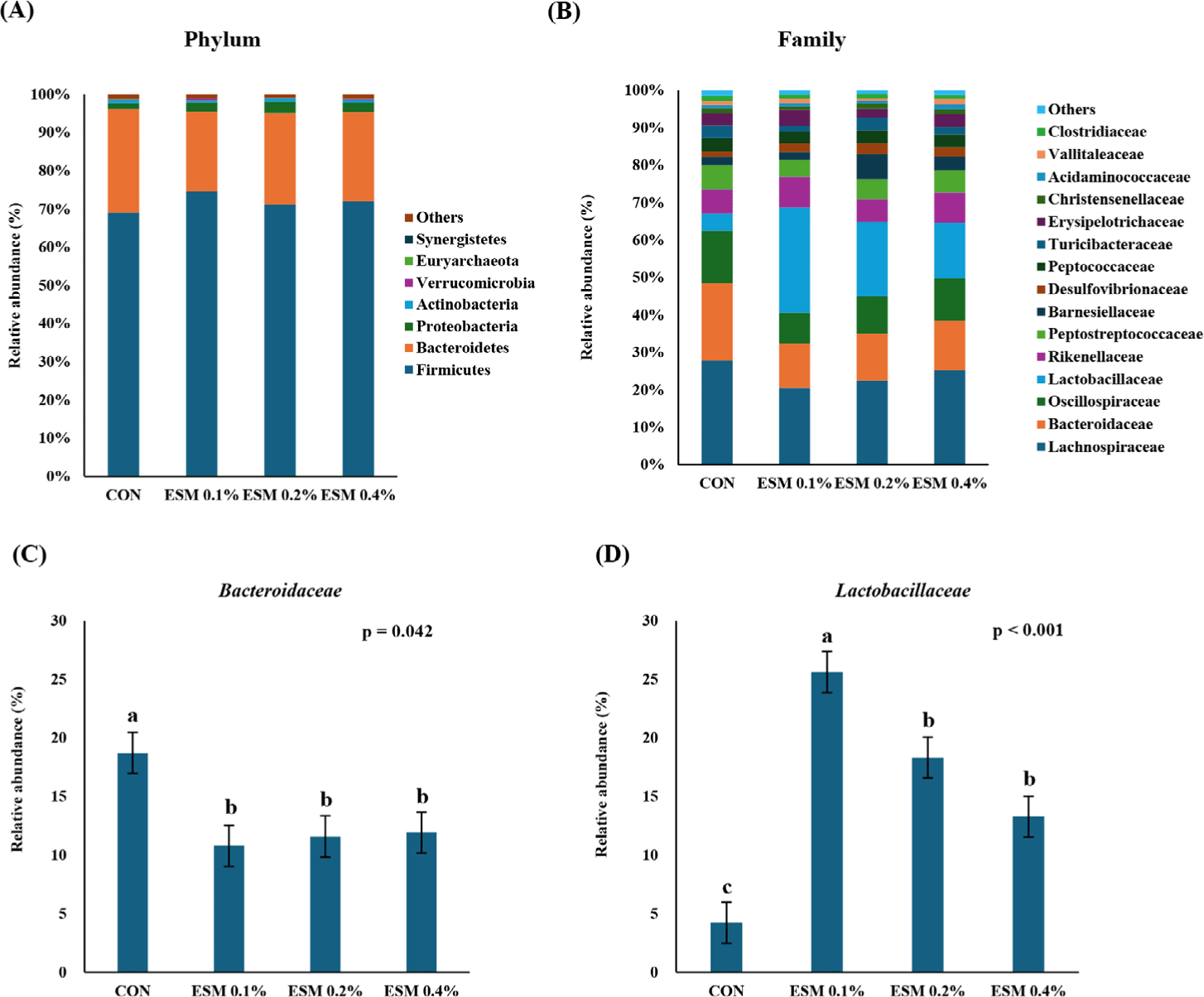
Table 8 shows the alpha diversity indices (ASVs, Chao1, Shannon, and Gini–Simpson) for the cecal microbiota of laying hens. Supplementation with ESM 0.4% showed higher alpha diversity (ASVs and Chao1) than ESM 0.1% and ESM 0.2% (p < 0.05), but ESM 0.4% was not significantly different from the control. Shannon and Gini–Simpson were not significantly different between the groups. Fig. 1 shows the result of the PCoA (beta diversity) and phylogenetic tree analysis representing the similarity of the microbial community. The results demonstrated that microbial communities in the ESM supplementation exhibited distinct clustering patterns compared to the control. Specifically, the ESM group samples were clearly differentiated by their microbial composition, indicating that the feed additive had a significant effect on the gut microbiota. Fig. 2 shows the cecal microbiota. Firmicutes (71.7%) was the most abundant phylum in the cecal microbiome, followed by Bacteroidetes (23.8%) as the second most abundant phylum (Fig. 2A). At the family level, the abundance of Bacteroidaceae was significantly higher in the control groups than in the other ESM groups (p < 0.05). Lactobaillaceae was significantly more abundant in the ESM groups than in the control group. ESM 0.1 showed the highest abundance of Lactobacillaceae (25.63%) (p < 0.01).
