INTRODUCTION
Antibiotics have long been used to prevent bacteria-related diseases while improving feed efficiency in the livestock industry [1,2]. This antimicrobial substance is active mainly against bacteria and protozoa and suppresses bacterial infections in animal bodies [3]. Despite the multiple positive effects of antibiotics from an animal disease control perspective, several emerging concerns regarding antibiotic-resistant and residue issues make an appearance. Various antibiotics, including penicillin, tetracycline, macrolide, aminoglycoside, and amphenicol, have been detected in animal products [4]. There is also an emerging issue with new types of antibiotic-resistant strains in recent years that have become prevalent in hospitals [5]. Therefore, increasing numbers of countries, including the European Union, banned antibiotics usage as feed additives [6,7]. South Korea has also restricted antibiotics usage in feed except for therapeutic purposes. In light of increasing concerns regarding antibiotic resistance and potential residues in animal products, there is a growing need to identify effective alternatives to antibiotics that can improve feed efficiency and growth performance in beef cattle without posing risks to human health. Several compounds have been proposed as promising alternatives, including probiotics, silicate agents, organic acids, herbal extracts, and propolis [8].
The phytoncide was defined as the secreted plant materials with disinfectant pathogens, pests, fungi, or emits to resist [9]. As a mean of suppressing activity of microorganisms, such as bacteria and fungi, relatively large amounts of phytoncide were released by Korean pines and other conifers into the environment [10]. There was the effect of stress reduction in a Human study [11]. The main component of phytoncide was consist of terpene and content with phenol compounds or alkaloids. The physiological activity of terpene in the plant was increased phytochemical activity in the tree and interfered with the growth of other plants. It was also known to effectively suppress inflammation make a tumor grow [12,13]. Although cypress (CYP) used in the study was known as the most abundant phytoncide in the plant, there was not many certain features or mode of action in the body were unknown. It is previously reported that mugwort (MUG), an aromatic perennial plant, possesses therapeutic activity such as sterilization, analgesic, anti-inflammatory, immune function improvement, and cholesterol-reduction effects in humans. In addition, the cineole component abundant in MUG is reported to kill or inhibit the growth of Escherichia coli [14].
This study was conducted experimental procedure of lipopolysaccharide (LPS) challenge after 90 days of CYP and MUG feeding trials. Lipopolysaccharide is materials located on the outer membrane in peptidoglycan surface of Gram-negative bacteria. Treatment of LPS in this study was induced by an endotoxin, which was simultaneously activated inflammatory response of Hanwoo cattle. The treatment of LPS challenge has been used as efficient testing method to analyze acute immune response for understanding a broader range of physiological and immune markers of beef cattle [1,15]. Previous studied indicated that LPS challenge was regulated by diets, breeds, and weaning age of beef cattle and ultimately affect the growth of beef cattle [16,17]. In this study, LPS challenge was conducted to evaluate immune responses of Hanwoo bulls treated with a natural substance of CYP and MUG byproducts.
MATERIALS AND METHODS
The National Institute of Animal Science Animal Care and Use Committee approved all procedures involving the use of animals for the present study (NIAS, Project No. PJ010065). The experiment was conducted at the Hanwoo Research Institute, National Institute of Animal Science at Pyeongchang, South Korea. Hanwoo bulls (n=15), initial bodyweight (BW) of control 196.00±5.33 kg, treatment 1 (CYP) 196.00±3.91 kg, treatment 2 (MUG) 196.00±3.31 kg were used for the experiment for 90 days. A randomized complete block design was used to evaluate the effects of supplemental CYP and MUG on animal performance (Fig. 1).
Animals were blocked into three groups based on BW and assigned into pens (5 heads per pen).
