Background
Consumption of meat and meat products have been increased dramatically probably due to their higher incomes in Korea [1]. Each Korean ate 9.5 kg of beef & veal, 28.4 kg of pork meat, 14.4 kg of poultry meat and 0.2 kg of sheep meat [2]. Especially, Korean meat consumers prefer for pork belly and Boston butt which contain high fat [3].
Production cost can be reduced by improving the market weight. To this end, The specific standards for nutritional requirements for swine were established early in 1940s by National Research Council (NRC) and in Korea, the National institute of Animal Science revised a feeding standard of swine in 2012, which adapted the ideal protein concept for pig production [4].
The source of odour from pig farming facilities is due to generation of large amounts of urine, feces and fermentation of this mixture during storage [5–7]. The odour of pig excreta is the result from anaerobic microbial degradation of the components of the feed in gut, especially some odours from proteins are more toxic and offensive sensation than the carbohydrates [8, 9]. Many types of odorants have been identified from samples of fresh and rotten swine manure [10] and these were classified into four main groups, namely straight- and branched-chain volatile fatty acids, indoles and phenols, ammonia and volatile amines, and the volatile sulfur compounds [6, 11]. Acetic acid, propionic acid and butyric acids are most common volatile fatty acids (VFA) produced from microbial conversion of dietary residues. The straight chain- VFA derived from both plant fibre and protein microbial degradation and the branched chain-VFA produced by anaerobic fermentation of amino acids such as leucine, isoleucine, lysine [7, 12]. Sulfur-containing volatile compounds such as hydrogen sulphide, dimethyl sulphide, carbonyl sulphide are originates from microbial degradation of amino acid substrates like cysteine and cysteine [13]. Aromatic compounds such as phenols, p-cresol, indoles and skatole are by-products of tyrosine and tryptophan degradation. These compounds are formed in the gut microflora and also during anaerobic storage of swine manure [7]. Nitrogenous compounds, ammonia and volatile amines are produced from protein degradation. Ratio of amino acids is an important element that affects the generation of odorous compounds in swine excreta [9, 14]. Several studies have been reported that decreasing crude protein (CP) level can be reduced faecal odorous compounds [15–17].
The term ideal protein can be defined as the protein which containing the minimum quantity of essential amino acids with maximum utilization to meet the exact nutritional amino acid requirements [18]. It refers to determine the required amounts of amino acids relative to lysine for maintenance, protein accretion, and growth performance of pigs [19, 20]. Munks et al. [21] first initiated the ideal protein concept to poultry for production of chicken egg. Later, Mitchell [22] was determined the requirement of amino acids for different animal species. In 1981, British agriculture research council (ARC) was first formulated feed based on ideal protein concept [19]. Since then, various new effective formulations have been developed based on ideal protein concept, such as Institute National de la recherche agronomique (INRA) in 1984 [23], NRC in 1988 [24], and Wang & Fuller in 1989 [18].
Until now, there is no reports on the effects of feed formulated based on the ideal protein concept on odorous compound generation from pig farm. Therefore, the objective of this study was to compare the excretion compounds and bacterial community from faeces of pigs fed diets formulated either by 2007or 2012 Korean feeding standards of swine.
Methods
A total eight (n = 8) barrows ({(Landrace × Yorkshire) × Duroc}] with an average bodyweight of 89.38 ± 3.3 kg were used for this study. These pigs were randomly allocated into two diet (2007 & 2012) groups. Pigs were housed individually in metabolism cages and the temperature was maintained at 25 ° C. The experiment was performed two replications. All procedures involving animals were approved by the National Institute of Animal Science Animal Care and Use Committee.
Feeds were formulated based on 2007 & 2012 Korean feeding standard for swine. The form of the feed was ground form, ingredient composition for diets were given in Table 1 and nutrient content are shown in Table 2. The Feed were fed in a limited manner, approximately 1.5 kg was fed to each pig per twice a day at 10.00 am and 16.00 pm. Pigs had free access to water throughout the experiment.
aDCP digestible crude protein
aDE Digestible energy, bME Metabolizable energy, cCP Crude protein
After 15-day adaptation period, fresh faecal samples were collected directly from pigs every week for 4 weeks. The 40 ml of each sample was taken into 50 ml tube and then stored in a refrigerator for further analysis.
