INTRODUCTION
The recent increasing public interest in animal welfare has aroused sentiments so that many countries have tightened the laws and guidelines of animal welfare and animal protection in stages. Although animal welfare certification standards differ among countries due to cultural and religious differences, cages have generally been banned for laying hens. The installation of nest boxes, perches, and sand boxes is basic and essential in a rearing system. The laying hen welfare certification standard in Korea includes banning cage rearing and requires less than nine head per m2, a 4 cm round type feeder space per head, 1 m2 or more of nest boxes per 120 head, and 15 cm or more of perch space per head. Floor type or free range type systems have been used as alternatives to an animal welfare system; however, these systems require more area to rear laying hens and more labor time to collect eggs. A multi-tier system is considered an alternative to floor type or free range systems, and has advantages of using a feeder, nest boxes, and a perch vertically and horizontally which help express hens’ natural behaviors and facilitate collect egg collection and manure removal.
Environment enrichment is an increasing method of research for improving welfare of domestic animal by maintaining of their natural behavior and productivity. It helps to reduce extreme stress, decrease abnormal or damage behavior, improve physical health and enhance immunity [1]. However, it can have some adverse effects on animal health due to lack of skill to navigate in complex environment [2] or overcrowding as insufficient space for feeding, drinking and dustbathing [3]. Also, early life stress has been shown to exert profound short- or long-term effects from rearing environment to adult environment [4]. For example, severe feather pecking of young hens during rearing period develop high levels of feather damage later in life [5]. It is possible to predict development of welfare problem during rearing period and reduce by providing appropriate environment [6]. It needs to evaluate the performance of the multi-tier system under production conditions with regard to behavior and productivity.
This study was conducted to investigate laying hens’ initial behavioral changes in a multi-tier system and to compare egg productivity and egg quality of the multi-tier system with those of the floor system.
MATERIALS AND METHODS
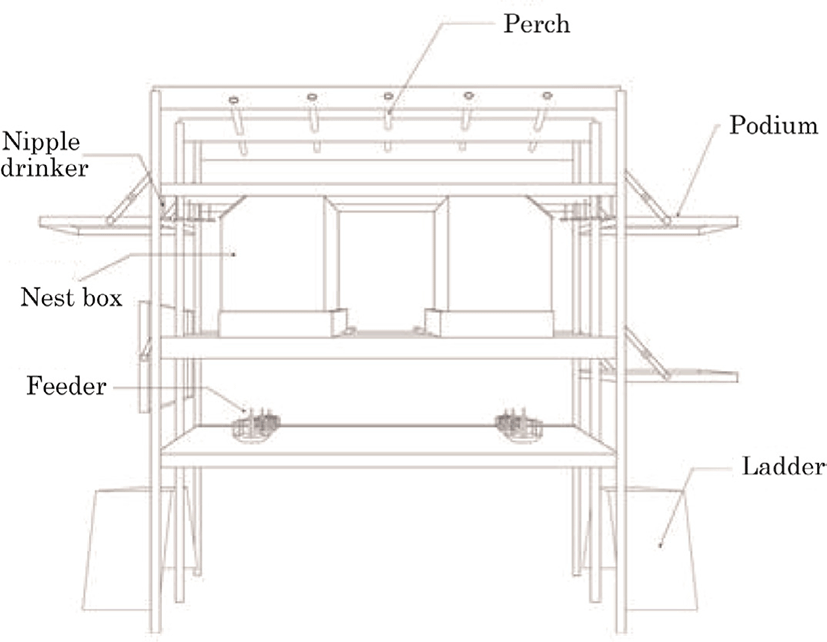
A total of 1,974 Hy-Line Brown hens were randomly divided into two groups and allocated to either the floor system or the multi-tier system at 11 weeks of age under commercial farm conditions (Fig. 1). Both housing systems were provided with perches, nest boxes, and litter on the same floor area (7 × 20 m), and the floor area was covered with 5 cm deep of wood shavings. The multi-tier system was equipped to automatic facilities, such as egg collection systems and manure removal belts. Commercial standard layer diets were provided at approximately 05:30 and 16:30 h and water was available ad libitum. The photoperiod was 14-h light: 10-h dark with the lights on at 5:30 am.

