INTRODUCTION
In recent years, pets have been considered as members of the family [1]. This trend increased the consumers’ demand for well-made pet food, and many efforts have been made to develop pet food with a variety of ingredients [2]. Pet food commonly includes a variety of animal and plant-based ingredients, such as chicken, beef, salmon, soy, grains, fats, oils, vitamins, and minerals to provide balanced nutrition and flavor [3].
Although adding different ingredients can provide excellent feed for pets, their involvement can also increase safety concerns for pet food. In the case of dry pet food, the most commonly used type, it undergoes a complex manufacturing process, including grinding, mixing, extrusion, drying, cooling, and packaging [4]. During these processes, the probability of contamination with various raw ingredients, using unhygienic equipment, and cross-contamination, especially by pathogens, can increase [5]. According to the US Food and Drug Administration (FDA) recall database, there were 3,691 pet food recalls in the United States between 2003 and 2022, often due to contamination by Salmonella serovars, Listeria monocytogenes, fungi, and mycotoxins. Such contamination can lead to symptoms like vomiting, fever, diarrhea, dehydration, and loss of appetite, and in severe cases, pose life-threatening risks on pet animals [6]. Especially, if ingested continuously, even small amounts of mycotoxins can accumulate to high levels in the liver, potentially inducing cancer. Therefore, preventing microbial and mycotoxin contamination in pet food before consumption is essential.
Meanwhile, irradiation may effectively decrease both microorganisms and mycotoxin in food products while minimizing nutritional loss and adverse changes in its quality, as it is conducted without heat [7]. Three different types of irradiation sources, namely gamma-ray, electron beam (EB), and X-ray (XR), can be applied in the food sector. Gamma-ray irradiation, despite its highest penetration capabilities, involves the use of radioactive isotopes, posing safety concerns [8]. In contrast, EB and XR technologies provide a safer alternative due to their electrical generation methods, ceasing emissions when it is not in operation [9]. This safety advantage drives increasing preference for EB and XR in the food industries and among consumers [10]. EB consist of electrons flowing directly, whereas XRs are generated when the motion of electrons interacts with atoms, transforming into electromagnetic radiation [11]. Generally, there is a difference in their penetration depth [12]. EBs interact directly with materials, causing them to lose energy quickly within the material. On the other hand, XRs are a form of electromagnetic wave with very short wavelengths and possess stronger penetrating power.
Several studies have explored the decontamination effects and physicochemical quality changes in various foods such as fruits, vegetables, grains, meats, seafoods, and dairy products following irradiation with EB and XR [13,14]. However, the impact of irradiation on the quality of pet food remains largely unexplored. Also, the differential effects on pet food quality attributable to the distinct generation mechanisms of EB and XR remain underexplored. Therefore, we evaluated the decontamination effects of EB and XR on the microorganisms and mycotoxins in dry pet food as well as the consequent changes to its physicochemical properties.
MATERIALS AND METHODS
The dry pet food in the form of extruded kibble (10 mm in diameter) was supplied by ATbio. The samples (100 g) were divided into air-impermeable bags and sealed for EB and XR treatments. Then, sample packs were stacked to a thickness of 5 cm to minimize deviations in the transmittance of the irradiation.
Before the irradiation process, two 5 mm alanine dosimeters (Bruker Biospin GmbH) were attached to the front and back of the sample packaging, perpendicular to the direction of irradiation treatment. The dosimeters were analyzed using an electron paramagnetic resonance analyzer (e-scanTM alanine dosimeter reader, Bruker BioSpin GmbH), following International Atomic Energy Agency standardization procedures.
EB irradiation was performed at the Advanced Radiation Technology Institute of the Korea Atomic Energy Research Institute using a 10 MeV linear electron accelerator (MB 10-30, Mevex). The beam was maintained at a constant level, and samples were exposed to EB doses of 2.5, 5, 10, and 20 kGy at ambient temperature. XR irradiation was conducted using a high-energy linear accelerator (MB10-8/635, UEL V10–10S, Seoul Radiology Services) with a beam energy of 7 MeV. Samples were exposed to XR doses of 2.5, 5, 10, and 20 kGy at a temperature of 25°C. A non-irradiated group (0 kGy) was used as the control.
After irradiation, the sample bags were opend and stored in aerobic conditions at 25°C and 70% relative humidity to mimic the consumer’s storing pattern. Each sample was collected for further analysis on days 0, 14, 28, 42, and 56. Since opened dry pet food is typically consumed within 4 to 6 weeks, we set a 56-day maximum to reflect realistic usage conditions.
After being irradiated, each 5 g sample was aseptically collected. Microorganisms were enumerated following the method by Park et al. [15]. The sample was homogenized for 2 min using a stomacher (BagMixer400P, Interscience) in sterile Whirl-Pak bags with 45 mL of sterile saline solution. The solution was serially diluted, and aliquots were spread onto plate count agar (PCA) and potato dextrose agar (PDA). PCA plates were incubated at 37°C for 48 h, and PDA plates at 25°C for 120 h. Colonies on PCA plates were counted as total aerobic bacteria (TAB) and those on PDA plates as yeast and molds (YM), expressed as colony-forming units per gram (CFU/g). Each distinct single colony was isolated and identified according to the method described by Lee et al. [16].
