INTRODUCTION
Owing to global warming, the rise in environmental temperatures presents ongoing challenges and difficulties for the livestock industry and poultry production [1]. In poultry, issues related to stress from rising temperatures and decreased productivity due to heat stress are becoming more severe [2]. Efforts to address these problems are intensifying and the use of feed additives is gaining attention as a potential solution. Research efforts to enhance the heat stress resistance of poultry through feed additives and to maintain or increase their productivity are increasing [3].
Among feed additives, taurine is a non-proteogenic amino acid and a by-product of cysteine metabolism, playing a crucial role in the health and physiological processes of animals. It significantly influences lipid metabolism and serves essential physiological functions in various animal species [4]. Taurine is extensively used to enhance the growth and performance of poultry [5]. Research indicates that, when applied to poultry, taurine offers protective effects against environmental and biological stress factors, leading to its diverse applications [6]. Taurine is used in the poultry industry as a water additive and feed supplement to alleviate stress in broilers. During periods of heat stress, the addition of taurine enhances muscle development and growth performance [7,8], reduces the expression levels of heat shock proteins (HSPs) [9], alleviates intestinal damage caused by heat stress, enhances the expression of appetite-related genes [10], provides a defence against oxidative stress [11], and offers protection against cell death [6,12]. Previous studies suggested that taurine mitigates heat stress in poultry. The majority of these studies primarily focused on broiler specimens, analyzing rudimentary weight and feed intake metrics, as well as general HSPs expression levels [7]. To date, studies investigating the effects of taurine on cells under heat stress are limited, indicating a gap in the current research landscape in this domain.
In this study, we explored the impact of taurine on broilers under heat stress using cell culture models. By applying two distinct culture temperatures to broiler satellite cells and assessing the role of taurine, we aimed to uncover new perspectives in poultry cell culture research and approaches to mitigating heat stress. Specifically, we investigated how these factors influence the morphology, survival rate, heat stress response, oxidative stress levels, cell cycle progression, and signaling pathways of broiler satellite cells. Through this analysis of both individual and combined effects, we sought to better understand the potential benefits of taurine for enhancing animal welfare and productivity in the poultry industry.
MATERIALS AND METHODS
Muscle tissues were collected from the leg, including the biceps femoris, semitendinosus, and semimembranosus, which provided sufficient muscle mass for isolating satellite cells from 18-day-old chick embryos (Ross 308), and cells were isolated using an established protocol in our laboratory [13]. Briefly, the eggs were disinfected with 70% ethanol and the embryos were euthanized and extracted. Muscle tissues were washed with phosphate buffered saline (PBS; Gibco) supplemented with 10% Antibiotic-Antimycotic (AA, Gibco, #15240062), and minced into small pieces. Subsequently, minced muscles were dissociated and disaggregated with DMEM/F12 medium supplemented with 1 U/mL of dispase II (Roche, #4942078001), 2 mg/mL of collagenase D (Roche, #11088858001), 0.25% trypsin-EDTA (Gibco, #25200–072), and 10% AA at 37°C for 1 h. Digested tissues were filtered through a 100 μm cell strainer, followed by a 70 μm cell strainer, and neutralized with DMEM/F12 medium containing 15% fetal bovine serum (FBS; Gibco, #16000–044). Suspension was centrifuged at 205×g for 5 min, and incubated with an ACK lysing buffer (Gibco, #A10492–01) at 4°C for 5 min. The supernatant was removed following centrifugation, and the cell pellets were resuspended in DMEM/F12 medium containing 15% FBS, 1% penicillin-streptomycin-glutamine (PSG; Gibco, #10378–016), and 5 ng/mL basic fibroblast growth factor (bFGF, Gibco, #13256–029). Cells were seeded into culture dishes pre-coated with 0.1% gelatin and cultured for 1 h. Unattached cells were transferred to new culture dishes for separation and purification of satellite cells.
CMSCs were stabilized in growth medium at 37°C for 24 h. After stabilization, cells were treated with taurine (Sigma-Aldrich, #T0625) at concentrations of 50, 100, 150, 200, and 250 μg/mL. The cells extracted are being embryo at egg-incubation, with the incubation temperature set as 37°C. The body temperature of newly hatched chicks is 38°C, while in adult chickens, it can rise up to 41°C. Therefore, the control temperature for the cells used in this study was set at 37°C, and accordingly, the control and high temperatures were established. The cells were then transferred to two separate incubators set at 37°C (control group) and 42°C (heat stress group), and cultured for 48 h. An appropriate dose of taurine was selected by comparing cell viability (Fig. 1).