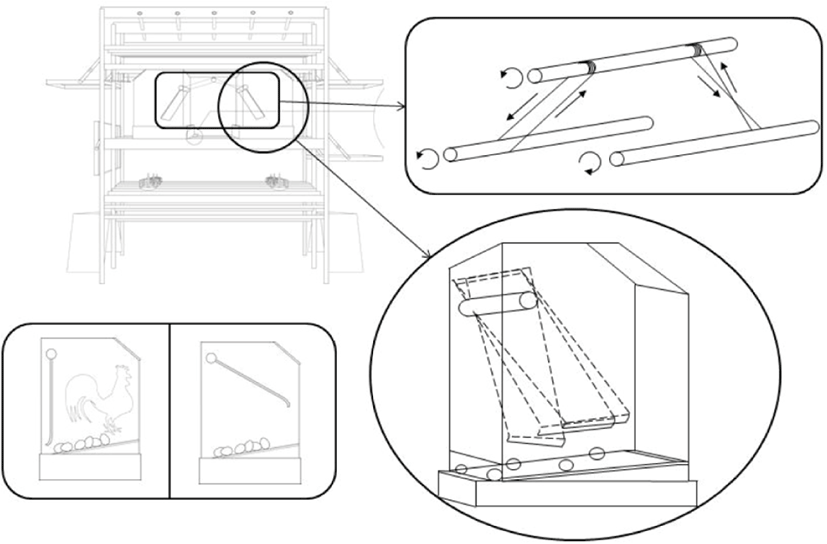
The floor system had feeders and drinkers placed in the middle and perches and nest boxes on its each side. The multi-tier system was equipped with three tiers with automatic chain feeders on the first tier, nipple drinkers and integrated nests on the second, and perches on the top tier. A schematic drawing of the housing system is provided in Fig. 2. Conveyor belts installed under the lower and top tier floors automatically removed droppings. Its height was within 2 m from the ground to the top and there was 30 cm in between tiers. Ladders or podiums were provided to go up or down the tiers. Perches allowed a 15 cm space for each bird, and nest boxes offered 1 m2 nest space per 100 hens. The doors of the nest boxes were automatically closed during the night and their ends were rounded to prevent injury (Fig. 3). The floor of the nest was made of a perforated rubber plate to prevent the hens from sliding down the slope (about 8°). Nipple drinkers were installed in front of the nests and one nipple was provided per 10 hens.
Behavioral observations were generally conducted by the focal sampling method after tied the races up of legs or dying the wings of a small group of laying hens. However, viewing of marked birds was often obstructed when they moved around the multi-tiers, and it was impossible to distinguish hens during the dark phase. In some studies, behavioral observations were extracted in the form of the total number of birds counted within marked sections [7]. Therefore, data were recorded for seven behaviors using CCD (charge-coupled device) cameras and a digital video recorder within a marked section. Hens’ behaviors are defined in Table 1. We monitored for 24 h per day for 10 days. General behavior (feeding, drinking, perching, and dust-bathing) was determined from instantaneous scan sampling and was continuously observed at 2-min intervals throughout the experimental period. Specific behaviors, such as nest visiting, feather pecking, and wing-flapping, were instantly counted at the time the behavior was exhibited.
Not all layers were in the egg laying period, so the number of visited nests was counted. Distinction between general feather pecking and severe feather pecking was not considered. All behavioral analyses were carried out by the same person.
Between 19 and 44 weeks of hens’ age, feed consumption and mortality were recorded. Eggs were collected daily, from which egg yield and the number of cracked and/or dirty eggs was recorded. Egg weight was measured after washing and drying them. Eggshell break strength was measured using an FHK eggshell strength tester (Fujihara, Tokyo, Japan). Eggshell thickness was measured by using a micrometer, and so were yolk color, albumen height and Haugh unit (HU) done with a QCM + System (TSS, York, England).