To prepare aflatoxin B1 (AFB1) solution sample, AFB1 (≥ 98.0%, Sigma-Aldrich) in powder form was dissolved in acetonitrile to obtain a concentration of 80.00 µg/L. Each 100 mL of this solution was transferred to nylon polyethylene/polypropylene bags and sealed. The bags were then irradiated with electron beam and X-ray at doses of 0, 2.5, 5, 10, and 20 kGy.
To prepare AFB1 spiked dry pet food sample, AFB1 powder was diluted to 0.004 µg/L in acetonitrile, and 1 mL of this solution was used to spike 50 g of dry pet food, reaching a final concentration of 80.00 µg/kg. Each 50 g of sample was then transferred to nylon polyethylene/polypropylene bag (size: 15 × 20 cm, thickness: 0.07 mm, wire diameter, Whirl-Pak® Inc.) and sealed. The bags were irradiated with EB and XR at doses of 0, 2.5, 5, 10, and 20 kGy.
Total AFB1 in the samples was determined by high performance liquid chromatography (HPLC) following extraction, purification, and qualitative & quantitative analysis. (i) Extraction: The homogenized dry pet food sample (25 g) was extracted with 100 mL of 70% methanol for 30 min, followed by centrifugation at 2,265×g and 4°C for 15 min. The solution was filtered through a 0.2 μm syringe filter, and 40 mL of 0.1% Tween 20 in phosphate buffered saline (PBS) was added to 10 mL of the filtrate. (ii) Purification: The sample solution (20 mL) was injected into the immunoaffinity column, with flow adjusted to 2–3 mL/min. After passing through, the column was washed with 10 mL of 0.1% Tween 20 in PBS and 10 mL of distilled water. To elute the bound AFB1, 1 mL of methanol followed by 1 mL of distilled water was used. (iii) HPLC analysis: The purified sample was injected into the C18 UG120 HPLC column (4.6 × 250 mm, 5 μm). The mobile phase consisted of acetonitrile, methanol, and distilled water in a 1:3:6 (v/v) ratio. The injection volume was 10 μL, with a flow rate of 1.2 mL/min. A fluorescence detector with wavelength of 360 nm for excitation and 450 nm for emission was used. The AFB1 concentration was calculated by comparing peak areas to a standard curve.
The pH was measured as described by Jung et al. [17]. The sample (1 g) was added to 9 mL of distilled water and homogenized for 30 s. After centrifuging the homogenate at 2,265×g (Continent 512R, Hanil Scientific), the supernatant was filtered (Whatman No.1, Whatman), and the pH was measured using a pH meter (Seven2GO, Mettler-Toledo).
The thiobarbituric acid reactive substance (TBARS) value was determined using the methods described by Park et al. [15]. First, 5 g of minced sample was combined with 15 mL of DDW and 50 μL of 7.2% 2,6-Di-tert-butyl-4-methyl-phenol in ethanol, then homogenized at 9,600 rpm for 30 s (T25 basic, IKA Works). The homogenate was centrifuged at 2,265×g (Continent 512R, Hanil), and the supernatant was filtered (Whatman No.4). A 1 mL aliquot of the filtrate was mixed with 2 mL of 20 mM thiobarbituric acid in 15% TCA, heated at 90°C for 30 min, cooled, vortexed, and centrifuged at 2,265×g for 15 min. The absorbance of the supernatant was measured at 532 nm using a spectrophotometer (M23, Molecular Devices). TBARS values were expressed as mg of MDA per kg of dry pet food, calculated using a standard curve.
The carbonyl content was measured using the method described by Lee et al. [18]. The dry pet food sample (1 g) was homogenized (T25 basic, IKA Works) in 10 mL of 0.6 M NaCl in 20 mM sodium phosphate buffer (pH 6.5) at 9,600 rpm for 30 s. The homogenate was divided into 2 test tubes, one for carbonyl content and the other for protein content. Each tube received 0.2 mL of homogenate and 1 mL of 10% TCA, then centrifuged at 1,000×g for 10 min, after which the supernatant was removed. For protein content, 1 mL of 2 M HCl was added to the pellet, reacted at room temperature for 1 h, followed by another centrifugation after adding 1 mL of 10% TCA, and the supernatant was discarded. Then, 2 mL of 6 M guanidine HCl in 20 mM sodium phosphate (pH 6.5) was added and the solution was diluted 5-fold. Absorbance was measured at 280 nm using a spectrophotometer (X-ma 3100, Human Corporation), and the protein content was quantified using a standard curve obtained with bovine serum albumin. To determine carbonyl content, 0.2% DNPH in 2 M HCl (1 mL) was added to the pellet, reacted at room temperature for 1 h, then centrifuged with 1 mL of 10% TCA, and the supernatant was discarded. To wash the DNPH color, 1 mL of ethanol and ethyl acetate (1:1, v/v) solution was added, followed by vortexing and centrifugation at 1,000×g, after which the supernatant was removed. This washing process was repeated three times. Then, 2 mL of 6 M guanidine HCl in 20 mM sodium phosphate (pH 6.5) was added, and absorbance was measured at 370 nm. Carbonyl content was expressed as nmol carbonyls mg−1 using a molar absorptivity of 22,000 M−1 cm−1.