Treatments were included:
Hanwoo bulls were fed concentrate diets twice a day (08:00 AM and 16:00 PM) and allowed to access forage diet ad libitum. Water and minerals were to be consumed at any time. Commercially formulated concentrate and rice straw were fed for fattening Hanwoo bulls. The composition of diet was collected each time as 2 kg and analyzed as AOAC methods (Table 1). Dry ground CYP and MUG as natural phytoncide additives were stored and used in feeding trials. Bulls were gradually acclimated from an early fattening diet to a middle fattening diet with topdressing dried CYP and dried MUG (0.5 mg/kg) or without treatment (control). BW was collected using a scale (CAS Korea, Newton HT-501A) before the morning feeding on days 0, 25, 52, and 90 of the feeding trials.
| Item | Concentrate | Rice straw |
|---|---|---|
| DM | 90.52 | 91.43 |
| CP | 14.08 | 4.39 |
| EE | 4.80 | 2.36 |
| CA | 9.41 | 13.07 |
| NDF | 28.05 | 70.21 |
| ADF | 11.10 | 38.13 |
Blood samples were collected at day 0, 25, 52, and 90 relatives to treatments. All samples were collected in the morning before feeding. Approximately 10 mL of blood was collected from the jugular vein of each cow in Vacutainer tubes (Becton Dickinson). Serum was stored at −80°C for subsequent analysis of albumin (ALB), glucose (GLU), total protein (TP), triglyceride (TG), phosphorus (IP), and non-esterified fatty acid (NEFA) and analyzed with a serum analyzer (Hitachi 7020, Hitachi High-Tech).
Following 90-day of dried CYP and MUG supplement, 15 bulls were transported to dirt-lot pens. Animals were allowed to access water ad libitum overnight.
The 5 bulls were assigned to each treatment:
After each bull was housed in a pen, a bull was fitted with jugular vein catheters. A small 1-2 cm incision was made in the skin and installed indwelling jugular catheters (16 G, 1.72 mm, JungWon Medics) consisting of approximately 1 m of sterile Tygon tubing with heparin contained saline. Blood samples were collected into blood tubes with no additives every 0.5 hour beginning 1 hour before and continuing 5 hours after administration of LPS (1 μg/kg BW of LPS from E. coli O111:B4; Sigma-Aldrich). Approximately 10 mL of blood was collected from the jugular vein of each cow in Vacutainer tubes (Becton Dickinson). Blood samples were centrifuged to collect serum samples, which were stored at −80°C until metabolites (ALB, GLU, TP, TG, IP, and NEFA) and proinflammatory cytokines (IL-1β, IL-6, and TNFα) test. Serum cytokines were determined by triplicated serum aliquots on 96-well microtiter plates with a colorimetric ELISA assay following the manufacturer’s guidance (Cusabio).
Data was analyzed in a randomized complete block design with PROC MIXED (SAS Institute). The model for the effect of treatments included the fixed effect of treatment, time, and treatment x time interaction. Each Hanwoo bull served as experimental unit for all performance, serum metabolites, and proinflammatory cytokine data. Treatment means were separated by a significant value as (p < 0.05) or a tendency value as (0.05 < p < 0.10) with the LSD procedure of SAS.
RESULTS AND DISCUSSION
There was body weight and ADG increase during 90 days of feed with both dried CYP and MUG (Table 2). However, there was no treatment effect or time x treatment interaction during the feeding trial of Hanwoo bulls. The average age of cattle was 15 months, and these fattening periods typically have dramatically increased body weight in beef cattle. Previous research indicated that there was numerically different bodyweight but not significant with chromium diet for 56 days [16]. These data indicated that the treatment of natural substance for 2–3 month was not enough time for changing body weight of beef cattle. Typical ADG of 15 month-old Hanwoo steer have 0.75–0.8 kg but our result shown 1.2–1.7 kg [18]. The difference of ADG of cattle may come from the difference between bull and steers.