After being tested for normality and circularity to meet the assumptions, square root transformation of the data was performed if necessary to meet those requirements. All data were statistically analyzed using the PROC GLM procedure of SAS (SAS Inst., Cary, NC, USA) for randomized design. Post hoc testing was performed using Fisher’s least-significant-difference (LSD). All differences were considered significant at p < 0.05. The results in tables are presented as means and standard deviations.
RESULTS AND DISCUSSION
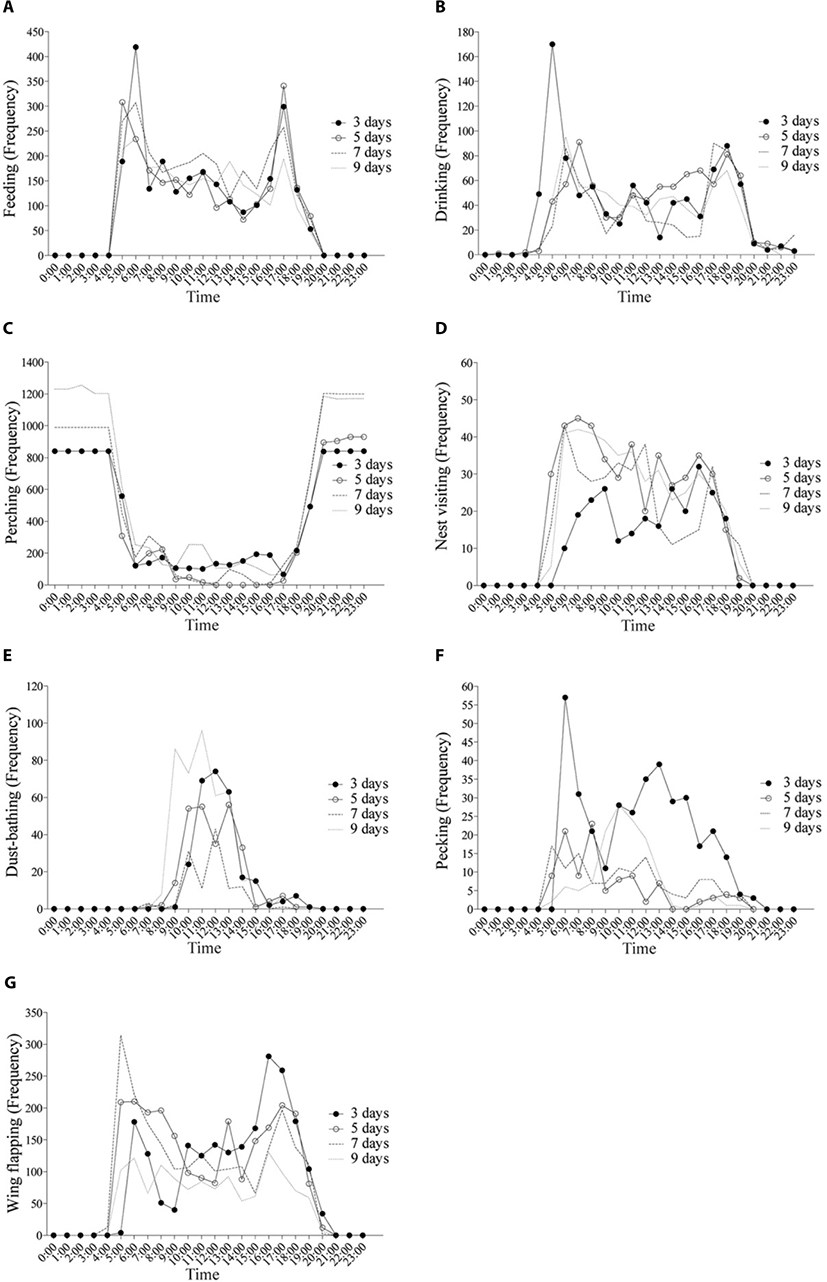
The hourly feeding pattern consistently showed two peaks during the experiment (Fig. 4a; p < 0.05); one in the morning (05:00–06:00) and the other in the afternoon (16:00–17:00), corresponding to the results of Choi et al. [8] and Jordan et al. [9]. The hens consumed about 60% of daily feed intake from 05:00 to 12:00 and ate the remaining 40% from 13:00 to 20:00, contrary to Hetland et al. [10]. Savory [11] reported a similar result that non-layers usually eat more in the morning than layers. Some evidence indicates that time of oviposition is negatively correlated with feed consumption. Feed intake decreases an hour or two prior to oviposition, but increases immediately afterwards [8,12,13]. In this study, feeding behavior was probably influenced more by other factors, and not time of oviposition, as the hens were immature. Laying hens were likely to attempt to feed when feeders were filled with a commercial diet.
