INTRODUCTION
In the poultry industry, the immune system and growth performance are governed by changes in the bedding conditions. Particularly, weight gain and gut health of poultry are critical to maintain a healthy population [1–3]. Age and environmental conditions also considerably affect microbial communities [4,5].
The suitability of various materials such as the bedding for chickens has been studied previously [6–8]. Growth performance, health, carcass quality, and welfare are directly affected by litter. Rice hulls can be considered a cost-effective litter source that can be used in place of traditional bedding in rice-producing areas. The use of thick sawdust or rice straw did not significantly affect weight gain and carcass weight [9]. Conversely, broilers reared on rice hull had lower weight gain than other groups [10]. All microorganisms were significantly higher in the rice hull treatment, except total yeast. however, body weight gain and mortality did not show statistically significant differences between treatment groups [11].
The avian gut microbiome varies considerably from that of mammalian. Litter, as bedding material, alters the microbial composition and diversity in the cecum of chickens [12]. Moisture promotes the growth of pathogenic microbes and ammonia production, which adversely affect weight and feed conversion in poultry. Additionally, litter supply and the gut microbiome are related to poultry performance [13]. Bacteroides and Eubacteria are established within 2 wk, and gut microbes take 6–7 wk for complete colonization in chickens [14]. The dominant phyla in the cecum of chickens throughout the life cycle are Firmicutes and Bacteroidetes [15–17]. In broiler chickens, gut microbiome colonization and function differ from 1–42 d [18–20].
Changes in gut microbial function and microbial metabolites, such as those of the immune system (cytokines), are simultaneously observed, depending on the litter. However, some studies have found no significant differences in peripheral blood leukocyte counts between cage- and litter laying hens [21]. In general, animals raised in outdoor environments have stronger immune functions [21,22]. Immune functions among animals vary with litter broilers exhibiting higher levels of interleukin-1β (IL-1β) and interferon-γ mRNA than those in caged chickens [12,23–25]. Free-range and semi-stocked chickens demonstrate higher titers of Newcastle disease virus and infectious bronchitis virus in peripheral blood than those in confined chickens.
A recent study on litter has revealed altered microbial composition and diversity in the cecum [26]. However, it is unclear whether litter and litter microbes can influence the cecal microbiota. This study aimed to determine whether litter affects broiler gut microbiota and growth characteristics.
MATERIALS AND METHODS
All animal experiments were approved and reviewed by the National Institute of Animal Science (NIAS) Animal Use and Care Committee (NIAS-2021-0508). All broiler chickens were managed according to the National Research Council specifications. One-day-old broiler chicks (Ross 308) were purchased from a commercial farm and divided into two groups. Each group was assigned to a floor pen (0.93 × 2.14 m). The size of mesh is 2.54 cm by galvanized steel wire, and bedding materials is used rice hulls. The chickens were fed using a graded feeding program (Table 1) consisting of starters (0–7 days), growers (8–21 days), and finishers (22–35 days); water was provided ad libitum. Feed was supplied as small pellets for the start-up phase and as pellets for the growth and finishing phases. The animals were randomly assigned to one of the six replicate pens per treatment. The experimental groups were divided into cage and cage-free groups, according to litter usage. Room temperature was monitored daily. The light-dark cycle was set from 18 to 6 h during the experimental period. All bedding materials are sterilized and UV irradiated. Additionally, all experimental equipment was brought into the room after a sterilized or sterilized products were used. Body weight and feed intake were recorded weekly. The weight gain and feed conversion ratio (FCR) were then calculated. At 7, 14, 21, 28, and 35 days of age, chickens in the treatment groups were euthanized by anesthesia with carbon dioxide. Blood was collected from the carotid artery or wing vein. Cecal digesta were placed in liquid nitrogen and stored at −80°C.
| Items (%) | Starter | Grower | Finisher |
|---|---|---|---|
| Crude protein | 24.15 | 23.47 | 23.01 |
| Crude fat | 9.41 | 6.13 | 4.65 |
| NDF | 9.23 | 12.35 | 8.51 |
| ADF | 4.24 | 3.94 | 3.66 |
| Ash | 8.35 | 5.87 | 6.01 |
Blood samples were collected from the carotid artery or wing vein using ethylenediaminetetraacetic acid tubes (BD Vacutainers, Becton Dickinson). An automated hematology analyzer (Mindray BC-5300, Mindray) was used to assess hematological parameters, such as red blood cell (RBC) count, white blood cell (WBC) count, packed cell volume, hemoglobin (HGB), mean corpuscular volume (MCV), mean corpuscular HGB, mean corpuscular HGB concentration, erythrocyte sedimentation rate, total protein, and absolute counts of heterophils, lymphocytes, monocytes, eosinophils, and basophils, according to the manufacturer’s instructions. Concentration of pro-inflammatory cytokines, including IL-1β, IL-6, and tumor necrosis factor alpha (TNF-α), were measured using commercial chicken enzyme-linked immunosorbent assay kits (EK780087, EK780053, EK780062, AFG Scientific) according to the manufacturer’s instructions.