Blood sample was collected from the vein with using syringe. After collecting the blood, 2 ml of collected blood was transferred into a tube containing anticoagulant (K2 EDTA 5.4 MG tube BD Vacutainer REF 367856) for haematology analysis, and a tube containing anticoagulant (Lithium Heparin tube BD Vacutainer REF 367884) 2 ml of blood was added and 8 ml of blood serum was stored in a serum separation tube (BD SST II AVANSAT REF 367953).
Initial and final body weight of pigs in each group were measured to calculate the average daily gain (ADG). Feed intake and excretion (faeces & urine) were recorded to determine the feed efficiency by calculating feed conversion ratio (FCR).
VFA analysis were performed by gas chromatography (GC) (6890 N, Agilent, Santa Clara, CA, USA), equipped with an HP - INNOWax column and a flame ionization detector.
To analyze the VFA, 5 ml of slurry sample, 25% phosphoric acid solution and 1 ml of saturated mercury solution (Sigma-Aldrich, St. Louis, Mo., USA) were taken into a 15 ml tube and then the solution was centrifuged at 3134 x g for 20 min. Thereafter, 1 ml of the supernatant was centrifuged at 13,800 x g for 10 min and filtered through a 0.2 μm filter (Whatman, Uppsala, Sweden). The filtrates were placed in 2.0 ml GC vials (Agilent, Santa Clara, Calif., USA) to measure the concentration of volatile fatty acids by GC.
0.2 μLwas the sample injection volume with a split ratio of 10:1.The temperature of the oven started from 80 °C, and then initially increased by 20 °C per minute and kept at 120 °C for 2 min, then the temperature was upgraded to 205 °C by increasing 10 °C per minute, finally, it was maintained at 205 °C for 2 min. The injection and detection ports were maintained at 250 °C.
The concentrations of phenols and indoles in slurry sample were quantitatively measured by Gas Chromatography. For this measurement, the slurry sample was centrifuged for 20 min at 3134 x g at 20 °C. 4 ml of the supernatant liquid was added to the 20 ml glass bottle which containing 4 ml of chloroform and 60 μm 4 M of sodium hydroxide solution. Then the mixture was centrifuged at 20 °C at 3134 x g for 20 min and then 2.0 ml of the chloroform layer was transferred to a GC vial to analyze the phenols and indoles by using GC equipped with flame ionization detector (FID) and DB-1 column (30 m × 0.25 mm × 0.25 μm, Agilent, Santa Clara, Clara, CA, USA). 0.2 μLwas the sample injection volume with a split ratio of 5:1. The temperature of oven was programmed as follows: initially started from 40 °C and held for 5 min and it upgraded to 230 °C by increasing 10 °C per minute. Then 230 °C was kept for 2 min. The injection and detection ports were maintained at 250 °C. The nitrogen was determined by Kjeldahl method [25].
The bacterial communities of pig slurry samples were analysed by PCR amplification based on 16S rRNA gene sequences by using the 27F and 518R primer pair.
In the first step, genomic DNA from slurry sample was isolated by Fast-DNA Spin Kit (MP Bio, Santa Ana, CA, USA) according to the manufacturer’s instruction manual. Humic acid, which is inhibiting PCR amplification was removed using the Power-Clean DNA Clean-Up Kit (MP Bio, Santa Ana, CA, USA). The amplification conditions of the PCR were followed: initially 1 cycle at 95 °C for 5 min and then 30 cycles of 30 s at 95 °C, 30 s at 55 °C, 72 °C for 30 s, and finally one cycle was performed at 72 °C for 7 min.
Pyrosequencing analysis was performed by Chunlab (Seoul, Korea) using the 454 FLX titanium System (Roche, Pleasanton, CA, USA). Sequencing reads of samples were assigned each sequence read to specific sample by their endemic bar codes. PCR primer sequences, barcode, and linker were then removed from the original sequence analysis. For the next analysis, the pyrosequencing reads were selected based on quality filtering process, which are containing more than 300 base pair with an average quality score of more than 25. EzTaxon-e database were used with the BLAST search tool to perform taxonomic alignment of bacterial high-quality sequence reads. Sequences that could not be matched the EzTaxon-e database, which is at 97% species level were used to a second-order UCHIME program to identify unrealistic sequences. Operational taxonomic units (OTUs) were generated using a CD-HIT program at with a similarity level of 97%. The Shannon-Weaver diversity index, Chao1 richness index and Goods library coverage were calculated using the Mothur program.