The daily feeding pattern was slightly reduced on day 6 (Fig. 5a; p < 0.05) which may have been a temporary fluctuation due to the sudden appearance of humans rather than the rearing environment. Feeding frequency increased the next day and did not differ during the remainder of the experimental period. It seemed that feeding behavior was not greatly impacted by the new environment. Several studies have found that feeding frequency is strongly influenced by social rank [14]. Dominant hens eat the most and are more aggressive at the feeder [15]. Low feed intake of subordinates causes a major drop in body weight as well as egg yield. Therefore, adequate feeder space should be provided so all hens can feed synchronously [16,17].
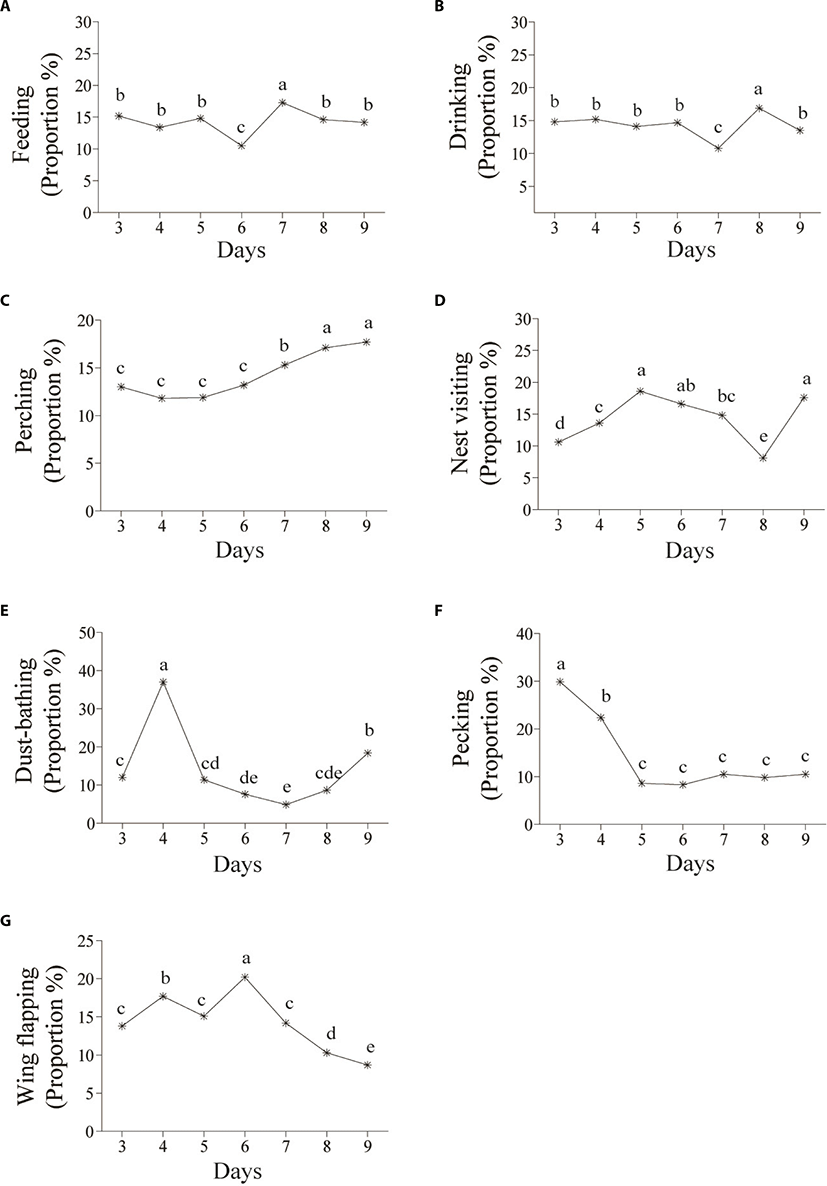
The hourly drinking pattern was similar to that of feeding. Drinking time peaked one hour (06:00–07:00) after peak feeding time (05:00–06:00) in the morning, except on day 3 (Fig. 4b; p < 0.05). The frequency of drinking suddenly decreased on day 7 (Fig. 5b; p < 0.05) because laying hens spent more time feeding, but their feeding increased slightly more than usual the next day. It was a reward-related behavioral pattern from the previous day.
Laying hens were willing to travel up to the middle tier to drink water after feeding, suggesting that drinking was mainly influenced by feeding behavior during the rearing period. The different tiers of feeders and nipple drinkers will cause the hens to move much more than when they are provided on the same tier. Therefore, adequate nipple drinkers should be provided to laying hens.
Birds sit in elevated perches by instinct to avoid predators during the night in the wild. Domestic hens also perch [18–20], and they prefer to rest on the highest perch [21,22]. Some evidence indicates reduced fearfulness and vigilance when birds are sitting on a perch [23,24]. As expected, our results showed that all hens were willing to perch at night. The hourly perching pattern decreased significantly beginning at 05:00, but increased after 19:00 (Fig. 4c; p < 0.05), which was consistent during this observation period. Other studies have reported that the presence of a perch reduces aggressive behavior between hens by allowing subordinates to avoid dominant hens and limits stocking density on the ground [25].