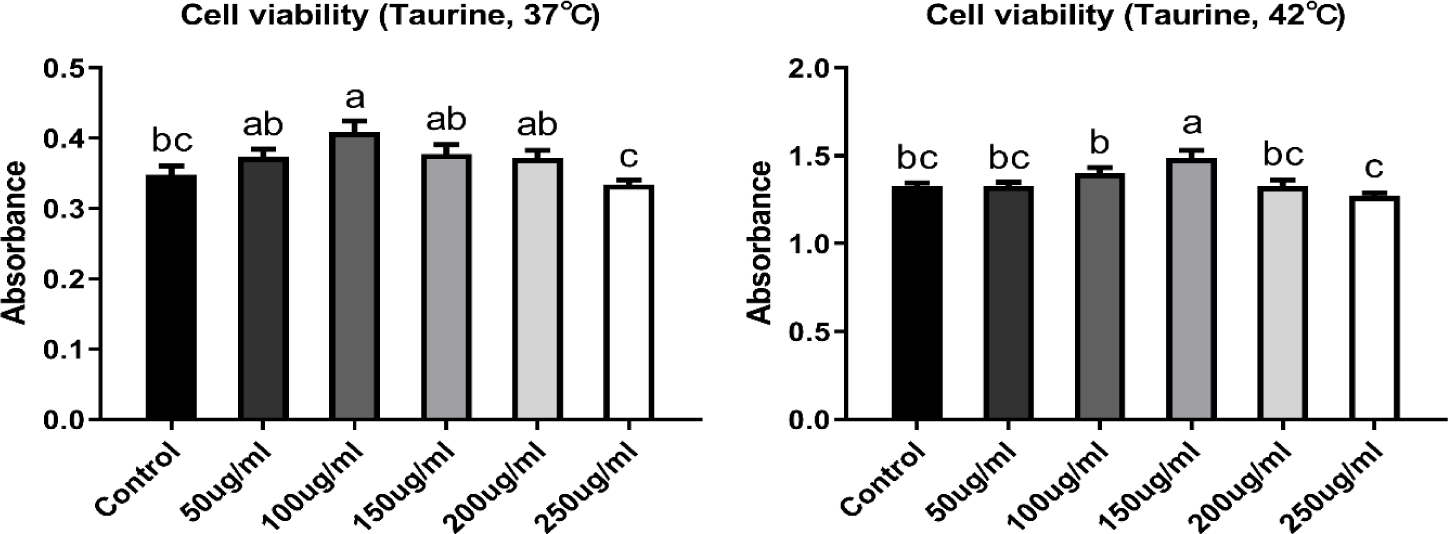
Cell viability was measured using the Cell Counting Kit-8 (CCK-8, Dojindo, #CK04-11). Briefly, the cells were seeded into 96-well plates at a density of 5 × 104 cells per well and cultured for 48 and 72 h, including 24 h of stabilization in the growth medium. The cells were then treated with CCK-8 solution and incubated at 37°C for 4 h. Cell viability was measured at 24 and 48 h after taurine treatment, and absorbance was detected at 450 nm using a microplate reader (Thermo Fisher Scientific).
CMSCs were fixed using 4% paraformaldehyde for 20 min at 4°C and washed three times with PBS. The cells were subsequently permeabilized and blocked using a blocking solution containing 3% BSA and 0.3% Triton X-100 in PBS for 1 h at room temperature. The cells were followed by an overnight incubation at 4°C with primary antibodies against Pax7 (Monoclonal, 1:50, DSHB), and MyoD (Polyclonal, 1:200, Proteintech). The primary antibodies were detected with secondary antibodies, and subsequently stained with 4′-6-diamidino-2-phenylindole (DAPI) for 5 min at room temperature. Immunostained images were acquired using a super-resolution confocal laser-scanning microscope (LSM 800, Carl Zeiss).
Total mRNA was isolated from chicken satellite cells using TRIzol (Invitrogen), according to the manufacturer’s instructions. The quality and purity of the total mRNA were calculated using a Nanodrop (Thermo Fisher Scientific) at 260 and 260/280 nm absorbances. Then, cDNA was synthesized using a cDNA synthesis kit (AccuPower RT Premix, Bioneer) according to the manufacturer’s instructions. RT-PCR was performed using the CFX96 Real-Time PCR Detection System (Bio-Rad). Broiler (gallus gallus)-specific primers were prepared using the Primer 3 program, and each gene was related to heat shock proteins, oxidative stress, cell cycle, and signaling pathways (Table 1). The final result was calculated through the 2-ΔΔCt method [14]. All data were normalized to the Ct value of glyceraldehyde 3-phosphate dehydrogenase (GAPDH), and normalized to the control (37°C, 0 mg/mL taurine).
HSP, heat shock protein; IL8, interleukin 8; Nrf2, Nuclear factor erythroid-derived 2-like 2, NFE2L2; CAT, catalase; SOD, superoxide dismutase ; Acox2, Acyl-CoA oxidase 2; ERK, extracellular signal-regulated kinase ; JNK, c-Jun N-terminal kinase; NFkB, Nuclear factor kappa-light-chain-enhancer of activated B cells; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Experimental data were analyzed using SAS 9.4 and expressed as mean ± SE. The differences of viability, and gene expressions between temperatures (37°C and 42°C) or taurine treatments (0 and 150 mg/mL) were analyzed using student’s t-test. The main (temperature and taurine) and interaction (temperature × taurine) effects were analyzed using two-way analysis of variance (ANOVA) with GLM. Differences were considered statistically significant at p < 0.05.