Concentration of odorous compounds data were analysed by using statistical analysis system [26] with using analysis of variance of GLM (General linear model). Significance difference among means of control and treatment groups were compared using Duncan’s multiple range tests [27]. Statistical analysis was also performed using Pearson’s correlation coefficient to determine the relationship between concentrations of odorous compounds and the relative abundance of bacterial species. Probability value less than 0.05 (P < 0.05) was considered for all measured variables.
Results and discussion
Although effects of CP levels on the concentration of odorous compounds and gut microbiota have been well established [17], little is known about the essential amino acids based diet impact on the microbial community changes in swine gut. Therefore, this study was performed to evaluate the impact of administration of diet formulated based on the ideal protein concept on growth performance, odorous compounds production, and gut microbial communities in pigs.
There was no significant difference in feed intake, BW, ADG, FCR, or amount of total faeces and urine excretion between treatment groups (Table 3). Lopez et al. [28] demonstrated that the ideal protein based diet improved the feed efficiency and carcass leanness in gilts but does not affect the growth performance. Similarly, Hong et al. [29] were also reported that feeding low energy and low protein diets with adequate amino acid supplements had no negative effects on growth performance of growing-finishing pigs. Other studies on piglets and grower-finisher pigs showed that dietary CP levels can be reduced by formulating pig diets with supplementary amino acids including Lys, Met, Thr, Trp, Phe, His, Val, Ile, and Leu without compromising their growth performance and feed efficiency when feeds were formulated by ideal protein concept [30, 31]. Hence, utilization of these supplements will reduce feed costs and improve profits for swine farmers as well as provide other benefits on environment by reducing N wastage and concentration of odours.
aMeans of 8 pigs per treatment with two replicates
bDM Dry Matter, cBW Body Weight, dADG Average Daily Gain, eFCR Feed Conversion RatiofSEM Standard errors of the means
The effects of pig diets formulated either by 2007 or 2012 feeding standard on levels of VOC are shown in Table 4. Phenolic volatile compounds are one of the major causes for odour in fresh slurry [32, 33]. Phenol was not detected in faecal samples of 2012 treatment group. Biochemical oxygen demand (BOD) levels were significantly decreased (P < 0.05) in 2012 group when compared with 2007 group. Levels of p-cresol and phenols were lower (P < 0.05) and pH tended to be lower (P = 0.07) in faeces from pigs fed 2012 diet compare to those fed 2007 diet. Other compounds including phenole, indole, skatole, total Kjeldahl nitrogen (TKN), and N also tended to decrease in 2012 group, suggesting odorous compound levels were decreased across the board by supplementation of pig diets formulated based on the ideal protein concept.
aVOC, volatile odorous compound; means of 8 pigs per treatment with two replicates
bPhenols = Phenol + p-Cresol, cIndoles = Indole + Skatole, dBOD, Biochemical Oxygen Demand, eTKN Total Kjeldahl Nitrogen
Canh et al. [34] have demonstrated that low levels of CP with essential amino acid supplements reduces nitrogen excretion and ammonia emission without compromising growth performance of growing-finishing pigs. Reduced CP content with adequate amino acids supplementation also showed reduced urinary energy loss as well as greater growth performance [35]. In the current study, CP levels included either in 2007 or 2012 diet were not statistically different, but adequate and appropriate ratio of amino acids were provided for 2012 diet (Table 2). There was abundant evidence that pigs fed ideal protein based diets showed maximum utilization of absorbed protein for maintenance and for tissue protein accretion (for growth) with minimum nitrogen (total N) excretion [36–38]. Reduced N excretion in pig slurry is closely associated with decrease in the odour emission from pig facilities [39]. Total Kjeldahl nitrogen (TKN) is the sum of organic nitrogen, ammonia (NH3), ammonium (NH4) and therefore to calculate total nitrogen, concentration of nitrate-N and nitrite-N needs to be determined and added to TKN [40]. Total N and TKN levels, interestingly, tended to be lower but ammonium nitrogen (NH4-N) was higher in 2012 group than in 2007 group in our study. Since conversion of TKN into certain type of N depends on the type of protein present in the sample, especially what fraction of the protein is composed of nitrogenous amino acids, further study will be necessary to evaluate the intermediate processes of N conversion.