The daily perching pattern increased significantly over time on day 7 (Fig. 5c; p < 0.05), indicating that the hens were able to have access to and use the perches with or without using the platforms and ladders provided. Early access to a perch can affect cognitive skills of adult hens in three-dimensional space [26,27]. Installing perches improves bone strength in birds compared to those housed in cages, reduce aggressive behavior by allowing subordinates to avoid dominant hens and ease stocking density [28,29]. However, there can be adverse effects on physical health due to falls, collisions, and deformities caused by perch design factors such as height, width, and materials. In addition, the farmer should pay particular attention to laying hens suffering pain from injuries.
Laying hens started to visit the nest boxes early in the morning such as 05:00 h with the highest frequencies on days 5, 7, and 9 whereas the peak frequency occurred in the afternoon on day 3 (Fig. 4d). Searching for a suitable nest site is an instinctual behavior of layer chickens. Therefore, providing nest boxes is important to improve animal welfare [30,31]. Nest site preference varies among individuals. Domestic hens generally favor elevated nests [32] and corner nests [33] which are also affected not only by surface materials, nest color and seclusion of the nest sites [34–38]. Dominant hens, which remained closer to the nest boxes, often gave aggressive pecks and laid eggs earlier in the most attractive nests [39], whereas subordinate hens were busy seeking an appropriate nest and watched for an opportunity to take an attractive nest, which made them more active prior to oviposition [40].
Nipple drinkers were placed in the front of the integrated nests, which may have stimulated the hens to enter the nest boxes at the beginning of the laying period. However, Lentfer et al. [41] demonstrated that placing nipple drinkers in front of the nest has no effect on the number of eggs laid in nests. A high number of hens on the nest platform can result in overcrowding causing agonistic behavior and laying of eggs outside nests [42]. Further research is needed to reduce the risk of overcrowding on nest platforms when hens are using nipple drinkers, as it could help to determine how to disperse the birds to the middle nests at peak laying time.