There was time difference in the serum parameters during 90 days of feeding at Hanwoo bulls (p < 0.05; Table 3). Level of ALB has time and treatment interactions (p < 0.05). According to the serum data, serum ALB was decreased both CYP and MUG treatment (p < 0.05). The level of serum parameters was used as indicators for predicting nutritional status during fattening periods of beef cattle [19,20]. Serum ALB was synergistically increased with synthesis of serum protein [21]. Serum ALB level in bulls were lower than those level of steers [22]. Our data indicated that serum ALB and TP were decreased during 90 days of feeding. This result supported previous researches that ALB and synthesis of protein were synergistic regulation in bovine blood. The level of serum TG was increased during 90 days of feeding (p < 0.05). Previous studies have reported that blood TG levels increase as growth progresses due to the increased intake of concentrate (grain-based) feed [23], and the present study showed similar results. According to the level of serum GLU, TG, TP, IP, NEFA in the blood, CYP and MUG diets were not affected the level of serum glucose and lipid metabolites (p > 0.05).
After feeding trials for 90 days, 15 bulls were housed at each pan and installed jugular vein catheters for 24 hours. Serum metabolites, cortisol, and proinflammatory cytokine were analyzed before and after injected LPS (Figs. 2 and 3). Serum ALB was known as negative indicator when beginning of inflammatory reaction in Human body [24]. Level of serum ALB was numerically decreased after LPS injection (p > 0.05). The redues of ALB ensured that the pathological condition was caused after LPS injection. A similar pattern in serum ALB levels was observed in Hanwoo heifers subjected to an LPS challenge, consistent with the findings of this study [25]. The CYP showed higher serum ALB levels overall than the CON (p < 0.05).
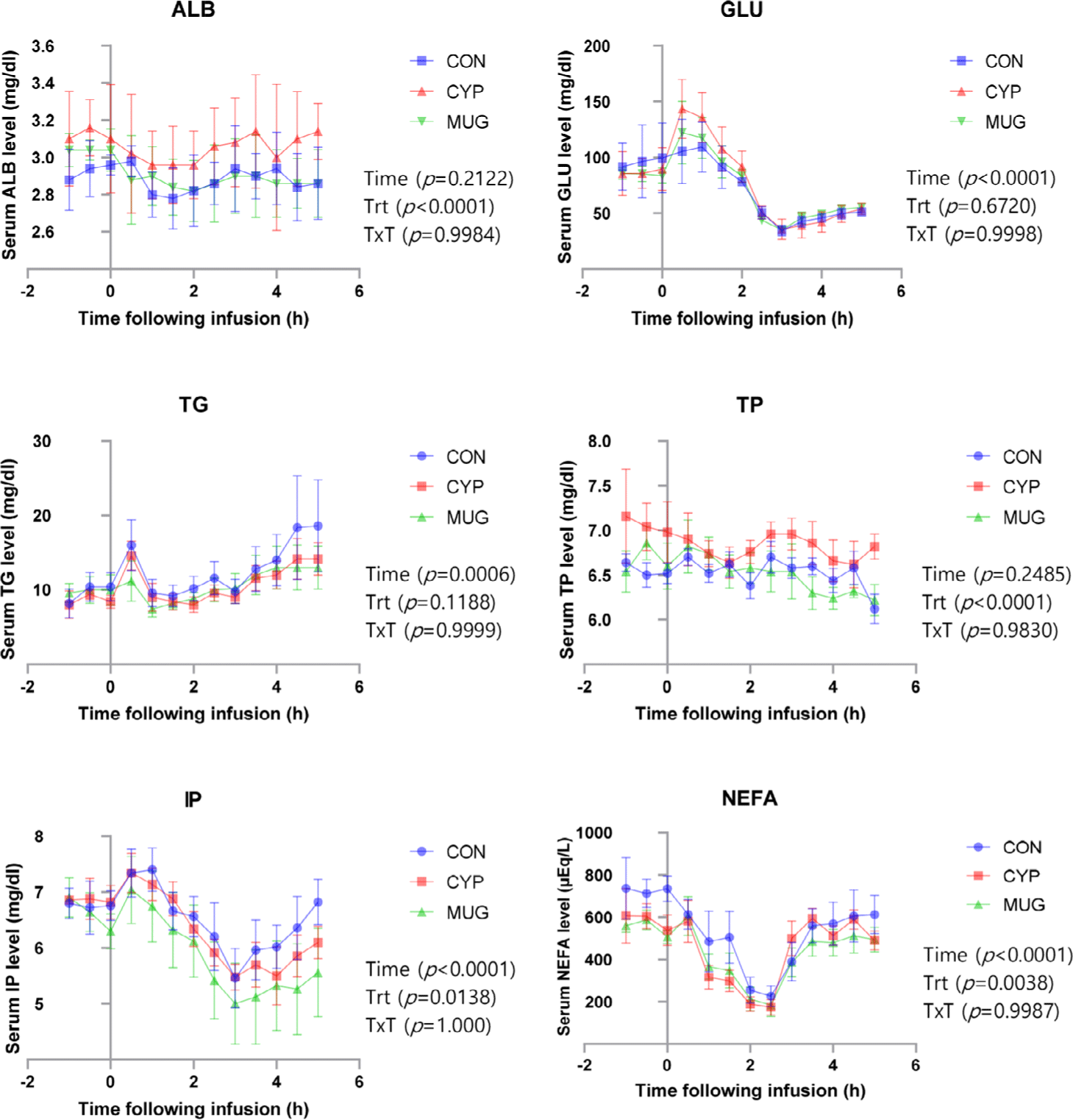
Serum GLU has been used as a metabolic indicator of pathogenic diagnosis or physiological conditions in human or livestock animals [26]. It also used as an indicator substance response with cortisol when animal exposed to stress environment. Previous study indicated that serum GLU was known as physiological indicator to environmental stress such as calf transporting to feedlot system [27–31]. Level of GLU was rapidly increased until 30 min after injecting LPS and decreased to previous level of serum GLU at 3 hours (p < 0.05).