RESULTS
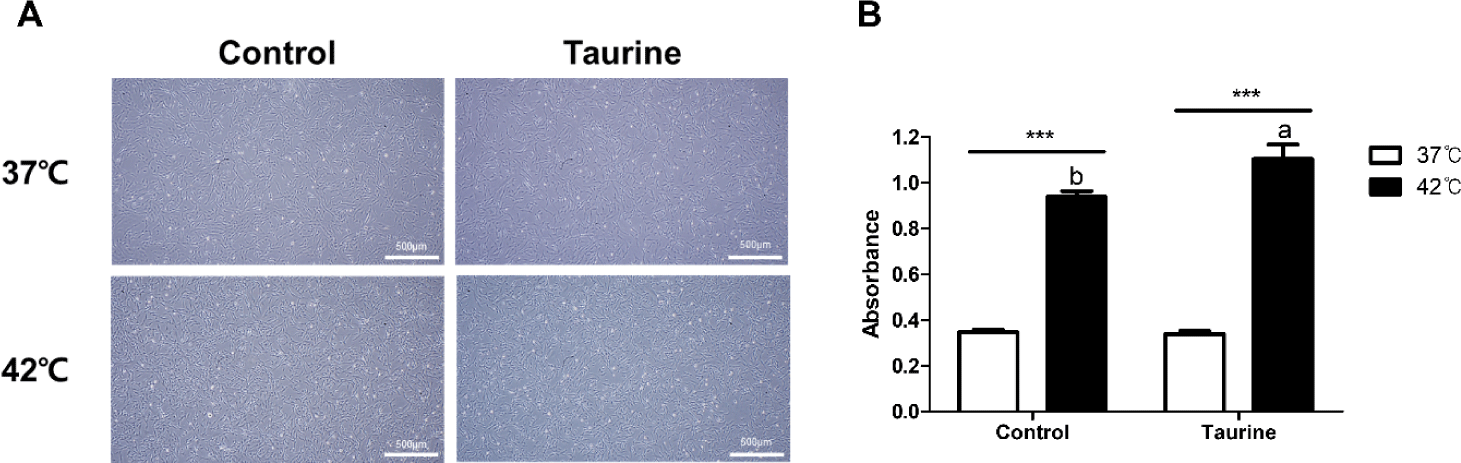
There were no significant differences in cell morphology based on the culture temperature or taurine treatment (Fig. 2A). Normal growth proceeded without any morphological changes in the cells across all treatment groups. The results of the CCK analysis indicated that cells cultured at 42°C exhibited higher cell viability than those cultured at 37°C, regardless of taurine treatment (p < 0.001; Fig. 2B). While taurine showed no effect on cells cultured at 37°C, a significant increase in cell viability was observed when taurine was added to cells cultured at 42°C.
Cells were extracted from the leg muscles of broiler embryos and Pax7 and MyoD expressions were analysed to confirm the identity of the extracted cells (Fig. 3). As depicted in Fig. 3A, the ICC results clearly demonstrated the expression of both Pax7 and MyoD, which are satellite cell markers. The expression rates of Pax7 and MyoD across all treatments in this study were 92.83 ± 0.85% and 98.06 ± 0.4%, respectively, when compared to total cell counts (DAPI; Fig. 3B). These results indicate the excellent purity of the satellite cells extracted in our laboratory.
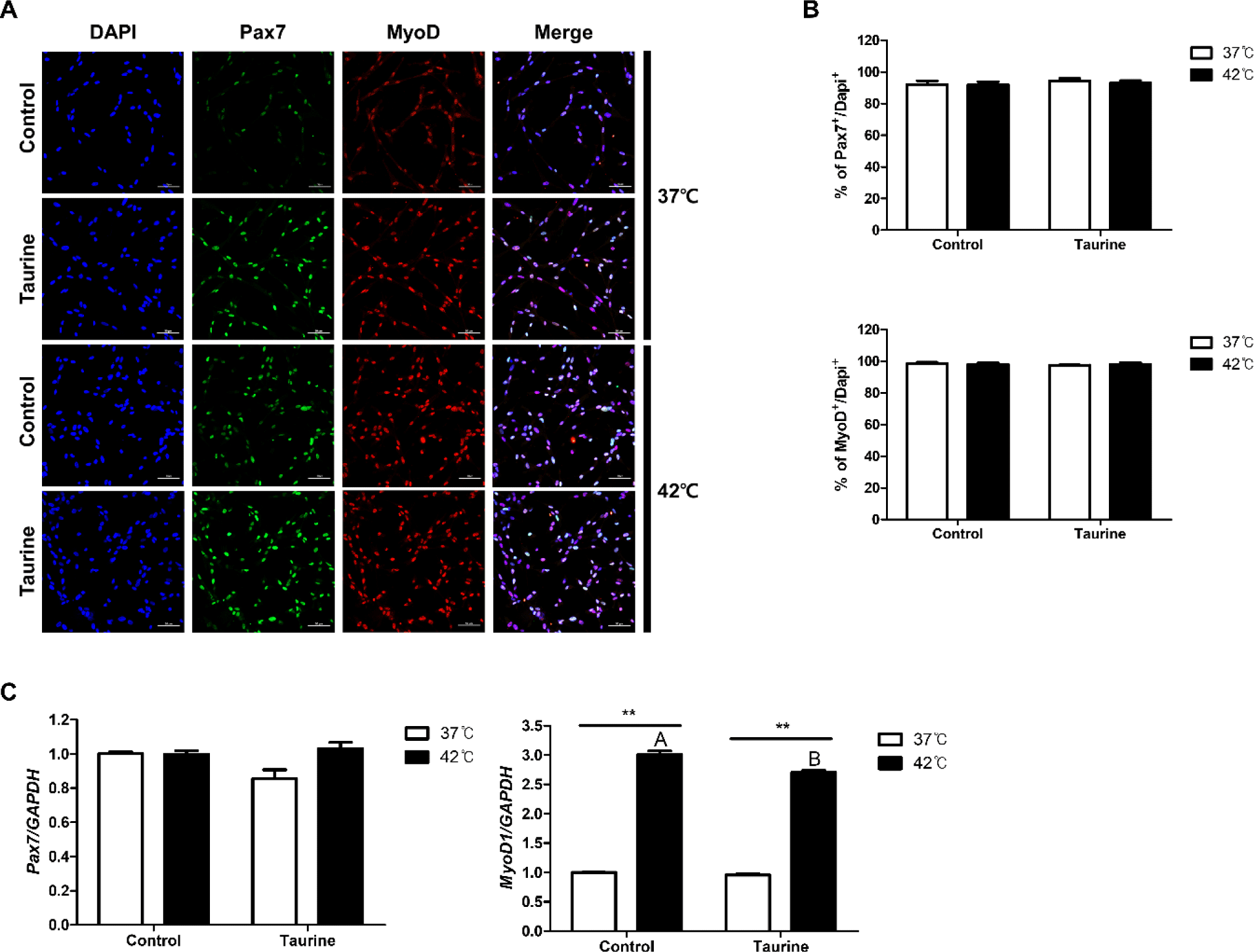
The results comparing the gene expression levels of Pax7 and MyoD are presented in Fig. 3C. There were no significant differences in the gene expression levels of Pax7 among the treatment groups. However, the expression level of MyoD1 was significantly higher in cell cultured at 42°C compared to those cultured at 37°C. Moreover, cells cultured at 42°C exhibited significantly higher MyoD1 expression levels when not treated with taurine (p < 0.01).