Laying hens prefer to spend time dust bathing around mid-day and usually perform one bout (20-30 min) every other day [43,44]. In the present study, most of dust bathing was observed between 09:00 to 14:00 h (Fig. 4e). However, the daily pattern was irregular and a significant increase was observed on day 4 of the experiment (Fig. 5e; p < 0.05). It was difficult to explain what stimulated hens to suddenly dust bath. Dust bathing is stimulated by multiple factors as light, heat stimuli [45,46], and visual stimulus of the substrate [47]. Among various substrates, peat moss and sand are more attractive than wood shavings and straws [43,48]. An early experience with a particular substrate during the rearing period can affect the hen’s choice for a particular dust-bathing substrate at a later age. Also, birds with no access to dust substrates (peat moss) during the rearing period are motivated to dust bath by other hens that had already experienced dust bathing [49]. According to Orság et al. [50], a hen’s motivation to dust bath in non-cage housing systems is enhanced more than in cage type systems.
Laying hens pull feathers out of other hens, which causes feather or skin damage, hemorrhaging, and some cannibalism can occur [51,52]. Feather pecking is a prevalent welfare problem, especially in non-cage systems [53]. In this study, feather pecking occurred intensively around feeding time in the morning (from 06:00 to 08:00) during the first seven days. The peak time was from 10:00 to 12:00 on day 9, while daily feather pecking declined significantly until day 5 and at a low level thereafter (Fig. 4f, Fig. 5f; p < 0.05).
Laying hens showed more activity in the morning, which was contrary to the findings of Channing et al. [7] and Ramadan and Von Borell [54]. Pecking was mostly observed on the floor. According to Nicol et al. [55], the location of the birds within the house has significant effects on feather pecking. Birds located on the floor are subjected to feather pecking [56], which is related to dust bathing or foraging and causes a crowded floor. Floor space must also be considered a factor related to feather pecking [55,57]. Laying hens showed low levels of pecking during the observation period, despite the fearfulness of unfamiliar conspecifics and the new environment. Wood-Gush [58] proposed that birds in large groups use other signals (body and comb size) to establish dominance relationships without fighting.
Wing flapping is also important to evaluate for poultry welfare [59-62]. Several studies have shown that a lack of space restricts wing stretching/flapping and causes poor humerus health [63–65]. Wing flapping patterns largely coincided with feeding frequency for the first several days (Fig. 4g), but the patterns became less distinctive over days, suggesting a possible adaption of hens to the environment.
Wood-Gush [58] reported that wing flapping is higher in males than females as a displacement behavior during courtship or aggressive encounter. According to Nicol [61], a significant negative correlation is observed between dominance and wing flapping. Subordinate hens may have difficulty accessing the feeder and show increased wing flapping as a response to frustration or conflict. However, one study showed that dominant hens wing flap significantly more than subordinates because they are able to learn more quickly [66].
Table 2 shows the production performance in three phases. Feed intake and egg production were consistently higher in the multi-tier system, whereas cracked and dirty eggs were more frequent in the floor system (Table 2; p < 0.001). As the hens aged, feed intake and the eggs production increased, whereas the numbers of cracked and dirty eggs decreased. Laying hens in the multi-tier system seemed to need more energy due to increased physical activity, because of the complexity of the housing system [67,68]. The separate spaces helped the layers exhibit specific behaviors while decreasing disturbance to others and promoting egg laying.
The reason for lower production in the floor system might be insufficient nest space. Crowded nest boxes caused a competition, especially for the more attractive nests [69]. Thus subordinate hens tended to delay laying eggs or laid eggs outside the nests, where stepping or pecking hens broke the eggs. An appropriate design of the nest box is very important in reducing cracked/dirty eggs and encouraging hens to use of the nest box [70,71]. The eggs rolled down a slope immediately after laying and were stored under the nests to prevent clutter in the nests. This helps prevent the laying hens from entering the nests at night and protect the eggs from accidents, egg pecking or breakage.