Serum TG has been used as diagnosis of vascular disease and diabetes for human [32]. Previous study indicated that LPS challenge to heifers decreased level of serum TG [33]. In the present study, levels of serum TG showed a transient increase following LPS injection, followed by a return to baseline levels (p < 0.05). Previous studies have reported a positive correlation between serum TG and NEFA levels in Hanwoo steers [34]. However, an LPS challenge conducted in Hanwoo heifers demonstrated a negative correlation between these two metabolites [25]. Similarly, in the current study, a negative correlation between TG and NEFA levels was observed after LPS injection and the onset of disease. To date, research investigating the relationship between serum TG levels and disease in Hanwoo cattle remains extremely limited. Therefore, further studies are required to elucidate the precise association between disease states and serum TG dynamics in Hanwoo. Additionally, there was no difference between the CON and CYP, MUG groups (p > 0.05).
Serum TP has been changed in physiological condition to protein turnover in the peripheral tissues. There was significance at 5 hours after treatment LPS. These results shown similar pattern as serum ALB and this may indicate serum TP related with serum ALB and globulin. The level of IP was increased for 1hours and steady decreased for 3hours after the LPS injections but there was no significant difference (p > 0.05). Serum IP has been known to play a key role in metabolic process in animal body. This study indicated that the level of IP in cattle peaked at 1hours and steady decreased until 3hours after LPS injection. Especially, treatment of MUG was numerically low compare to CYP treatment. This result indicated that the level of IP was increased with immunological treatment under pathogenic conditions. This result may support another LPS challenge study.
Serum NEFA has shown to decreased and recover to original level after LPS injection. Serum NEFA has been known as energy sources which was binding with ALB and transfer to peripheral tissues. In general, levels of NEFA increased under condition with starving, cold, fear, and metabolic disorder [35]. Previous study has been shown that the level of NEFA increased under treatment with LPS treatment [16,36] but this result shown opposite response with LPS injections. Previous research has indicated that serum NEFA levels in ruminants are closely associated with Insulin and GLU levels, as increased insulin secretion following feed intake suppresses lipolysis, leading to a reduction in circulating NEFA [37]. In the present study, serum GLU levels increased following LPS injection, while NEFA levels decreased, consistent with this metabolic relationship. A similar pattern of NEFA reduction after LPS challenge has also been reported in Hanwoo heifers [25]. These results suggest that such a response may represent a breed-specific characteristic of Hanwoo, highlighting the need for further confirmatory studies. Compared to CON, blood NEFA levels were generally lower in the CYP and MUG groups (p < 0.05). Feeding natural phytoncides appears to lower serum NEFA levels.