Volatile compounds in dry pet food were analyzed using the solid-phase microextraction and gas chromatography-mass spectrometry (SPME-GC-MS) method described by Ismail et al. [19]. The dry pet food sample (3 g) was placed into a 20-mL headspace vial and sealed with a PTFE-faced silicone septum. For volatile extraction, the vial was warmed to 40°C for 5 min, then a 65 μm polydimethylsiloxane/divinylbenzene fiber (Supelco) was exposed to the vial’s headspace for 60 min. The collected volatiles were desorbed at 270°C in the gas chromatograph’s injection port (Trace 1310, Thermo Fisher Scientific) in splitless mode. Helium served as the carrier gas at a flow rate of 2 mL/min, facilitating the separation of volatile compounds in a fused silica capillary column (DB-Wax, 60 m × 0.25 mm i.d., 0.50 μm film thickness; Agilent Technologies). The GC oven temperature started at 40°C, increased to 180°C at a rate of 5°C/min, then rose to 200°C at 2°C/min and held for 5 min, before increasing to 240°C at 10°C/min, held for 10 min. The triple quadrupole mass spectrometer (TSQ 8000, Thermo Fisher Scientific), directly connected to the column, operated in electron ionization mode at 70 eV and 250°C. Mass spectra were acquired over a scan range of 35 to 550 m/z at 0.2 s intervals. Volatile compounds were identified by matching their mass spectra with the National Institute of Standards and Technology mass spectral library.
For assessing the effect of irradiation treatment on microbial activity and quality attributes, all samples were analyzed in triplicate. Data was analyzed using SAS software (Version 9.4, SAS Institute). A one-way ANOVA with Tukey’s test was utilized to identify significant differences between the means (p < 0.05).
RESULTS AND DISCUSSION
The initial count of TAB in the dry pet food was 2.84 Log CFU/g (Fig. 1). Irradiation showed a dose-dependent inactivation effect, with TAB significantly reduced from 5 kGy of EB and 2.5 kGy of XR. At 10 kGy, no bacteria were detected in EB- and XR-irradiated samples on day 0. This reduction is due to highly reactive free radicals generated by irradiation, damaging bacterial cell membranes and DNA [20]. Since different bacterial species have varying sensitivities for irradiation, the bacteria present in the samples before and after irradiation were identified (data not shown). From the non-irradiated samples, 14 different bacteria were observed: Acinetobacter radioresistens, Bacilus cerues, Bacilus glycinifermentans, Bacillus haynesii, Bacillus inaquosorum, Bacillus licheniformis, Bacillus sp. (in: firmicutes), Bacillus sp. THJ-DT1, Bacillus subtilis, Bacillus tequilensis, Priestia megaterium, Rummeliibacillus sp., Rummeliibacillus stabekisii, and Staphylococcus sp. BCRC 81404. Among them, Bacillus cereus and Bacillus licheniformis are known as pathogenic bacteria. Both pathogens were eliminated from the dry pet food when 2.5 kGy of EB and XR were treated. One and three different bacteria remained in EB- and XR-treated samples up to 5 kGy, respectively, however, all bacteria were sterilized at 10 kGy of EB and XR.