Metagenomic DNA was extracted from broiler cecal samples using the bead-beating (repeated bead-beating plus column) method [27] via a QIAamp DNA kit (Qiagen).
Artificial sequences and low-quality bases in the generated reads were removed using Trimmomatic and TruSeq3-PE. fa:2:30:10:2:True, LEADING:5, TRAILING:20, MINLEN:250 parameters [28]. After raw data QC, the filtered reads were analyzed using QIIME2 [29]. The remaining adapter sequences in the filtered reads were removed using the cutadapt module in the QIIME2 with --p-front-f CCTACGGGNGGCWGCAG and-p-front-r GAC-TACHVGGGT ATCTAATCC parameters [30]. The denoising step was conducted using dada2, a denoise-paired module in QIIME2, with parameters–p-trunc-len-f 230 and–p-trunc-len-r 220 [31]. Taxonomic assignment was conducted using the classify-sklearn module with a pretrained silva-138-99-nb-classifer. qza as provided by QIIME2 [32]. After taxonomic assignment, taxa assigned to the mitochondria and chloroplasts and those whose assigned level did not represent the minimum phylum were filtered out.
The align-to-tree-mafft-fasttree module [33,34] was used for tree construction of the representative amplicon sequence variant (ASV), and alpha- and beta-diversity were calculated using the diversity module in QIIME2 [35]. For functional pathway prediction of the microbial community, PICRUST2 was employed with a frequency table exported from QIIME2 [36]. Principal Component Analysis (PCA) plots and statistical tests for the predicted pathways were conducted using STAMP with the Kruskal-Wallis test [37]. Differential abundance taxon analyses were conducted using Linear discriminant analysis effect size (LEfSe) [38]. Significant differences in blood results and growth performance were determined at p < 0.05, using Prism ver. 9 software.
RESULTS
The effects of environmental bedding conditions on the growth performance of broiler chickens are shown in Table 2. Final body weight, weight gain, and FCR were higher in chickens housed in cages (without litter) than those in conventional conditions (with litter) for 5 wk (p < 0.01). However, the average daily feed intake did not differ significantly between the conventional and cage groups.
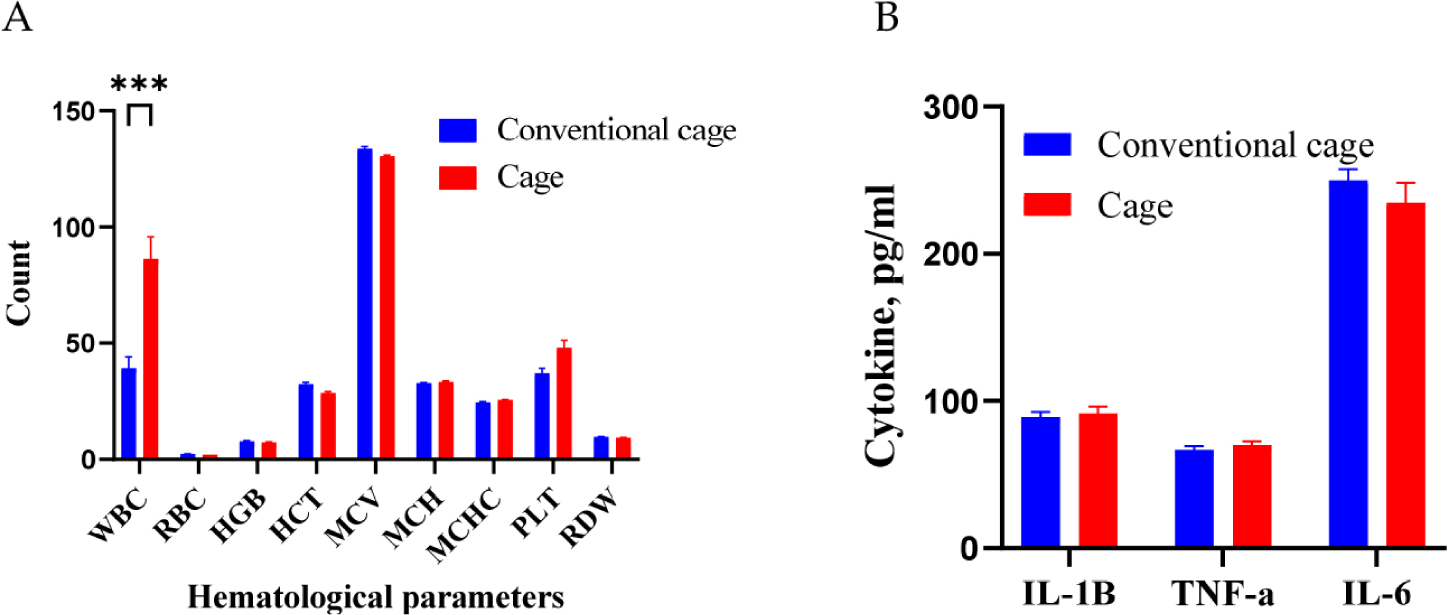
Blood hematological and cytokine analyses were performed for the different bedding environmental conditions (Fig. 1). Under different conditions, the WBC counts were higher in the cage group than those in the conventional group (p < 0.001). The observed increase in WBCs is proposed to represent a defensive mechanism against external disease or inflammation. However, the RBC count, HGB level, and MCV were not significantly different between the two groups. In addition, TNF-α, IL-1, and IL-6 levels were not differentially regulated between the conventional and cage groups.