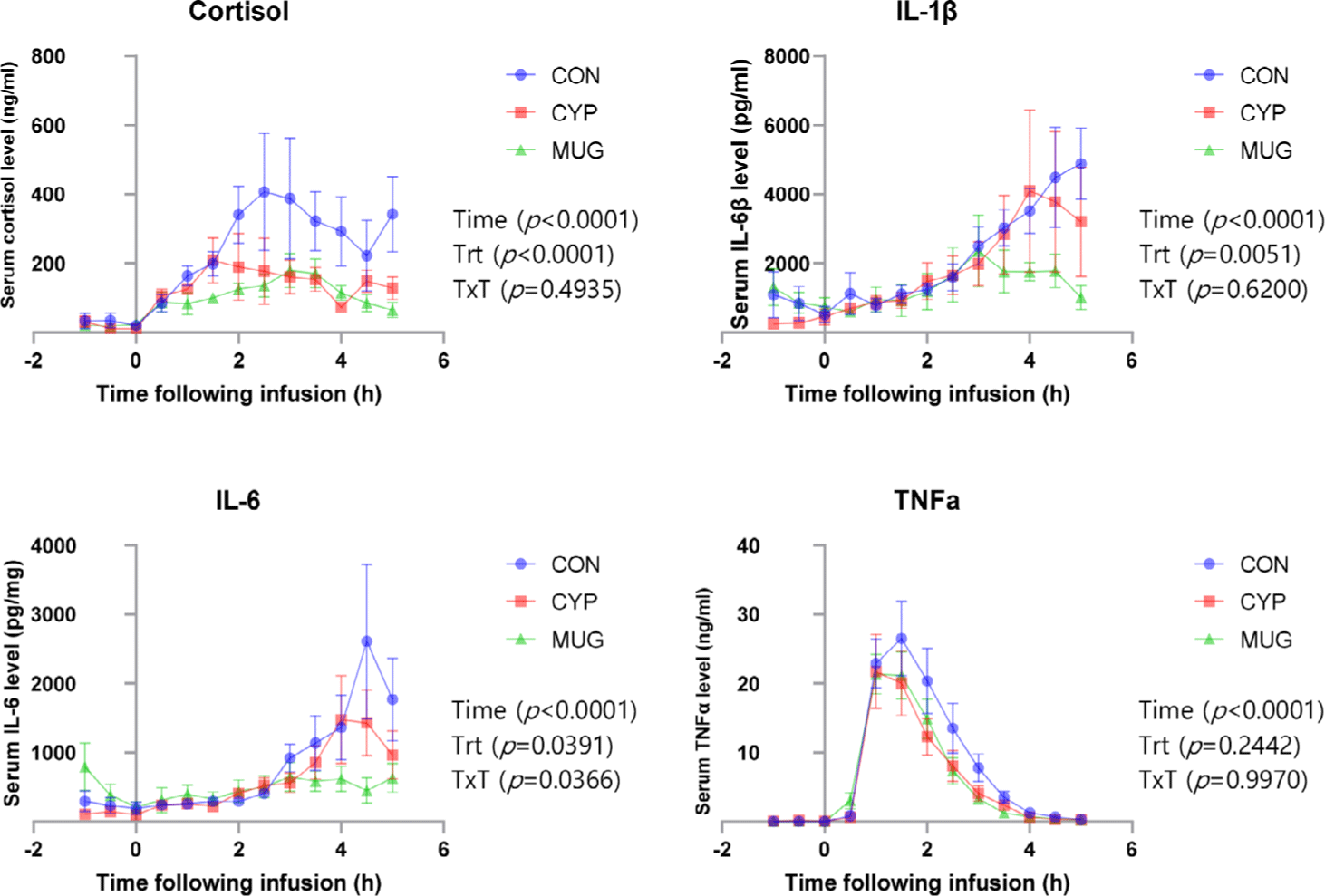
Cortisol level gradually increased between 1 hour 30 min and 3 hours after LPS injection and decreased after 4 hours. Level of cortisol has been demonstrated not only response hormone under acute immune stress but also support a energy for the body against the stress [11,38]. After LPS injection, level of cortisol were increased at control, MUG, and CYP and these results supported previous studies that LPS injection rapidly increased level of cortisol at beef cattle [1,33,39,40]. Previous studies indicated that the level of cortisol was peaked after 2-3 hours after LPS injection in the Angus or Brahman. Our data using Hanwoo bull also shown similar peaked with previous result indicated that there should not be different between cattle breed types [1]. This data also indicated that the level of cortisol have greater concentration in control demonstrated CYP and MUG diet may reduced cortisol secretion in Hanwoo bulls.