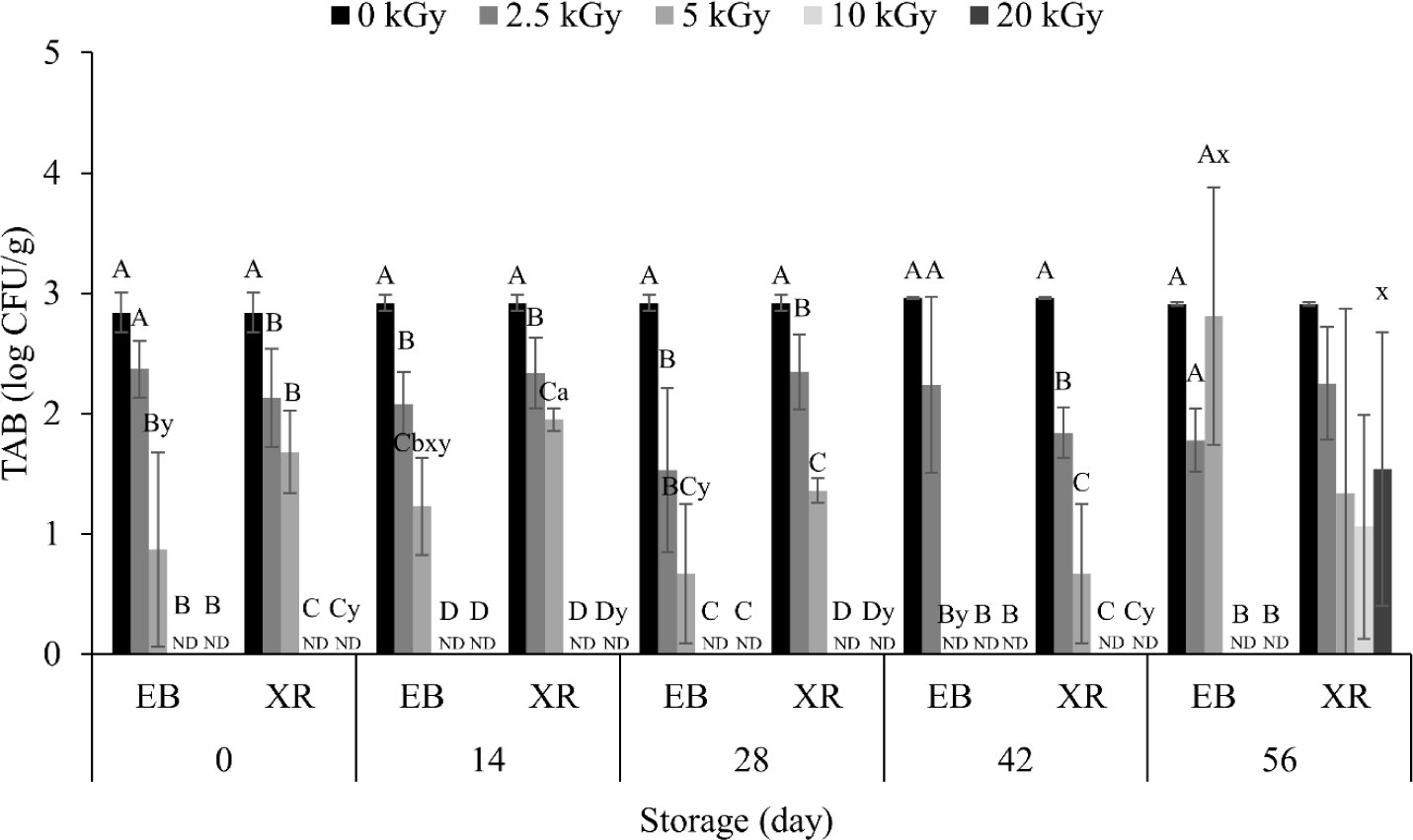
On the other hand, there was no significant difference in TAB counts between EB and XR during the whole storage period (Fig. 1). Generally, XR penetrates deeper than EB [12], however, our results did not reflect this, possibly due to the location of TABs in the dry pet food. Both EB and XR may sufficiently penetrate when TABs are at shallow depths. In this study, the height of the samples was 5 cm during irradiation. Additionally, penetration depth does not always correlate with high inactivation, as charged particles from EB are known to interact more intensively with matter than photons from XR [21]. This phenomenon is also supported by other studies, such as Jung et al. [22], which found the D10 value of EB was lower than XR, indicating a higher inactivation effect with EB.
In different food resources, TABs can grow with increasing storage period [23]. However, most TAB counts in dry pet food did not change significantly throughout the storage period, except for XR on day 56 (Fig. 1). The initial TAB count was 2.84 Log CFU/g and did not exceed 2.92 Log CFU/g despite of long-term storage. This low TAB level in dry pet food may be attributed to its low water activity (ranged 0.4–0.5, Table 1), as most bacteria require water activity above 0.9 to survive [24].
Different letters indicate significant differences (p < 0.05) between different irradiation dose treatments.
Before irradiation, the number of YM were 2.17 Log CFU/g (Fig. 2). This count was not significantly reduced with 2.5 kGy of EB and XR. However, 5 kGy sterilized all YMs in the dry pet food on day 0, regardless of irradiation type. Previous studies have shown that the inactivation effect on YM is due to an increase in chitinase activity and a decrease in chitin content within fungal cell walls, leading to their collapse [25]. In addition, irradiation can increase intracellular H2O2 content, inducing oxidative stress, further contributing to the inactivation of YM. Here, we also confirmed the effect on different YMs. A total of six YMs, Aspergillus sydowii, Cladosporium parasphaerospermum, Diaporthe eres, Penicillium brevicompactum, Schizophyllum commune, and Schizophyllum sp., were detected in non-irradiated samples (data not shown). However, both EB and XR eliminated all YMs except Schizophyllum commune. Similar to the result in TAB (Fig. 1), we also found no significant difference for YMs between EB and XR (Fig. 2).