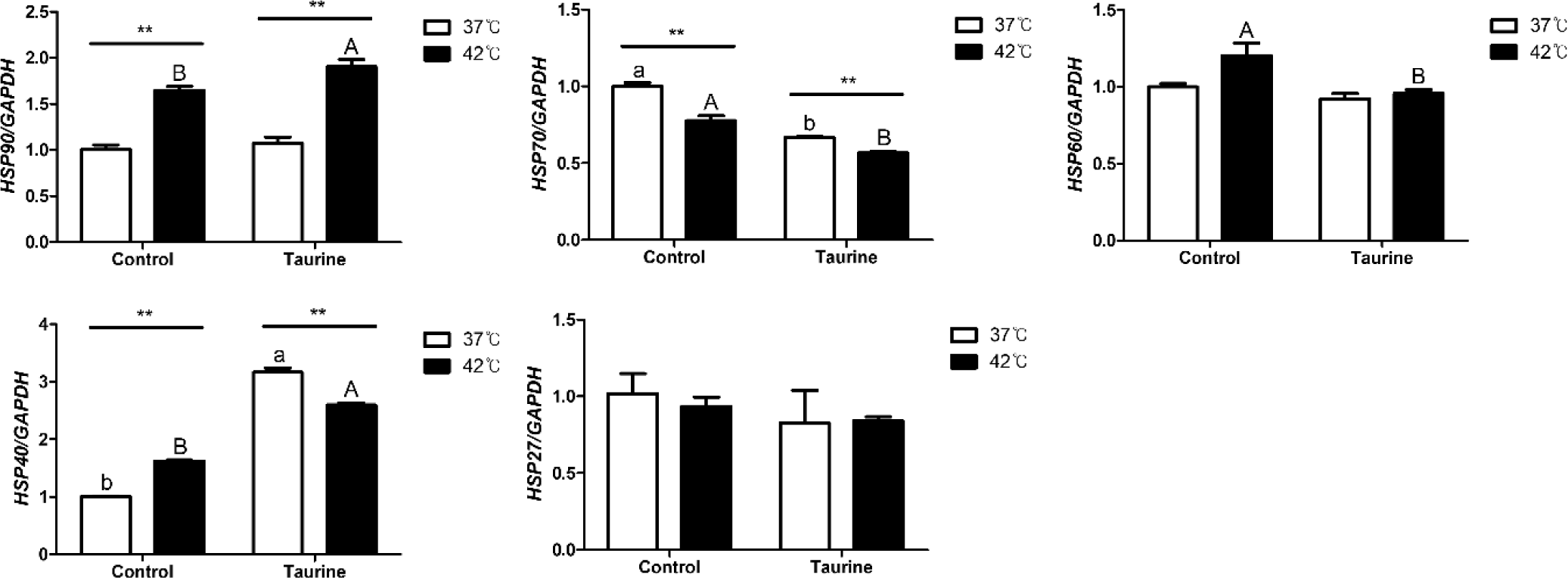
This represents the results of comparing the expression levels of HSP gene expressions based on cell culture temperature as well as the impact of taurine according to temperature (Fig. 4). HSP90, HSP70, and HSP40 exhibited significant differences in expression levels depending on the culture temperature, irrespective of the presence of taurine. However, no significant differences were observed in the expression levels of HSP60 and HSP27 attributable to the temperature. The expression levels of HSP70 and HSP40 decreased and increased in the presence and absence of taurine, respectively. In contrast, significant differences due to taurine were observed only in cells cultured at 42°C for HSP90 and HSP60. HSP90, HSP60, and HSP27 did not yield significant results related to taurine treatment in 37°C.
As a result of analyzing the effect of temperature, taurine, and their interaction on HSPs gene expression are shown in Table 2. High culture temperature as a main effect has significant effect on HSP90, HSP60, and HSP40 as up-regulation, but down-regulation in HSP70. In taurine treatment, HSP90 and HSP40 were significantly up-regulated but HSP70 and HSP60 were down-regulated. However, HSP27 showed no significant effects owing to variations in taurine, temperature or interaction. Notably, HSP70 and HSP40 demonstrated an interaction effect between culture temperature and taurine. Especially in HSP40, according to their interaction effect, temperature and taurine was up-regulated its expression but 42°C with taurine group down-regulated the HSP40 gene expression.