Administration of diets formulated by 2007 or 2012 feeding standard affected the levels of VFA (Table 5). Compare to pigs fed 2007 diet, those fed 2012 diet numerically increased levels of all kinds VFAs analysed in the current study, including acetic acid, propionic acid, butyric acid, valeric acids, iso-butyric acid and iso-valeric acid, and branched chain fatty acids (BCFA). Level of SCFA, especially, was significantly increased (P < 0.05) in 2012 group (759 ppm) when compared with 2007 group (708 ppm). Dietary proteins and carbohydrates are substrates for microbial fermentation in large intestine [41]. Fermentation of undigested proteins may produce potentially harmful end products including phenols, indoles, ammonia and BCFA [17]. In our current study, however, levels of these odorous compounds tended to be lower by supplementation of 2012 diet, which was formulated with well-balanced amino acid composition. Other studies showed that adequate amino acid supplements or addition of fermentable carbohydrates in pig diets helped reduce malodorous chemical generation and also improved gut health by conversion of fermentable carbohydrates into SCFA by microbes residing in the large intestine [42, 43].
aVFA, volatile fatty acids; means of 8 pigs per treatment with two replicates
bSCFA, Short Chain Fatty Acid = Acetic acid + Propionic acid + Butyric acid
cBCFA, Branched Chain Fatty Acid = Iso-butyric acid + Iso-valeric acid
dSEM Standard errors of the means
Bacterial communities in animal gut play important roles in degradation of undigested protein and carbohydrates, influence the gut healthiness by inhibiting colonization of pathogens, and maintain gut microbial ecosystem, which is essential to maintain normal gut physiology [44]. Results of 16S rDNA sequence analysis performed by using 454 FLX titanium multiplex bar-coded pyrosequencing system to determine the microbial community changes in the faecal samples of pigs fed 2007 or 2012 based diet are shown in Table 6. The total number of valid readings obtained 13,264 in the 2012 specification and 14,018 in the 2007 specification. Among these, 635 and 672 OTUs were detected in 2012 and 2007 group, respectively. Richness and diversity indices of communities are also showed in Table 6. Significant difference was not appeared in any item, but OUT counts tended to be lower in 2012 group when compared with 2007 group. Bacterial communities of 2007 manure samples showed more Jackknife numbers than those of 2012 group. Values of Shannon–Weaner index (H) were 3.95 and 4.11 in 2012 and 2007, respectively. There was no significant difference in Simpson’s index (D) or Goods library coverage between two diet groups.