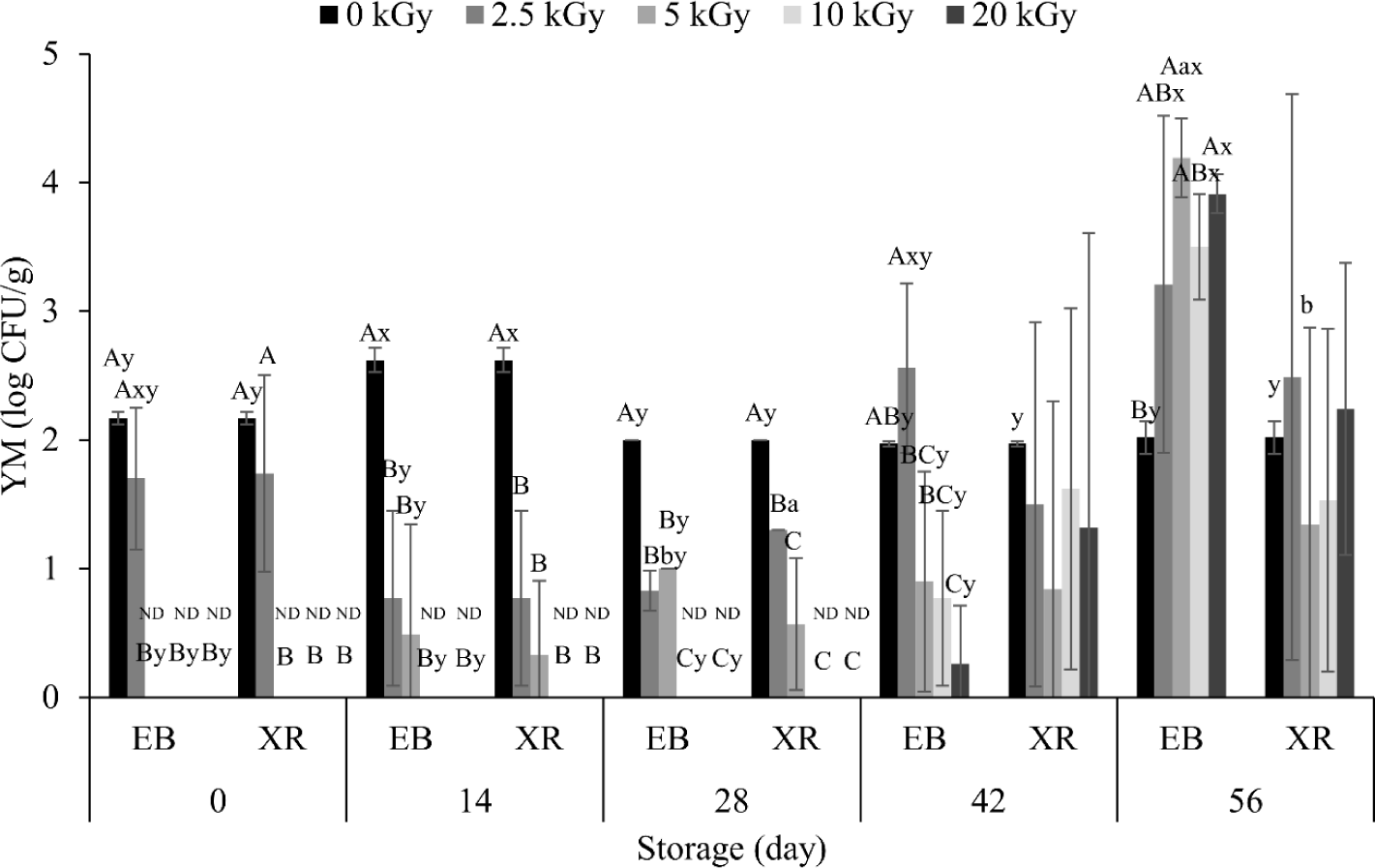
On the other hand, significant increase in YM counts were observed over the extended storage period. In non-irradiated samples, YM numbers slightly increased on day 14 and decreased thereafter. However, variations were small, with counts mostly ranging from 1.97–2.62 Log CFU/g during the whole storage period. In the irradiated dry pet food, YM counts remained lower until day 42, with an increase in EB-treated samples on day 56. This increase in YMs may be due to various factors, including penetration depth, survival condition, and recontamination.
AFB1 is a fungal toxin, and pet food, especially dry, is prone to its contamination [26]. When pets consume AFB1 in pet food, it can cause poisoning symptoms and serious liver damage, potentially leading to cancer with long-term exposure [27]. The effects of EB and XR on AFB1 decontamination were examined in both AFB1-inoculated solution and samples (Fig. 3). EB and XR could reduce AFB1 concentration in solution (80.00 µg/L), but were not effective in dry pet food (80.00 µg/kg). In solution, a higher dose resulted in greater AFB1 reduction. When treated with 5 kGy of EB and 10 kGy of XR, AFB1 in the solution was eliminated. Similar to the previous studies, this result showed the potential of these treatments for AFB1 reduction [28,29]. Irradiation can generate free radicals that damage the structure of AFB1, reducing its mutagenicity and cytotoxicity [30,31]. Wang et al. [32] reported that EB irradiation degraded AFB1 into two different products, C14H12O5 and C17H14O5.