In this study, we analyzed the effects of taurine and culture temperature on the expression of genes involved in cell cycle regulation and apoptosis (Fig. 5). The expression of p53, a G1 regulator of the cell cycle, was significantly reduced at 42°C compared to 37°C. However, its expression increased significantly at 37°C in the presence of taurine. The expression of p21 was not significantly altered by taurine, but it was notable upregulated at 42°C compared to 37°C. CyclinD1, a gene crucial for the transition from the S phase to the G1 phase and for inducing cell proliferation, was significantly upregulated upon taurine addition. Moreover, in the taurine-treated group, cyclinD1 expression was elevated at 42°C compared to 37°C. B-cell lymphoma 2 (Bcl2), which inhibits cell apoptosis, was upregulated at 42°C compared to 37°C. In contrast, caspase 3, an apoptosis promoter, was not significantly affected by temperature variations but exhibited decreased expression in the presence of taurine.
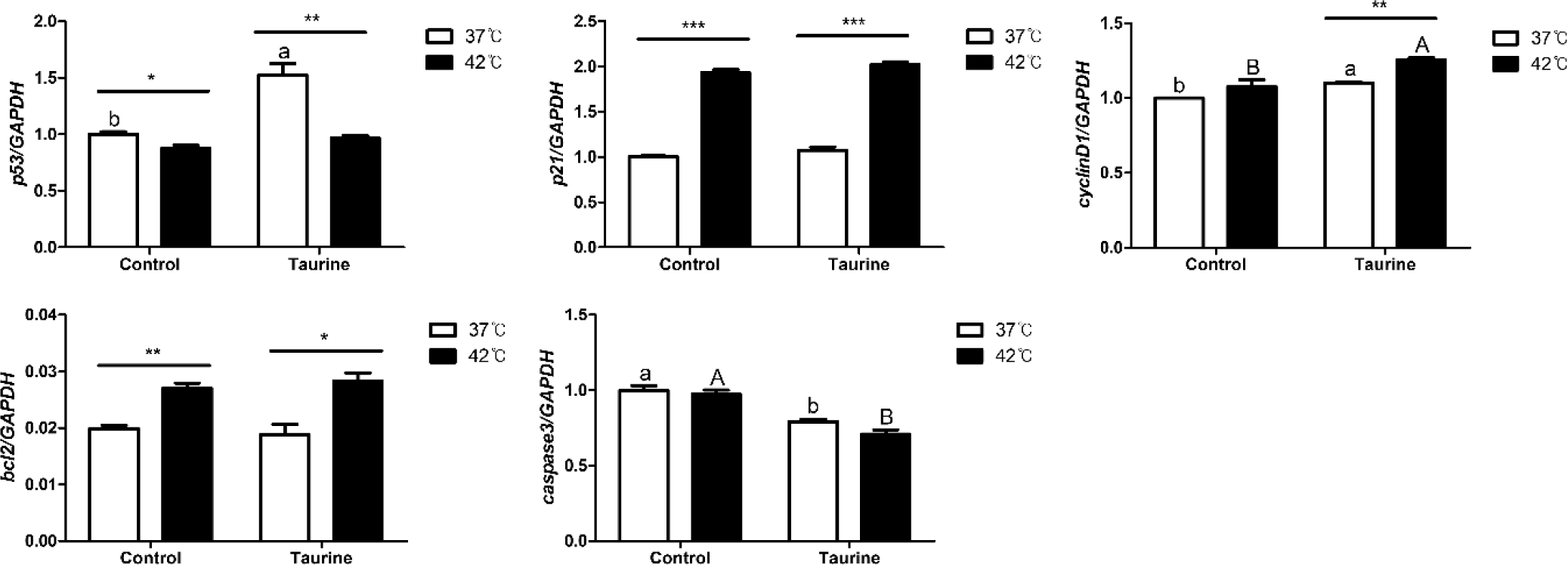
We analyzed the influence of temperature, taurine, and their combination on the expression of genes associated with cell cycle regulation and apoptosis (Table 3). The expression levels of p53, p21, and Bcl2 were significantly affected by temperature. In contrast, the expression of cyclinD1 and caspase 3 showed marked differences after taurine supplementation. Notably, a significant interaction between temperature and taurine was observed on the p53 gene. As the temperature and taurine significantly up-regulated p53 gene expression, but the 42°C with taurine treatment group was down regulated compared to 37°C with taurine group.
In this study, we investigated the effects of temperature and taurine supplementation on the expression of several enzymes and inflammatory markers (Fig. 6). The expression of superoxide dismutase (SOD), an antioxidant enzyme, was notably elevated at 42°C, and this upregulation was further pronounced with taurine supplementation under the same temperature condition. In contrast, catalase (CAT) exhibited a pattern distinct from that of SOD, whereas temperature variations did not significantly affect its expression. Taurine addition led to a significant decrease. Acyl-CoA oxidase 2 (ACOX2) expression increased substantially at 42°C compared to that at 37°C, and further elevation was observed at 37°C upon taurine supplementation. Calpain exhibited reduced expression at 42°C but was upregulated with the addition of taurine. Meanwhile, interleukin 8 (IL8), employed as an inflammatory marker, remained unaffected by temperature or taurine changes.