Serum cytokines has been reported to regulate immune function, infectious diseases, hematopoietic, and tissue recovery and finally induced not only production of antibodies to the antigen but also control the defense system in body. Thus, the action of neutralization antigen was known to produce an immune factor against pathogenic molecules [41,42]. Previous study indicated that the level of IL-1β after LPS injection was gradually increased until 3 hours then decreased [1]. Crossbred steers (Angus × Brahman) in this study were dramatically increased at 1 hours after LPS injection. However, Hanwoo bulls in this study shown gradually increased 3 hours after LPS injection. Our data also indicated that IL-1β was numerically increased until 5 hours then decreased. The difference of serum IL-1β may be caused by the breed types. In this study, the serum IL-1β in MUG has lower than those of CON (p < 0.05), then this data indicated MUG decreased serum level of IL-1β. However, there was no difference in CON and CYP.
The level of IL-6, similar as IL-1β, numerically increased until 5 hours after LPS injection and CYP has lower than those of CON. Serum IL-6 was known to be an major mediator of fever and acute response [43]. In this study, LPS injection were increased serum levels of IL-6 after 3hours. However, previous study reported that LPS injection was increased serum levels after 1 hour [1,39]. The injection concentration used in previous study was 1 μg/kg, which was same treatment as our trials. Because of same dose in the trials means it may not affect the difference respond in IL-6 level. However, breed types may cause different sensitivity to serum level of IL-6. The response to LPS injection has been slowly increased in Hanwoo bull. The treatment with MUG and CYP decreased serum IL-6 after LPS injection. thus MUG and CYP diet may decrease inflammatory response to expose pathological environment in Hanwoo bull.
The level of TNFα gradually increased from 30 min to 2 hours after LPS injection (p < 0.05). Although there was no treatment affect in serum TNFα, numerically treatment has low level compare to CON (p > 0.05). TNFα has been known as not only reducing virus duplication but also inducing signal-indicate suicide of tumor cells in the body [44]. Previous study indicated that LPS injection dramatically increased TNFα after 30 min [1,33,36,39], our result also supported the response between 30 min to 1 hour of LPS injection to TNFα. The CON reaction compare to treatments has greater peak, this means MUG and CYP diet has been reduced stress in Hanwoo bulls.
CONCLUSION
This study has been used dried CYP and MUG as natural phytoncide additive to feed Hanwoo bulls. Data from the metabolic analysis indicated that CYP supplement enhanced the glucose response to a LPS challenge, but oppositely affect the NEFA and TG response. The CYP and MUG-fed group also reduced serum cortisol, a typical known as stress hormone and tend to increased proinflammatory cytokines compare to control. Currently, Hanwoo industry did not support alternative antibiotics after prohibition of antibiotic supplement at 2009. LPS challenge may supported the technique for determine immune function to Hanwoo cattle and pytoncide such as CYP or MUG should improved immune activity in the Hanwoo industry.