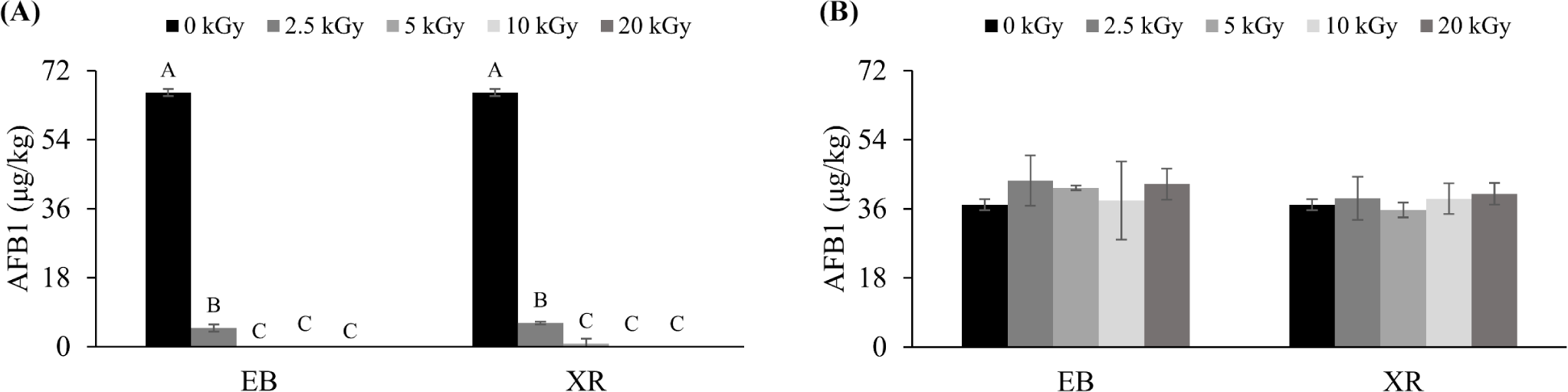
However, both EB and XR did not reduce AFB1 in dry pet food (Fig. 3), possibly due to the low moisture content. Moisture affects mycotoxins degradation, as radiolysis of water during irradiation generates highly reactive hydroxyl radicals (H• and HO•) [33]. Liu et al. [34] found that AFB1 degradation in peanuts increased with moisture content. Woldemariam et al. [35] found no significant AFB1 reduction in red pepper irradiated with 30 kGy of EB. This suggests that AFB1 in dry pet food may be difficult to decontaminate, and the irradiation dose used may not be sufficient to achieve significant reduction. Temcharoen et al. [36] suggested that very high doses, ranging from 50 to 100 kGy, are needed to deactivate aflatoxins in certain foods. Liu et al. [34] observed the degradation of AFB1 in peanut meal with EB up to 300 kGy. However, achieving such high doses of irradiation is impractical for commercial applications due to the cost, potential damage to the food product, and regulatory limitations.
Instead of AFB1, controlling fungal growth in the dry pet food and its ingredients may significantly lower the risk of mycotoxins. Since AFB1 is primarily produced by Aspergillus flavus [37], controlling such fungi through irradiation can prevent AFB1 occurrence. For instance, reducing Aspergillus flavus in Brazil nuts with 5 kGy and 10 kGy of EB and gamma rays also reduced aflatoxin levels [38]. Zhang et al. [39] used gamma rays at 10, 20, and 30 kGy on soybeans to control Aspergillus flavus, achieving significant AFB1 reduction. Therefore, it is essential to deactivate mycotoxin-producing fungi, including those responsible for aflatoxin production like AFB1, through irradiation before toxin formation occurs.
Water activity represents the availability of water for biochemical reactions and is expressed as the ratio of the vapor pressure in a substance to the vapor pressure of pure water [46]. Until day 14, irradiation did not change the water activity in dry pet food, except for XR on day 0 (Table 1). From day 28, water activity varied with irradiation types and doses, but no specific trend was observed. The range of water activity in dry pet food, from 0.437 to 0.560, was not conducive to microbial growth [47]. Generally, bacteria do not grow below 0.91, and most molds do not grow below 0.80 [48], which explains the lack of significant increase in microorganisms over time as shown in Figs. 1 and 2. Meanwhile, water activity fluctuated with storage days without any consistent trend, likely due to the variable temperature and humidity conditions at the measurement site during the storage period.