| 2012 | 2007 | |
|---|---|---|
| Total valid reads | 13,264 | 14,018 |
| bOTUs | 635 | 672 |
| Ace | 815 | 824 |
| Chao 1 shared richness | 774 | 777 |
| JackKnife | 829 | 849 |
| Shannon–Weaner index (H) | 3.95 | 4.11 |
| Simpson’s index (D) | 0.1 | 0.1 |
| Goods library coverage | 98 | 99 |
aFour samples from each treatment were analyzed for pyrosequencing data
bOUT Operational Taxonomic Units, Shannon-Weaver (diversity index), Chao1 (richness index) and Goods library coverage were calculated using the Mothur package
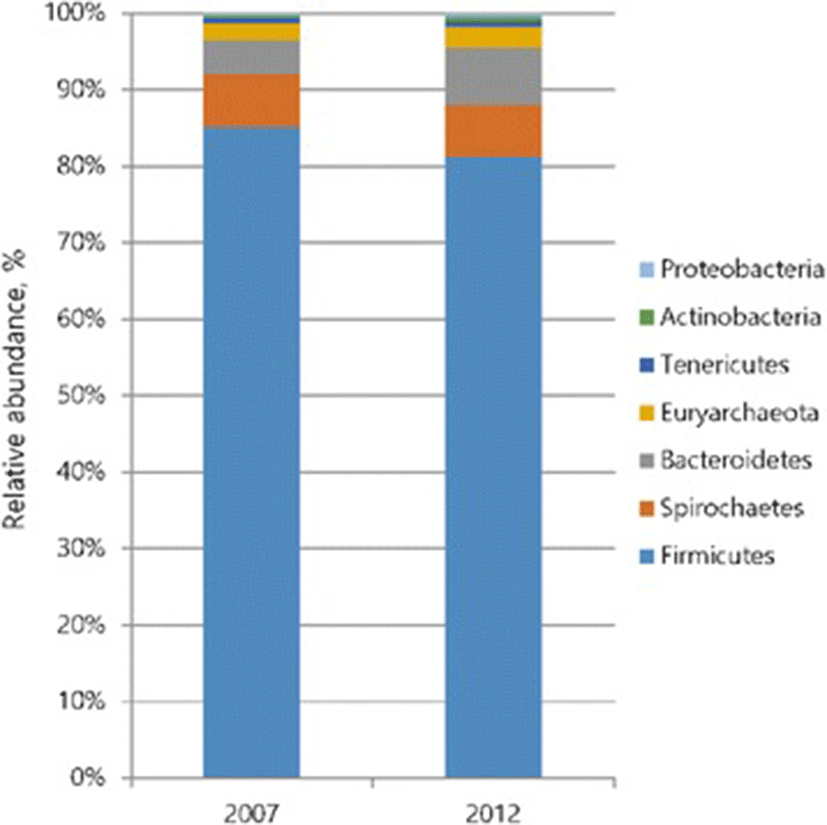
Bacterial taxonomic compositions in phylum level of feces from pigs fed 2007 or 2012 are shown in Fig. 1. Firmicutes and Bacteroidetes are major microbial phyla in mamals gut [45, 46]. Concomitant to these reports, results from our current study showed that Firmicutes are most dominant phyla in both groups, followed by Spirochaetes, and Bacteroidetes. At phylum level, the proportion of Firmicutes decreased, while that of Bacteroidetes were significantly increased (P < 0.05) in 2012 group. The microbiota in the gut produce SCFA such as acetate, propionate, and butyrate by fermenting nutrients from pig diet. Pigs fed 2012 diet showed a greater level of SCFA, which is known to positively influence the odor concentration [8]. The Bacteroidetes mainly produces propionate and acetate, therefore this phylum has been linked with the higher levels of total SCFAs in 2012 group [47, 48].
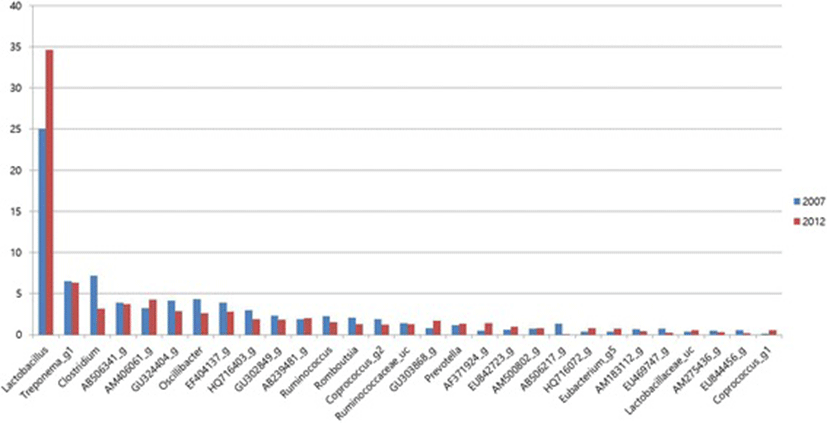
Among the total genera detected, the bacterial genera including Bacillus, Coprococcus, and Bacteriodes were increased (p < 0.05), while Oscillibacter, Clostridium, Ruminococcus, and Coprococcus were less in 2012 group than in 2007 group Fig. 2. In species level, Lactobacillus species were increased (P < 0.05) in 2012 diet fed pigs than control. Lactobacillus species are well known to provide beneficial effects on gut function and health [49]. Moreover, the pathogenic bacteria Clostridium were reduced in 2012 group.
