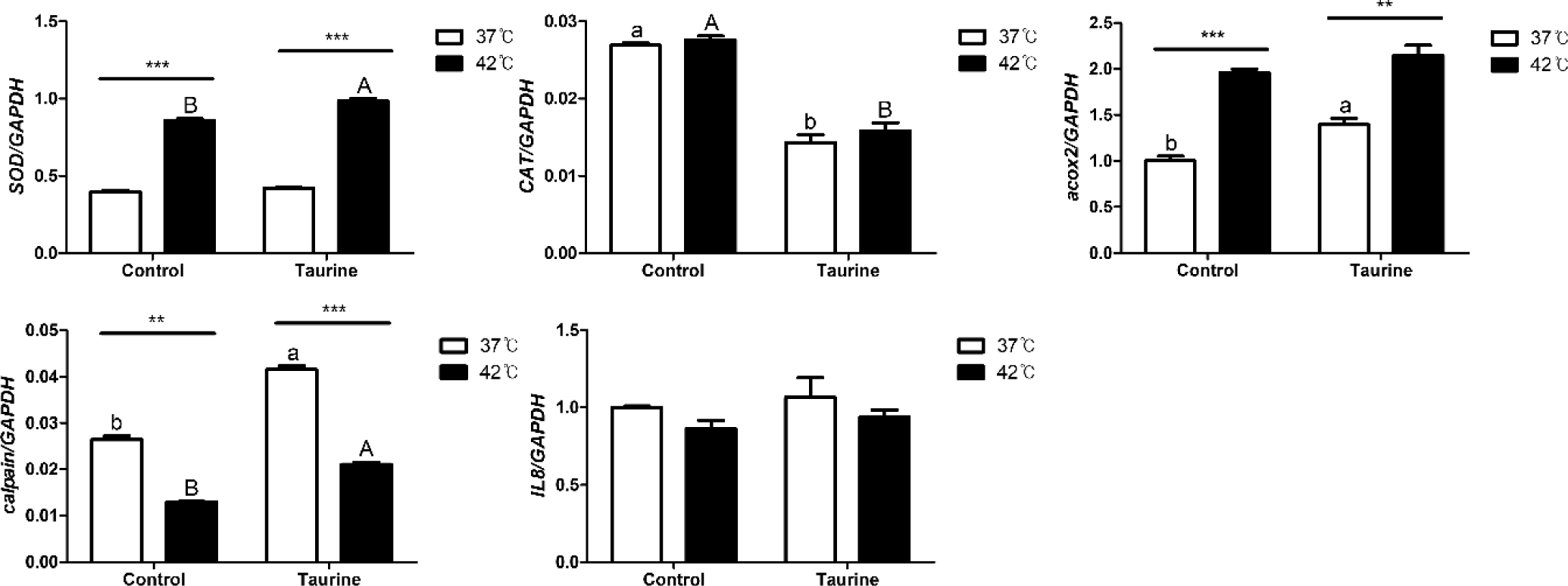
Among the examined factors, SOD and ACOX2 were most influenced by temperature, whereas CAT expression was predominantly affected by taurine (Table 4). Both temperature and taurine significantly affected the expression of SOD and calpain, with an observable interaction between these two factors. SOD exhibited significant individual effects from both taurine and temperature. However, while no significant difference was observed between the presence or absence of taurine at 37°C, a significant effect became evident after temperature treatment, indicating an interaction effect between taurine and temperature. However, IL-8 did not exhibit any significant effect on temperature, taurine, or their interactions.
We analyzed the expression levels of factors integral to the MAPK/ERK-Nrf2 intracellular signalling pathway (Fig. 7). Extracellular signal-regulated kinase (ERK) exhibited significant downregulation upon taurine treatment; however, its levels in 42°C increased compared to the 37°C when taurine was supplemented. c-Jun N-terminal kinase (JNK) showed an upregulation with taurine addition, but in the taurine-treated group, its expression decreased significantly at 42°C compared to 37°C. p38 exhibited lower expression at 42°C than at 37°C, but was upregulated with taurine supplementation. Nuclear factor kappa-light-chain-enhancer of activated B cells (NFkB) expression significantly diminished with taurine treatment, yet the expression at 42°C significantly decreased in the taurine treatment groups. Lastly, nuclear factor erythroid 2-related factor 2 (Nrf2) expression declined notably with taurine addition, but in the control groups, there was a marked increase at 42°C.