DISCUSSION
Interest in environment-friendly feed additives is increasing, and there is considerable research into the effects of probiotics and agricultural by-products on laying hen productivity and health. In this study, the four-week duration of the feeding trial was conducted to evaluate the immediate effects of the feed additive on laying performance in aged laying hens before slaughter. This period was designed to capture short-term impacts on productivity, egg quality, physiological changes, and economic benefits. Aged hens were selected to evaluate their potential for sustained productivity, providing insights into the practical and economic benefits of using feed additives to enhance performance in older birds.
In this study, supplementation with ESM improved egg weight and egg mass during the experimental period. Lee et al. [33] reported that supplementation with eggshell coarse (ESC) improved the egg weight, egg mass, and FCR, compared to other calcium source treatments. Similarly, eggshell meal supplementation increased the average egg weight, egg mass, and FCR, compared to bone meal treatment, or the inclusion of eggshell meal and bone meal [34]. The main composition of eggshell is calcium. Eggshells also have high protein concentrations, due to the egg membranes. Appropriate calcium supplementation can produce stronger eggshells and help to reduce the production of soft-shelled or shell-less eggs, thus improving the laying performance and FCR [35,36].
Multi-probiotics are known to contain bioactive compounds and secondary metabolites, and have been used as a potential feed additive [12,13,37]. Many studies have reported that supplementation with multi-probiotics improved egg productivity, egg weight, egg mass, and FCR. Ma et al. [23] reported that either 1% Ligustrum lucidum or Schisandra chinensis supplementation improved egg production and FCR to laying hens (57 weeks of age). In contrast, body weight and FCR in layer chicks were not affected by either 1% Ligustrum lucidum or S. chinensis treatment [38]. Some studies reported that S. chinensis and probiotics made no significant difference in laying performance [39,40]. The discrepancy in outcomes may be attributed to the impact of various factors on productivity, including age, diet, fermentation method, and farm environment.
The findings of this study indicate that ESM 0.1% and 0.2% are associated with an increase in egg mass. The observed increase in egg mass associated with ESM may be attributed to various physiological mechanisms. The bioactive compounds in ESM may improve gut health or enhance nutrient absorption, thereby increasing the effective use of nutrients for egg formation. However, it is important to acknowledge some limitations of this study. The research was conducted within a specific farm environment, which may limit the generalizability of the results. Further studies in diverse farm settings are needed to confirm these findings, and the long-term effects of ESM supplementation at different stages of the laying cycle should also be investigated. Such additional research would provide a more comprehensive understanding of the effects of ESM on egg production.
Eggshell powder contains protein and minerals (Ca, Fe, Mg, Zn, Cu, and Se), and the balanced mineral content of the diet can influence egg quality. Among the minerals, calcium plays an important role in eggshell formation and increases eggshell strength [10,41,42]. Several studies have shown that eggshell powder with high calcium content can improve eggshell quality. Lee et al. [33] showed that supplementation with eggshell coarse improved egg weight among dietary treatments. The oyster shell or eggshell coarse group had a higher albumen height than the cockle shell group, and egg yolk color was the highest in laying hens fed eggshell fine. Kismiati et al. [43] observed that a 7.5% eggshell flour group or mixture of 5% eggshell flour and 2.5% limestone increased eggshell weight. This suggests that increasing the concentration of ESM improves egg quality.
In contrast to previous studies, this study found no significant differences between the ESM groups. This lack of effect might be related to several factors, including the possibility that the amounts of eggshell powder in the ESM treatments were insufficient, or that the trial duration was not long enough to observe measurable changes in egg quality. Additionally, the specific physical and chemical properties of the ESM used in our study may differ from those in previous studies, potentially influencing the outcomes.
The general health of laying hens could be assessed by blood analysis. ALT, AST, and LDH are commonly used biomarkers of liver damage in laying hens [44]. In this study, ESM did not affect the levels of ALT, AST, and LDH in laying hens, suggesting that the ESM diet did not adversely affect liver health.
Albumin is a protein produced by the liver, and albumin concentrations can indicate liver and kidney function. BUN and creatinine in blood tests can indicate kidney function and health [45,46]. These are nitrogenous end products of metabolism. A high ratio of BUN to creatinine leads to reduced filtration by the kidneys, due to reduced blood flow to the kidneys [47]. In this study, albumin, total protein, BUN, and creatinine were not affected by ESM supplementation, suggesting that the ESM treatments did not have a detrimental effect on protein metabolism.
The range of serum TC levels can vary depending on factors such as age, diet, and genetics. Several studies reported serum total cholesterol ranges of (107.29−116.67) mg/dL [26], (103.8−157.8) mg/dL [33], amd (157.81−170.53) mg/dL [24] in laying hens, although there was no significant difference between the treatments. In this study, although total cholesterol and LDL+VLDL levels were lower than in other studies, these may not represent a general standard. Therefore, in the present study, no hens died during the experiment, suggesting that the ESM diet was non-toxic, metabolically stable, and had no adverse effects on the health of laying hens.