Changes in the pH can affect the flavor, texture, and color of food by altering the acidity and impacting the structure of components like pigments, fibers, and proteins [40]. In this study, XR did not change the pH value in dry pet food during the whole storage period (Table 2). However, the pH of EB-treated samples increased significantly with higher doses, occasionally surpassing that of XR-treated samples (p < 0.05). Generally, the pH increase with irradiation is attributed to the influence of free radicals [41]. According to Paul et al. [42], pH changes are attributed to protonation stimulated by radical reactions, potentially affected by ionic interactions. While EB generally has a shallower penetration compared to XR [43], high-energy charged particles from EB interact more intensively with materials than XR photons [21]. This more intense interaction may result in greater pH increases when using EB compared to XR (Table 2).
Different letters indicate significant differences (p < 0.05) between different irradiation dose treatments.
Over the storage period, the pH value of non-irradiated samples significantly increased. However, both EB and XR remained stable pH levels during storage, except for EB at 20 kGy. The rise in pH observed during storage could result from protein degradation, forming small nitrogen-containing components with alkaline properties [44]. This increase may also be due to microorganisms in dry pet food degrading proteins and producing nitrogen compounds like ammonia, leading to higher pH levels [45]. Therefore, it can be said that EB and XR irradiation contributed to inhibiting microbial growth, thus helping prevent changes in pH.
The pH changes were practically small, ranging from 6.35 to 6.41, and there were no significant differences in color and volatile basic nitrogen (VBN) value due to irradiation or the storage period. Additionally, the measured range of proximate composition, including moisture (5.30%–6.58%), crude protein (34.29%–34.66%), crude fat (10.49%–13.84%), crude fiber (3.34%–4.39%), and crude ash (7.55%–7.97%), showed minimal differences, indicating that EB and XR up to 20 kGy and a 56-day storage period did not significantly affect the overall quality of the dry pet food.
Thiobarbituric acid reactive substance (TBARS) values measure the level of malondialdehyde (MDA), which is a product of lipid peroxidation [49]. This indicates lipid spoilage progression, which can affect the sensory quality of food, impacting taste, odor, and overall acceptability [50]. On the whole, EB- and XR-treated samples showed higher TBARS values than the control during 56-day storage period (Table 3). Also, their values were largely increased with higher doses (p < 0.05). Specifically, the non-irradiated sample had 3.58 mg MDA/kg, while 20 kGy of EB and XR increased this to 5.31 mg MDA/kg and 5.33 mg MDA/kg, respectively. This increase is possibly by free radicals produced during the irradiation process [51]. Lipid oxidation by free radicals involves initiation, propagation, and termination stages. In initiation, reactive oxygen species create lipid radicals from unsaturated fatty acids. During propagation, these radicals form lipid peroxyl radicals that react with other lipids to produce unstable lipid hydroperoxides (ROOH). These hydroperoxides then degrade into aldehydes, ketones, and alcohols, affecting the taste, smell, and overall quality of food, until termination stabilizes the radicals [52]. On the other hand, no significant differences were observed between EB and XR treatments.
Different letters indicate significant differences (p < 0.05) between different irradiation dose treatments.
In all irradiation doses, TBARS values tended to increase over the storage period, indicating the accumulation of lipid oxidation products [53]. A slight decrease in these values was observed on day 56 (Table 3). This phenomenon could be attributed to microbial metabolism or binding to the other substances [54,55]. In summary, the increase in TBARS values with irradiation was more pronounced than the effects of storage time, as EB and XR can significantly promote lipid oxidation. This should be considered since lipid oxidation can deteriorate to safety and sensory qualities of food [56].
Protein carbonyl usually originates from the oxidation of amino acid side chains or the breakdown of peptide chains with oxidation [13]. During the storage period, protein carbonyl content in irradiated samples was significantly higher compared to the control (Table 4). The content increased with higher irradiation doses (p < 0.05). Free radicals produced during irradiation can cause protein oxidation, generating protein carbonyl content [57]. Feng et al. [13] reported that raw ground beef treated with EB irradiation develops higher protein carbonyl content than the control. Li et al. [58] also found that irradiation increases protein carbonyl levels in a pork meat emulsion system. Furthermore, it has been reported that many lipid-derived radicals and hydroperoxides also contribute to the formation of carbonyl contents by accelerating protein oxidation [59]. Therefore, the increase in lipid oxidation levels shown in the TBARS results (Table 3) could also be linked to the increased carbonyl contents in EB- and XR-treated samples (Table 4).
Different letters indicate significant differences (p < 0.05) between different irradiation dose treatments.
Comparing EB and XR, their carbonyl contents were not significantly different, except at 10 kGy (Table 4). However, as the storage period increased, XR tended to have a greater effect compared to EB (p < 0.05), with carbonyl content increasing over the storage days. This suggests that free radicals generated from irradiation continued to impact over time. Furthermore, since XR has a deeper penetration depth compared to EB, resulting in a lower scattering at the surface [43], could lead to higher carbonyl content in the XR-treated samples than that in the EB-treated samples. Thus, it can be concluded that over storage time, XR increased protein oxidation more due to deeper penetration in dry pet food and the persistent effect of irradiation-induced radicals.