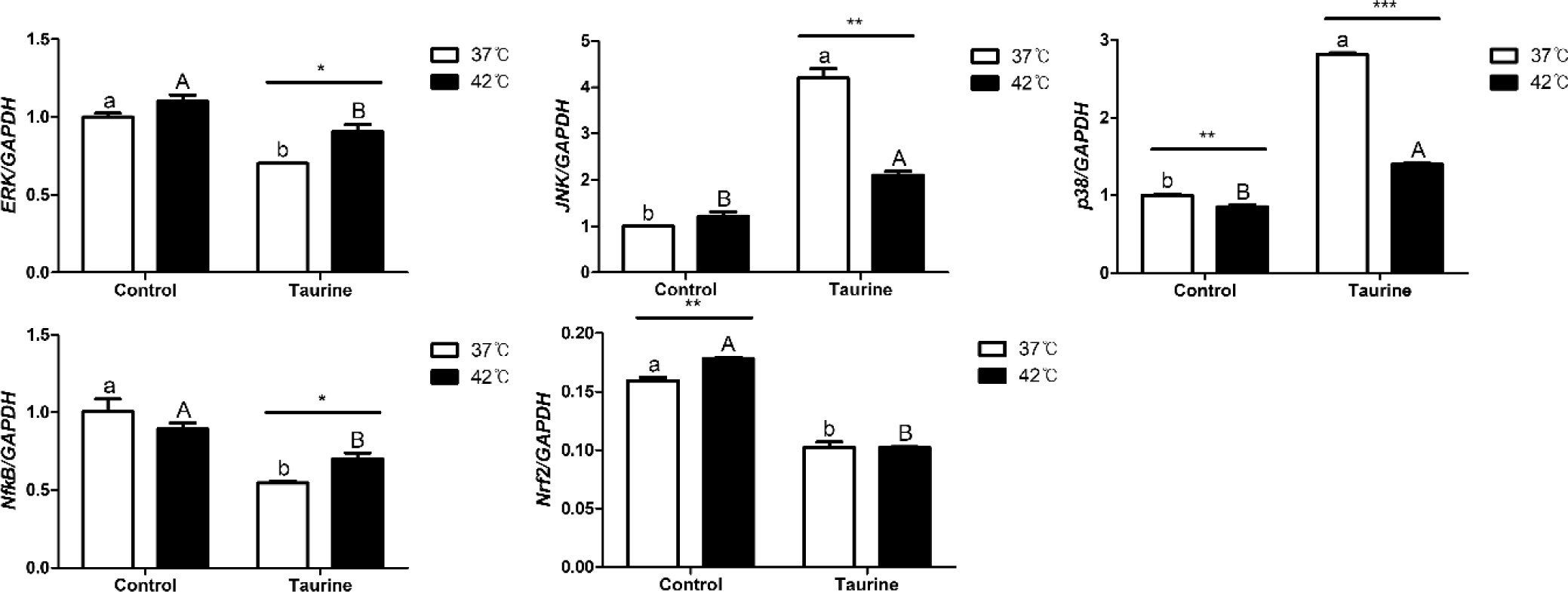
In the MAPK signaling pathway, ERK, JNK, p38, NFkB, and Nrf2 all exhibited significant response to taurine supplementation (Table 5). Notably, ERK, JNK, p38, and Nrf2 levels were significantly affected by temperature changes. Furthermore, a significant interaction between temperature and taurine levels was observed to JNK, p38, NFkB, and Nrf2. By the interaction effect, NFkB was significantly up-regulated by temperature with taurine.
DISCUSSION
In this study, we assessed the effects of taurine on satellite cell growth, heat shock protein expression, oxidative stress, and MAPK signaling at varying culture temperatures in broilers. We postulated that taurine promotes muscle growth and maturation while mitigating the deleterious consequences of heat stress.
Cell viability is the most widely employed parameter for verifying the suitability of various drugs and culture conditions [15]. Generally, the optimal temperature for mammalian cell culture is known to be 35°C‒37°C [16]; thus, in this study, the control group temperature was set at 37°C. To impose heat stress on broiler satellite cells, a culture temperature of 42°C, which is not sustainable for cell growth, was chosen and used as a treatment group for the study [17]. From the cell viability analysis results, we observed a significantly higher cell survival rate at 42°C than at 37°C. While the standard culture temperature for various cells, such as human, mouse, monkey, and cow, is known to be 37°C [18], research has suggested that due to the unique characteristic of a chicken’s body temperature being 41°C, a temperature of 41°C might be suitable for chicken cells [19,20]. The findings of this study revealed that when culturing chicken satellite cells, cells adhere more quickly at higher temperatures [21], and taurine significantly influenced cell viability or attachment speed at 42°C. Further studies on the exact cause, considering the cell culture temperature, attachment rate, growth rate, and survival rate, which appear to contribute to the high cell viability observed, are needed.
ICC analysis was conducted to verify the purity of the extracted cells and to check whether the satellite cells retained their characteristics. Pax7 protein is strongly downregulated during the differentiation of satellite cells, and due to its inability to be detected in non-muscle cell lines, is widely used as a marker for satellite cells [22]. MyoD possesses characteristics similar to those of Pax7 [23]. The ICC results demonstrated high marker expression across all treatments. This confirmed that the cells used in our experiment were high-purity satellite cells. Furthermore, no discernible differences or changes in temperature or taurine levels were observed. MyoD and Pax7 serve as satellite cell markers [24]. However, their mRNA expression can be influenced by external stimuli [25]. In a study examining the time-dependent expression of Pax7 and MyoD after skeletal muscle wounding in an animal model, both genes exhibited upregulation on the 5th day (for Pax7) and between days 3‒7 (for MyoD), followed by a subsequent decline [26]. This spike in expression likely facilitates skeletal muscle differentiation and promotes muscle recovery after injury [27]. In our investigation, we observed a significant elevation in MyoD mRNA expression at 42°C, suggesting that the elevated culture temperature might induce cellular damage. However, the addition of taurine mitigated this damage to some extent.
HSPs, known as stress markers, rapidly increase in cells in response to various stress factors, particularly high temperatures [28]. They are highly prevalent in various organisms and play important roles in cell protection and homeostasis maintenance [29]. When constitutive HSP70 is highly expressed, pyrogenic organisms can survive in hot environments or cope with sudden changes in the core temperature. Therefore, the higher the basic environmental temperature, the more HSP70 is found in each individual under stress-free conditions. In organisms originating from hotter climates, normal protein system function can occur without changes in HSPs at high temperatures; however, at lower temperatures, HSP70 may increase. This increase is not a response to cell damage or protein denaturation, but is due to the need for an adaptation period to the temperature, as HSP70 was produced in the original high-temperature environment [30]. This could imply that the lower levels of HSP70 at 42°C in this study suggest that the optimal cell culture temperature for broiler might not be 37°C. Hence, consistent research on the optimization of cell culture temperatures in broilers is warranted.