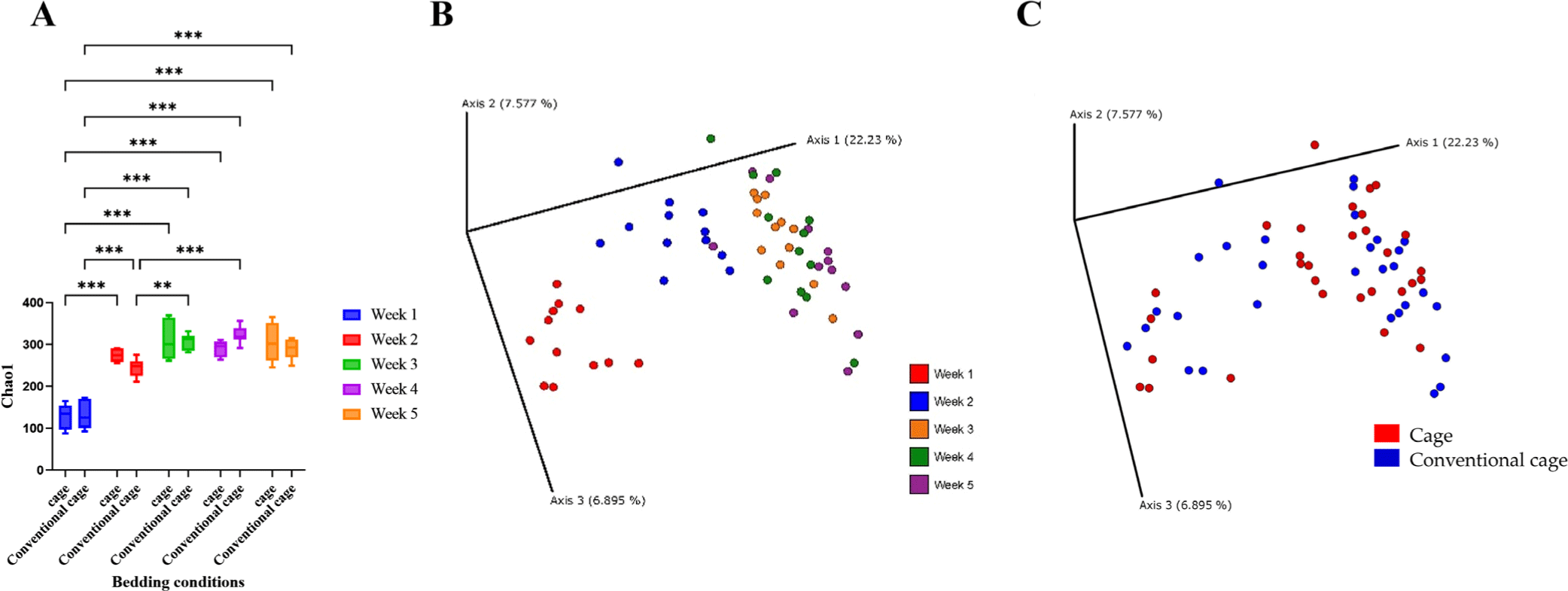
In the broiler cecum, changes in alpha diversity were confirmed over 5 wk (Fig. 2). The alpha diversity was significantly different from 1 to 2 wk. However, the diversities at wk 3, 4, and 5 were similar. In the bedding environment, the alpha diversity was not significantly different. Beta diversity clustered from 1 to 5 wk, similar to the alpha diversity pattern. Beta diversity determined using the Principal Coordinates Analysis (PCoA) plot was independent of the presence or absence of litter.