Volatile compounds were analyzed to assess the impact of EB and XR on odor changes in dry pet foods (Table 5). Among the many peaks, 33 oxidation-related volatile compounds were identified, including 16 hydrocarbons, 9 aldehydes, 3 ketones, and 5 alcohols. On day 0, significant increases in hydrocarbons, aldehydes, ketones, and alcohols were observed when EB and XR were applied to dry pet food. These increases in volatile compounds are related to oxidation and significantly affect food flavor [60]. It is known that irradiation can generate highly reactive species that accelerate oxidative processes in proteins and lipids, producing many secondary and volatile compounds [61]. In this regard, the increase in volatile compounds aligns with the increase in the TBARS value (Table 3) and the carbonyl content value (Table 4). Moreover, the changes in volatile compounds varied between EB and XR treatments (Table 5), highlighting inconsistent differences between the two irradiation methods.
Among the identified hydrocarbons, saturated straight-chain alkanes (n-octane, n-nonane, n-decane, n-dodecane, n-pentadecane, and n-tetradecane) and unsaturated hydrocarbons (1-octene, 1-decene, and 1- undecyne) are known radiolytic products which can be originated from fatty acids [62]. Branched alkanes (2,6,10-timethyldodecane, 5-ethyl-2,2,3-trimethylheptane and 2,6,8-trimethyldecane) significantly increased with irradiation. In addition, several alkane and alkene contents (1-butyl-2-methyl cyclopropane, n-decene, n-octane, n-nonane, 1-decene, and 1-octene) were significantly higher in EB-irradiated samples compared to XR-irradiated samples. The formation of alkanes and alkenes involves ionization and cleavage near carbonyl groups, leading to radical reactions that determine whether alkanes or alkenes are produced based on the cleavage site [21].
Irradiation also increases aldehydes and ketones due to free radicals promoting dehydrogenation reactions within molecules. This process includes the oxidation of primary alcohols to aldehydes and secondary alcohols to ketones [63,64]. These oxidation processes increase the content of carbonyl groups (aldehydes and ketones), which aligns with the increase in carbonyl content (Table 4). All 9 detected aldehydes were found in greater quantities in EB- and XR-treated samples compared to non-irradiated ones. Specifically, 2,4-heptadienal, 2-methyl butanal, 3-methyl butanal, hexanal, octanal, and pentanal were higher in XR-treated samples, while 2-heptanal, heptanal, and nonanal were higher in EB-treated samples. The increase in aldehydes indicates lipid oxidation. Aldehydes like heptanal, octanal, nonanal, pentanal, and hexanal are responsible for the unpleasant odors in poultry products [65]. This increase can cause bitter, metallic, and sour taste [61], making the product unpleasant and indicating quality deterioration.
The quantities of all 3 detected ketones were higher in EB- and XR-treated samples compared to the control group, with higher levels in XR-treated samples. 2-butanone and 3,5-octadien-2-one maintained this trend after 56 days, while 2-propanone showed no significant difference between EB and XR treatments. The total amount of ketones decreased by day 56, mainly due to a reduction in 2-propanone. It was reported that 3,5-octadien-2-one is a principal compound causing off-flavor in isolated lentil protein [66]. It is known that this increase in ketone can cause rancid, fruity, acetone-like odor [61]. These odors can give the food a chemical-like smell, which can be unpleasant.
All 5 detected alcohols (6,9-pentadecadien-1-ol, 1-hexanol, 1-octen-3-ol, 1-penten-3-ol, and 2-methyl-2,3-pentanediol) increased significantly with both EB and XR treatments (Table 5). This increase could be due to structural changes in carbohydrates, reduction of aldehydes, and the breakdown of fatty acids during irradiation [62]. These alcohols can serve as precursors to MDA [63]. Also, Mielnik et al. [67] noted that 1-penten-3-ol correlates highly with TBARS values, markers of lipid oxidation. The increase in alcohols due to oxidation can impart an alcoholic or chemical odor, potentially overwhelming the food’s original aroma and leading to an unpleasant sensory properties.
Therefore, it is necessary to verify how volatile substances produced by such oxidation actually affect the sense of smell perceived by pets and whether they have any negative effects through sensory evaluation.
CONCLUSION
Both EB and XR treatments demonstrated excellent efficacy in microbial decontamination of dry pet food without compromising its quality. Furthermore, there were no significant differences between the applications of EB and XR in this study. While higher doses achieved greater decontamination, they also induced oxidation and altered the volatile compounds in the dry pet food. In conclusion, employing EB and XR treatments in dry pet food effectively reduced TAB and YM without compromising its quality. However, given the potential for oxidation, further research is necessary to assess whether these oxidation products adversely affect the safety and sensory qualities of the food.