Changes in the structure and size of organs are related to their development, including gut immunity and digestive function, and can be used to assess their health status [48]. In general, as the size of an organ increases, the energy required to maintain it increases, reducing the energy available for productivity [49]. In addition, the spleen is small, and is an important lymphoid organ in the immune system. However, infections, liver and blood diseases, and a rapid immune response can lead to an enlarged spleen [50]. The liver is a large organ responsible for toxin removal, digestion, metabolism, and immunity. An increase in liver size is a sign of health problems, such as fatty liver disease, hepatitis, and cancer [51,52].
Kim et al. [24] observed that 2% S. chinensis supplementation showed the lowest liver weight and abdominal fat, but S. chinensis treatments had no effect on spleen weight. Supplementation of whole hatchery waste meal including eggshell showed no significant difference in abdominal fat and internal organs (liver, lung, heart, and gizzard) in broiler [53]. This indicated that eggshell powder might not be affect the organ characteristics.
In contrast, our study revealed that ESM supplementation did not significantly impact organ characteristics. These findings suggest that ESM supplementation does not negatively affect organ characteristics, indicating its safety with respect to organ health. Additionally, it is possible that the bioavailability or the specific components of ESM were insufficient to elicit measurable changes in organ characteristics under the conditions tested. Further research could explore different dosages or durations of ESM to determine whether any conditions might reveal potential benefits or effects on organ characteristics.
Recently, there has been increasing interest in improving BMD and bone quality in laying hens. The bones of laying hens play an important role in mobility, productivity, and overall health. Calcium is an essential component of bone, and this influences bone quality and breaking strength [54,55].
Several studies have reported the effect of eggshell powder supplementation on tibia bone characteristics and BMD. Lee et al. [33] observed that supplementation with oyster shell or coarse eggshell particles showed higher BMD in the proximal, distal, and total tibia. Kismiati et al. [43] found that 5% eggshell flour supplementation had the highest calcium rate in the tibia, while eggshell flour had no effect on the tibia length and weight. Similar to previous studies, this study showed that when ESM 0.4% was fed to laying hens, total and tibial neck BMD were improved. Eggshell powder is known to have a high calcium content, so supplementation with eggshell may have an effect on BMD increase and bone quality in laying hens.
The gut microbiota plays a critical role in maintaining overall health and influencing digestive system health, immunity, and resistance to pathogens. In this study, analysis of alpha diversity metrics, including ASVs and the Chao1 index, showed that supplementation with ESM 0.4% increased microbial diversity in the cecum compared to ESM 0.1% and ESM 0.2%, although not significantly compared to control. ASVs provide high-resolution insights into the composition of microbial communities, and highlight the diversity and possible functional roles of the microbiota [56]. Similarly, the increased Chao1 index indicates richer species diversity [57], suggesting a more complex and potentially resilient ecosystem under ESM 0.4% treatment.
PCoA is used to determine the beta diversity analysis. PCoA plays a critical role in assessing variation in species composition across samples, and provides valuable insight into the effects of dietary interventions on microbial community structure [58]. In this study, PCoA showed that ESM groups had a more similar composition of cecal microbiota, compared to the control. This suggests that ESM groups influence the composition of the gut microbiome.
Furthermore, Firmicutes and Bacteroidetes were the most abundant strains in the cecum of laying hens in the study presented. ESM supplementation increased the relative abundance of the Lactobacillaceae family of Firmicutes, and decreased the Bacteroidaceae family of Bacteroidetes. This suggested that ESM supplementation increased the Lactobacillaceae family, while decreasing the composition of Bacteroidaceae in the cecum. Ren et al. [16] reported that combinations of phytobiotics and probiotics increased the lactobacilli and decreased ESBL-producing E. coli in the gut of young broiler chickens. These lactic acid bacteria are known to have many beneficial effects, including stimulating the immune system, producing lactic acid, inhibiting the growth of pathogens, and contributing to the overall health of laying hens [58–60]. Therefore, it is proposed that the ESM intervention alters the structure of the cecal microbiota and increases its diversity in the gut. ESM supplementation may be a promising strategy to enhance gut health by improving the balance of gut microbiota.
An improvement in gut microbiota is closely linked to enhanced immunity, digestive efficiency, and overall poultry productivity [61,62]. A growing body of evidence indicates that modulation of the gut microbiota can exert a beneficial influence on a number of key aspects of poultry production, including growth, feed efficiency, immune function, and disease resistance [63]. [64] reported that fermented plant product interventions can improve productivity, egg mass, Haugh unit, gut health, and alter the cecal microbial community in laying hens. Therefore. These findings emphasize the crucial role of gut microbiota in supporting poultry health and productivity.
CONCLUSION
Supplementation with ESM resulted in significant increases in egg weight, egg mass, tibial BMD, and cecal microbiota diversity. In addition, ESM did not affect blood characteristics or visceral organ properties, suggesting that it does not adversely affect the overall health of laying hens. Notably, ESM has not previously been studied as a feed additive for poultry, which may highlight its novel application. The observed improvements in egg weight, bone health, and microbial diversity underscore the potential value of ESM as a beneficial feed additive to improve egg performance and gut health in laying hens.