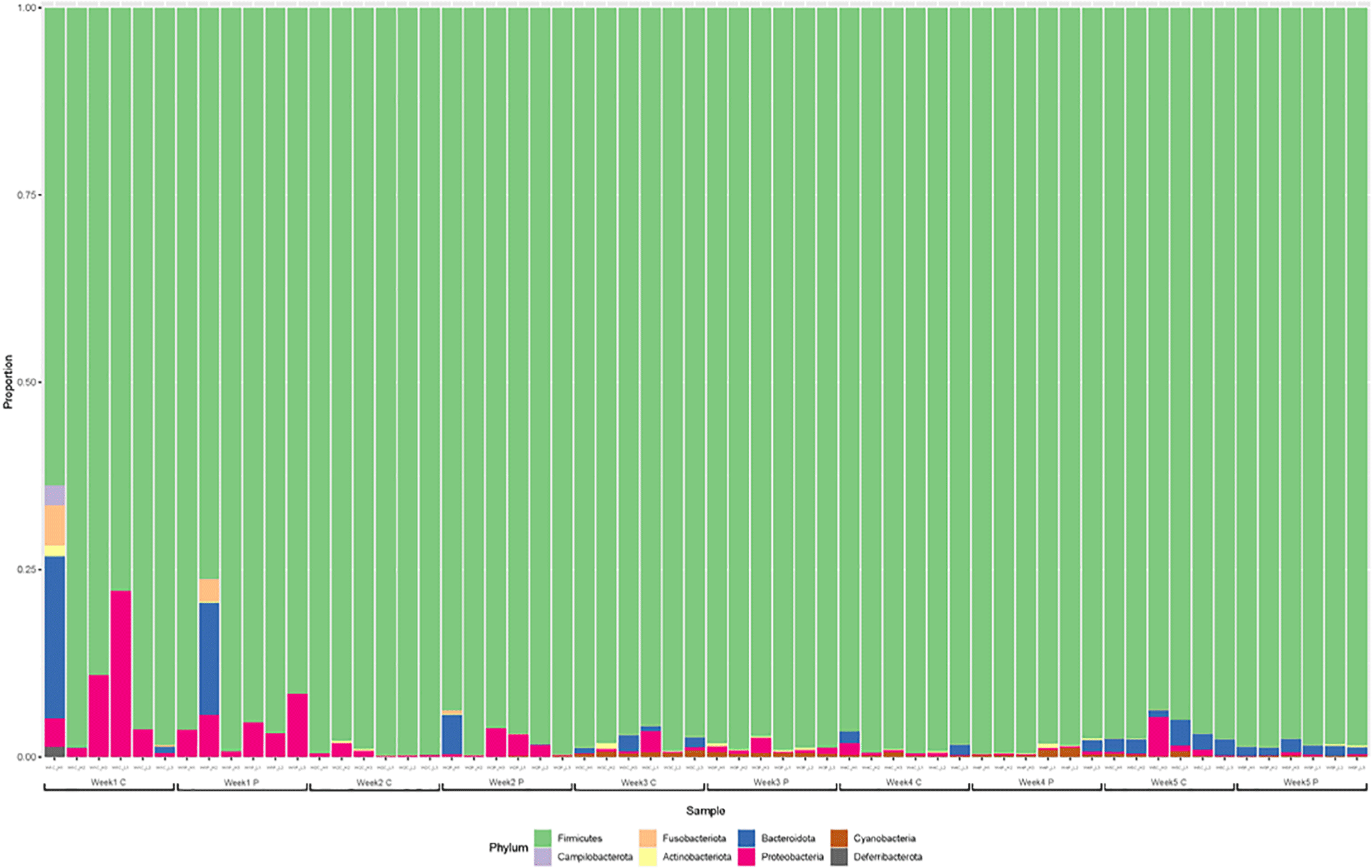
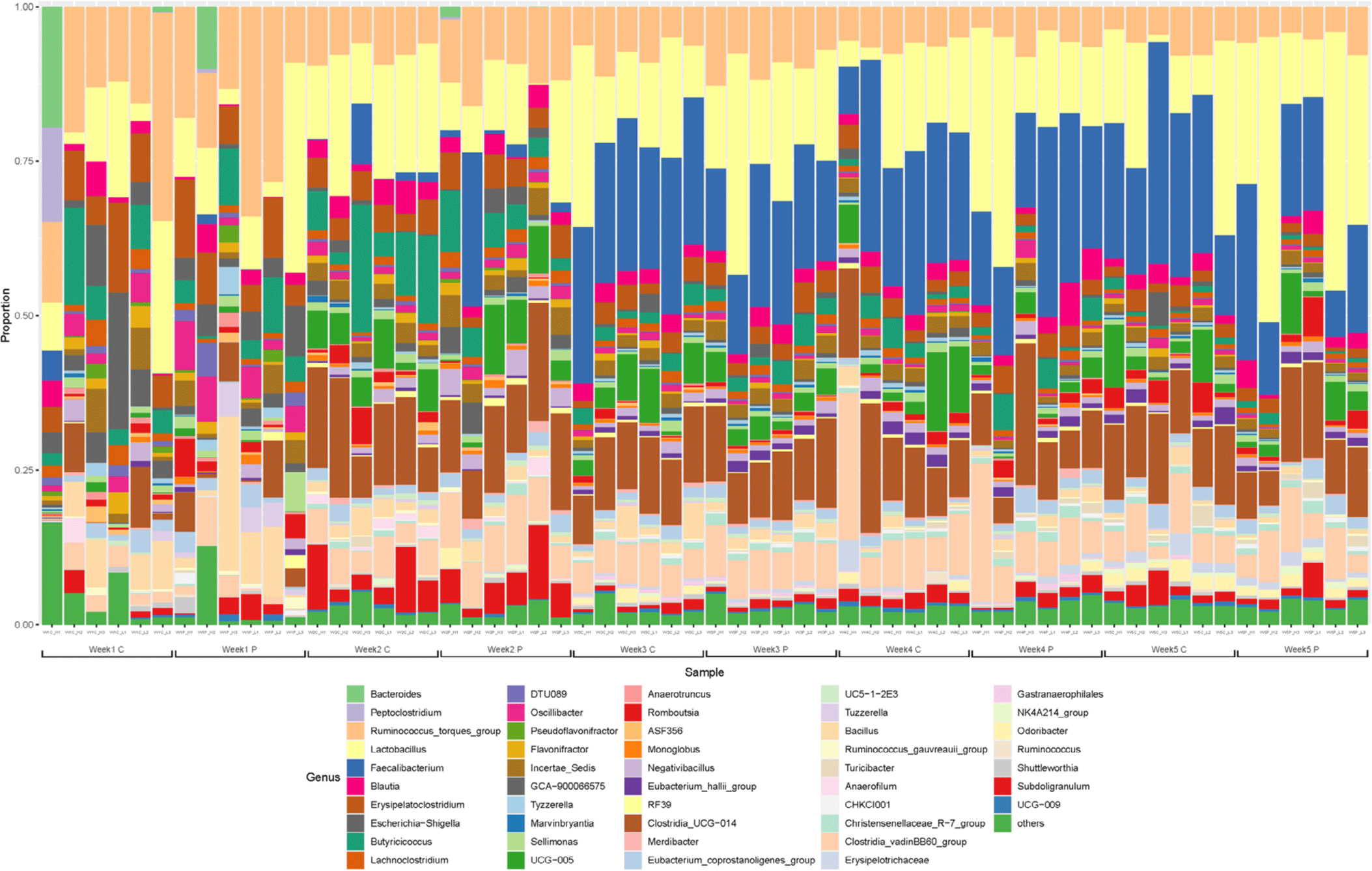
The gut microbiota was dominated by Firmicutes, Proteobacteria, and Bacteroidetes at the phylum level at 1 and 2 wk, especially, the Firmicutes account for greater than 98% (Fig. 3). The gut microflora composition marginally varied between the two groups after one wk. Ruminococcus was the predominant genus in majority of the samples. In addition, Lactobacillus and Bacillus corresponding to lactic acid bacteria, Escherichia-Shigella including Escherichia coli, and Erysipelatoclostridium and Clostridium were identified as the major genera at 1 wk. The gut microflora at 2 wk was not significantly different from that at the first wk. Faecalibacterium was dominant at 3 wk of age. At 4 and 5 wk, the predominant genera were Faecalibacterium, Lactobacillus, and Clostridia, accounting for more than half of the total population (Fig. 4).
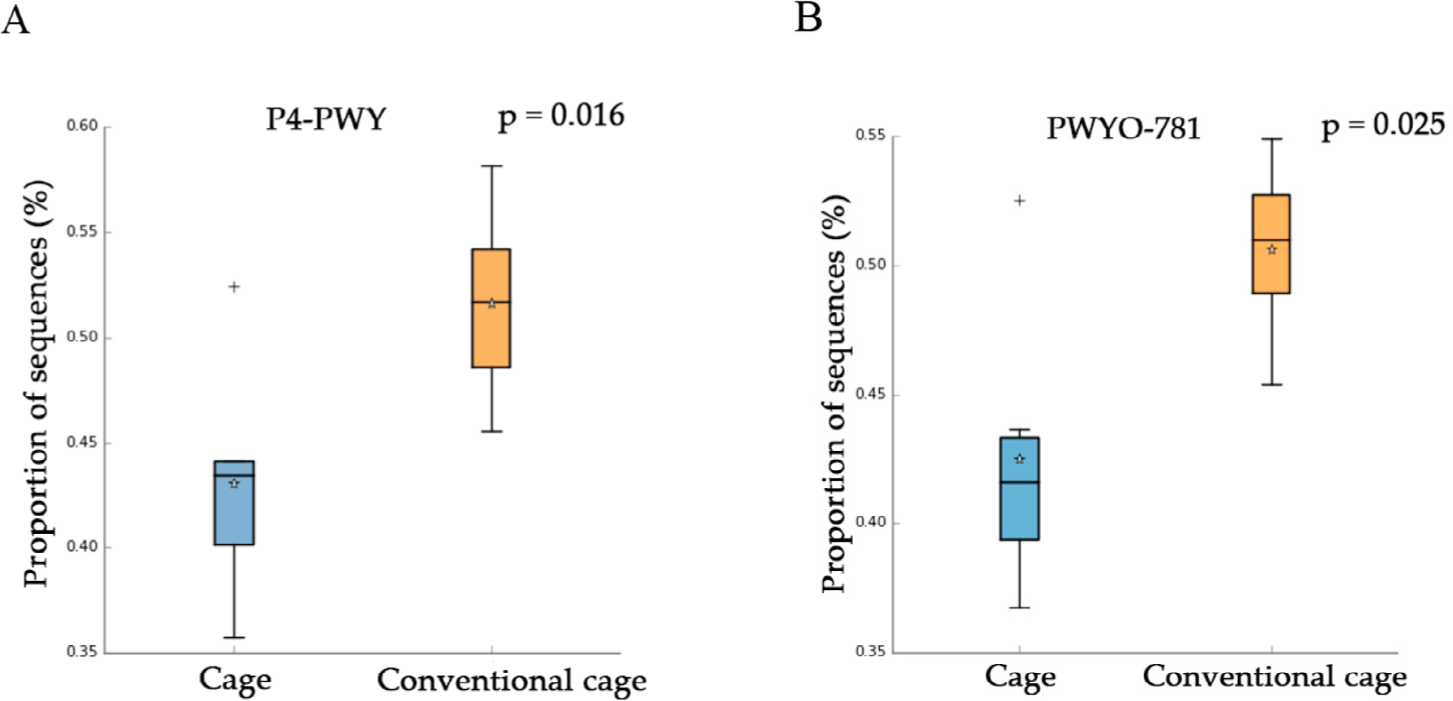
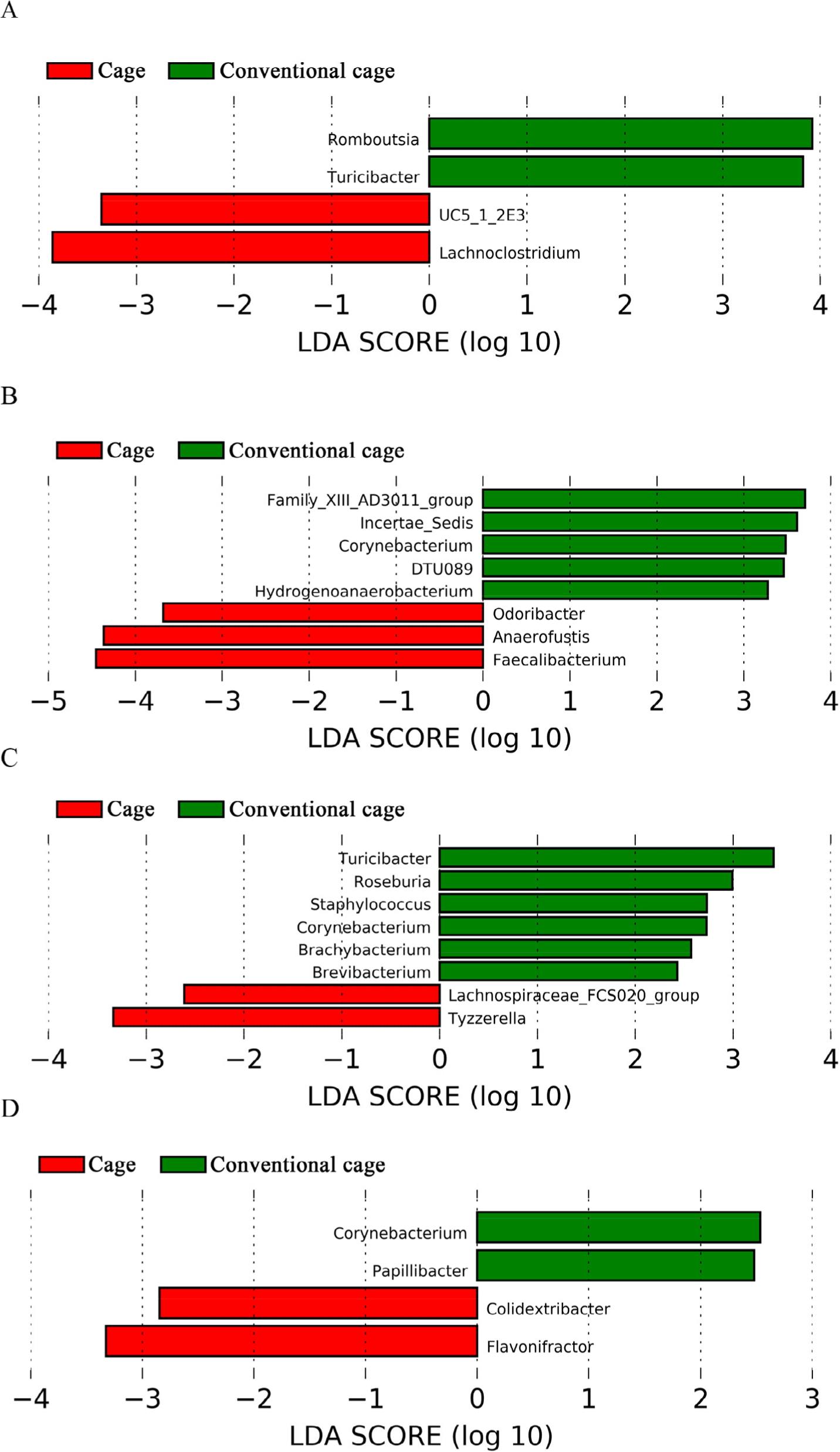
This study attempted to identify significant pathways in individual pathway units. P4-PWY (superpathway of L-lysine, L-threonine, and L-methionine biosynthesis I) and PWY0-781 (aspartate super-pathway) were upregulated in the control group with litter after 1 wk (Fig. 5). A total of 48 pathways