Table 3 shows the egg characteristics in three phases. The housing system did not affect egg quality, except albumen height at 23 weeks of age (p < 0.05), which was higher in eggs from hens in the floor system than the multi-tier system. Egg weight, eggshell weight, and albumen height increased significantly at 44 weeks of age in the floor system. Other parameters used to assess egg quality (eggshell thickness, eggshell strength, and HU increased significantly in the multi-tier system. Overall, egg quality improved with age between 23 and 44 weeks of age, so it was influenced by age rather than the housing system [72–74]. The mean weight of eggs at 44 weeks of age was > 64 g in both rearing systems being similar to others [75]. Many studies have compared production performance and egg quality between conventional cage and other housing systems. Studies showed no difference in these parameters between cage and aviary systems [76,77], higher egg weights in aviary vs. cage systems [78], and greater egg weights in the free-range system [74,79].
Eggshell color is important for consumer appeal. Korean consumers prefer brown eggs to white eggs [80]. No overall differences were found in eggshell colors, but the color decreased significantly with age of hens in the multi-tier system. It is shown that stressed laying hens retain their eggs in the shell gland beyond the normal oviposition time which causes brown eggs to appear paler [81,82]. In summer, eggshell color is lighter due to heat stress [83]. In addition, eggs laid in the morning are paler [84]. Walker [85] demonstrated that nest box design can influence eggshell color.
In the present experiment, eggshell quality, including eggshell weight, eggshell thickness, and eggshell strength increased with hen age regardless of the housing system. Eggshell weight increased by 2 g from 23 to 44 weeks of age in both housing systems. Eggshell thickness increased significantly in the multi-tier system. Yannakopoulos and Tserveni-Gousi [86] reported that the egg shell thickens with hen age, which is in agreement with our results. On the other hand, Silversides and Scott [87] reported a negative effect of age on eggshell thickness. Other studies have reported no significant effect [74,88]. Eggshell strength was higher in the floor system, than in the multi-tier system that possibly because of the different activity levels of the hens.
Yolk color also increased with age (p < 0.05). The average yolk color was 10.8 at 44 weeks in both housing systems. A preferred yolk color is 11 on the Roche scale in Australia Roberts [89]. The main contributing factors to yolk color are diet [90] and age and age [91,92]. Yolk color is greater in alternative systems compared to that in cage system [91,93] and, in particular, free range hens have a darker yolk color because of xanthophyll intake from plants [74].
Our study demonstrated that albumen height did not differ between the housing systems, but influenced more with age. However, we did not find the exact cause as to why albumen height suddenly dropped at 30 weeks in hens from the floor system. All management was the same, and the data analysis was conducted by the same person. In some studies, ammonia within the housing system affected albumen height [92,94] and Koerkamp et al. [95] reported that ammonia is higher in the floor system compared to a multi-tier system.
HU is an objective measure of egg quality that is influenced by age, diet, bacterial infection, ammonia, storage time, and storage temperature [89]. In our study, no differences were detected in HU between the two housing systems, although HU decreased at 30 weeks in hens with a reduced albumen height. HU in the multi-tier system increased with hen age (p < 0.05).
CONCLUSION
We observed that laying hens might need at least seven days to adapt to the multi-tier system. We also didn’t find any problems in access to perches and nests. Moreover, the egg production was higher in multi-tier system than in the floor system, whereas the cracked and dirty eggs were lower in multi-tier system than in the floor system. These results suggest that the multi-tier system evaluated here is expected to be suitable as an alternative housing system to the floor system for laying hen in Korea, as the system increased productivity and improved welfare of the hens. There are still potential conflicts between animal welfare and farm economics. However, further study on the welfare assessment of laying hens in this multi-tier would be useful.