Cyclin D1 and bcl2 are genes that promote cell division and proliferation, while p53, p21, and caspase3 are associated with mechanisms that recognize cell damage, initiate repair, or induce apoptosis [31]. In response to external stimuli such as heat, UV light, or toxic substances, the increased expression of cyclin D1 and bcl2 can enhance cell proliferation, whereas the activation of p53, p21, and caspase3 leads to apoptosis [32–34]. These opposing processes can occur simultaneously, and the balance of gene expression influences the rate of cell proliferation.
In the results of gene expression regulating the cell cycle, the addition of taurine in chicken satellite cells was found to activate the cell cycle and inhibit apoptosis. Based on the results of different culture temperatures, it was observed that cell cycle progression is induced more at 42°C than at 37°C, and there is also an effect of inducing apoptosis inhibition at 42°C. In this study, exposure to high temperatures resulted in increased expression of p21, which induces apoptosis, as well as increased expression of bcl2 which inhibits apoptosis, and cyclin D1 which promotes cell proliferation. Additionally, when taurine was added, there was a further increase in cyclin D1 expression, coupled with a decrease in caspase3 expression, indicating reduced cell apoptosis. This finding aligns with previous research demonstrating that taurine suppresses caspase3 expression, thereby inhibiting cell apoptosis [35].
The mechanism of apoptosis involves the recognition of DNA damage by p53, followed by p21 mediated cell cycle arrest. If the damage is irreparable, apoptosis is triggered through caspase3 [36]. In this study, although p21 expression was significantly elevated due to heat stress, taurine supplementation inhibited caspase3 expression, thereby preventing apoptosis. These complex regulatory mechanisms suggest that heat stress simultaneously promotes both cell proliferation and apoptosis, but the addition of taurine mitigates cell death, resulting in a significant increase in cell viability and proliferation under heat stress conditions.
The extent of stress regulator gene expression in cells can vary depending on whether there is no mild or excessive stress [37,38]. Differences in these responses also depend on the adaptation of the cells to external stimuli [39].
Cells exposed to external stimuli such as heat stress can experience oxidative stress, protein denaturation, and membrane damage, triggering intracellular stress responses. Cells cultured under high-temperature show increased reactive oxygen species (ROS) production, leading to oxidative stress. Oxidative stress occurs due to an imbalance between the generation and removal of ROS within the cell [40]. To counteract this, various antioxidant systems are activated, with SOD and CAT being the key antioxidant enzymes. SOD, in particular, acts as the initial antioxidant enzyme by catalyzing the breakdown of ROS into less toxic H2O2, making it a crucial marker for oxidative stress and cellular defense capacity [41]. Several studies have demonstrated that SOD expression increases with rising temperatures [41–43].
SOD and Acox2 significantly increased at 42°C, while calpain significantly decreased at the same temperature. SOD and calpain were upregulated by taurine, whereas CAT showed the opposite trend. From these results, it is inferred that cells extracted from broiler embryos have a higher defensive capability against external stimuli at relatively higher temperatures like 42°C, and taurine appears to enhance this defensive capability.
The MAPK/ERK-Nrf2 signaling pathway, greatly affected by heat stress, is a signaling or transcription factor involved in various cellular processes [44]. It responds to extracellular signals, such as external stimuli, hormones, and growth factors, and conveys these signals inside the cell to elicit a response. Depending on the type and intensity of the stimulus, these factors can either induce or inhibit each other’s activities and can be regulated through indirect mechanisms. ERK is a protein whose expression increases in response to extracellular signals, promoting various cellular functions, such as cell proliferation, survival, and differentiation [45]. The change in ERK expression appears to be a result of external stimuli. In this study, taurine downregulated ERK. However, at a temperature of 42°C, an increase in expression was noted. This increase in ERK expression corresponds to the theory that it can enhance cell proliferation and survival, which is consistent with the cell survival rate data in this study [46]. JNK, which increases in response to external stress or cell death signals, was upregulated by taurine but significantly decreased at a 42°C. NFkB, another factor that is upregulated as a defensive response to external stimuli, was significantly reduced by the addition of taurine, consistent with previous research findings [47]. According to the [48], following the induction of oxidative stress and the addition of taurine, the expression of proteins involved in the MAPK pathway, including ERK, JNK, and p38, was upregulated, along with the activation of their phosphorylated forms, p-ERK, p-JNK, and p-p38. These findings align with the patterns observed in the present study. Additionally, it is believed that cells cultured at 42°C with added taurine can mitigate the response to external stimuli. Nrf2, also a factor that regulates defence against oxidative stress [49], was significantly reduced in the taurine added groups, and it showed a significant upregulation at 42°C compared to 37°C. Integrating these results, it can be inferred that satellite cells extracted from broilers, when cultured at 42°C as opposed to 37°C, are likely to induce cell proliferation and differentiation, or have a significantly lower potential for cell apoptosis. Moreover, taurine can alter the response of cellular signaling factors to high temperatures.
Previous studies have shown that taurine has a positive effect on heat stress under broiler rearing conditions. Similarly, in the present study, culture media supplemented with taurine demonstrated a significant positive effect on cells cultured under heat stress conditions. Additionally, it was determined that the effects of taurine vary depending on environmental temperature.
In conclusion, at 42°C, cell viability was significantly increased, accompanied by the up-regulation of both cell-promoting factors (cyclinD1) and inhibitory factors. however, the addition of taurine significantly further enhanced cell viability, primarily through the doen-regulation of inhibitory factors and the up-regulation of cell cycle-promoting factors. consequently, the high temperature environment stimulated overall cell growth and metabolic processes. Taurine suppressed the expression of negative factors, such as cell cycle arrest and inflammatory markers, thereby promoting beneficial effects under heat stress conditions.