were affected by litter use, among which 32 pathways were upregulated and 16 were downregulated at 2 wk (p < 0.05). At 3 wk, 21 pathways showed significant differences with respect to litter use, among which 16 pathways were downregulated and five were upregulated in the conventional group (p < 0.05). The relative distribution of functional pathways within the intestinal microbial flora was determined to identify clustering patterns between groups using PCA at 3 and 4 wk. On supplying litter at 3, 4, and 5 wk, the mycolyl-arabinogalactan-peptidoglycan complex biosynthesis pathway was upregulated in the conventional group (Table 3). LEfSe was used to identify differences depending on litter use. Four differentially abundance taxa at the genus level were discovered at wk 1 using LEfSe (Fig. 6). The abundance of Romboutsia and Turicibacter increased in the conventional-litter group whereas that of Lachonoclostridium increased in the cage group without litters. In the second wk, no significant differences were observed between the cage and conventional cage groups. Relative abundance was not detected at the genus level at wk 2 (data not shown). Eight differentially abundance taxa were detected at the genus level at 3 wk, including five differentially abundance taxa with clear genera. Among these, the abundances of Corynebacterium and Hydrogenoanaerobacterium were increased in the litter use group, whereas those of Odoribacter, Anaerofustis, and Faecalibacterium were increased in the cage group. Eight differentially abundance taxa were detected at the genus level at 4 wk. The increased abundances of Turicibacter at 1 wk and Corynebacterium at 3 wk further increased at 4 wk. The abundances of Roseburia, Staphylococcus, Brachybacterium, and Brevibacterium also increased in the litter-treated groups, whereas that of Tyzzerella increased in the cage group. In the fifth wk, the four differentially abundance taxa showed differences at the genus level, depending on the litter. The abundances of taxa Corynebacterium and Papillybacter increased with litter use. In particular, the abundance of Corynebacter increased at both 3 and 4 wk. In the absence of litter, an increase in the abundances of two differentially abundance taxa (Colidextribacter and Flavonifractor) were observed. Relative abundance at the genus level differed based on the type of bedding. The cage and conventional groups are indicated in red and green, respectively. The bacterial taxa were statistically significant (p < 0.05) in terms of relative abundance.
DISCUSSION
Body weight gain in broiler chickens is influenced by various environmental conditions, including aging, nutrients, microbiome, immunity, and bedding materials [39,40]. In this study, growth performance generally showed a significant difference with or without litter (i.e., cage vs. conventional cage). In particular, although the FCR decreased in broilers in the cage at an early phase, it was ameliorated during the growing phase. Broiler weight gain from days 0–28 was not significantly different between the cage and conventional groups, similar to the findings of a previous study [41].
The productivity and intestinal microbiota were influenced in caged chickens, thus promoting the growth of beneficial microbes and preventing harmful bacteria. Therefore, we investigated the effects of litter use on the gut microbiota of chicken in cages (without litter) and conventional conditions (with litter). The most abundant phyla in the broiler cecum was Firmicutes, which is consistent with previous findings [42,43]. Firmicutes, associated with chicken weight gain, produce compounds in the intestinal wall as an energy source. In this study, the abundance of Firmicutes increased marginally under litter conditions. The abundance of gut bacteria was relatively low in the litter-treated group, as reported in previous studies [44,45].
Ruminococcus was significantly more abundant at all ages. The abundance of Bacteroides and Ruminococcus is associated with gut health [46]. The increased abundance of Lactobacilli may inhibit pathogens by producing vitamins and organic acids [47] Increasing the proportion of Faecalibacterium in the intestinal microflora positively affects growth [48]. Faecalibacterium produces short-chain fatty acids such as acetate, propionate, and butyrate, which are major products of intestinal microorganisms and commensal bacteria [49]. It also produces shikimic and salicylic acids, which are involved in its anti-inflammatory activities. Faecalibacterium spp. isolated from chickens with strong immunity may also serve as potential probiotics. Lysine, threonine, and methionine amino acids (AAs) are essential during the early chick phase [50]. The intestine-related inflammatory response can be attributed to β-galactomannan contained in soybeans of broiler fed. The increasing mannan degradation functions in the conventional group improved the abundance of gut microbiota in chickens, which changed with a decrease in intestine-related inflammatory reactions. Mannans are a type of hemicellulose found in a variety of cereals and industrial byproducts utilized in animal feed. While mannans can potentially be detrimental to animals, smaller portions of them offer benefits. The fermentation of mannan polysaccharides and oligosaccharides has been observed to alter the intestinal microbiota. Therefore, the varying sizes and monosaccharides present in mannan polysaccharides may influence the intestinal microenvironment [51]. Mitigation can improve productivity and alleviate mortality. The abundance of Faecalibacterium increased in the cage group compared to that in the conventional group. Therefore, it is expected to play an important role in the health of individual species at 3 wk of age owing to increased immunity. Increasing AAs in chickens housed without litter can enhance chicken health through intestinal microbial flora.
Five microbes were detected at the genus level. The abundances of Corynebacterium and Hydrogenoanaerobacterium increased in the conventional group while those of Odoribacter, Anaerofustis, and Faecalibacterium were enhanced in the cage group at 3 wk. Corynebacterium can cause diseases in various livestocks [52]. After the third wk, the use of litter for 3 wk induced Corynebacterium growth. Brachybacterium and Brevibacterium species at 4 wk associated with growth performance are frequently found in the microbial flora of dust and feces [53]. Forty-eight pathways showed significant differences after two wk. Among these, 32 pathways were upregulated in the conventional group with litter and 16 pathways were downregulated in the cage group without litter. The upregulation of biosynthesis-related pathways and downregulation of decomposition-related pathways were observed.
In this study, the pathways identified based on the graphical analysis at wk 3, 4, and 5 did not significantly affect the intestinal microbial flora during litter use. However, the three common pathways influencing the mycolyl-arabinogalactan-peptidoglycan complex biosynthesis increased at wk 3, 4, and 5 compared to 1 and 2 wk. However, this pathway is unlikely to be directly related to the effect of litter, since it is specific to cell wall synthesis. Romboutsia was an uncharacterized bacterial genus. However, the fungal species in the gut microbiota of young hens showed differences when Astragalus was used as a feed additive [54]. Romboutsia is the major genus involved in functioning of the intestinal microbial flora of chicken [55]. In addition, Turicibacter is present at residual levels in the feed intake of chickens [56]. Feed intake and average weight gain of groups depended on litter use. The Lachnoclostridium strain can be used to regulate body weight and drip loss associated with meat quality and body weight in broilers [57]. This suggests that meat quality can be improved by regulating the intestinal microbiota. The genus Corynebacterium can cause diseases in various animals and its growth is positively reduced by lactic acid bacteria or feed additives [58]. Therefore, if the abundance of related species increases in the intestinal microbial flora, litter use may not be considered positive after the third wk. In this study, the abundance of Odoribacter, a key bacterial species in feed additives consisting of phages, increased in the conventional groups without litter. Anaerofustis is related to energy metabolism and is positively correlated with the accumulation of abdominal fat in chickens [59]. Although this genus needs further evaluation, it is unlikely to positively affect growth rate. Faecalibacterium positively affects the growth of intestinal microflora [48]. In this study, Faecalibacterium was established as the dominant species in the cage group without litter from 3–5 wk. During this period, the unuse of litter is preferable based on the existing known intestinal microorganisms.
Brachybacterium is mainly found in dust and fecal samples from poultry farms with poor breeding performance [60]. However, increase in the abundance of this species in litter has not been evaluated. In addition, Brevibacterium is also abundant on farms with poor performance [60]. Herein, considering these bacterial species markers to evaluate the use of litter in intestinal microorganism research may not yield good results. Papillibacter is a pathogenic bacterium with considerably reduced abundance in chickens when Lactobacillus casei is used as a feed additive. Increase in litter use did not positively affect intestinal microorganisms, even in the fifth wk. Therefore, various evaluations may be necessary for related bedding, depending on the use of litter from the third wk onwards.
In summary, all the bacterial species that increased in abundance in the cage (without litter) group are known to be associated with generally beneficial functions, such as improving growth performance or regulating immune responses. However, in this study, the intestinal microbial flora composition was more remarkably affected by the growth period than that by bedding use. In particular, chicken intestinal microbial flora was established, and the major dominant species did not change after the third wk. In particular, the abundance of Cornynebacterium increased in the litter group from 3–5 wk. Increased bacterial abundance in the litter had a negative effect in this study. Hence, it is necessary to consider the benefits of using litter by analyzing the intestinal microbiota. In contrast, improvement in the FCR and relative abundance of beneficial gut microbiota was observed in cages (without litter) compared to those in conventional-supplied litter. Hence, it is recommended that the use of litter should be avoided after three wk when intestinal microorganisms are established.
