INTRODUCTION
Heat stress (HS) causes a decrease in the growth of pigs causing in major losses to the pork production. Especially, pigs are affected by HS in tropical or subtropical regions with hot seasons. Pigs suffering from HS show decreased feed intake [1], increased body temperature [2], reduced growth and increased veterinary costs [3]. Also, the temperature inside piggery is changed by the external temperature. Under HS, pigs increase their peripheral blood circulation to advance body thermolysis. The peripheral blood vessels are dilated with occurred vasoconstriction to the entire splanchnic blood vessels [4]. HS is seriously affecting the small intestine that is major tissues [5] to upregulate heat shock proteins during heat stress [6] and reduce oxygen and nutrient supply to gastrointestinal tract tissue [7] which may cause serious damage to the intestinal epithelia. Decreased intestinal villi height is generally observed in HS pigs [8] with cellular proliferation and membrane function changes [9]. In addition, dramatic decrease was observed in the genes coding the synthesis of amino acid transporters in the small intestine of HS pigs [10]. HS transmutes organism physiology, metabolism, homeostasis and increases the intestinal temperature [11]. Reduced proliferation of intestinal cells [9] and increased creation of reactive oxygen species (ROS) [12] are also caused by HS. Some amino acids such as arginine (Arg) and methionine (Met) seem to contribute to restoring intestinal epithelia [13] as well as destroy ROS [14]. Thus, we investigated the effect of Arg and Met supplementation on nutrient use in growing pigs in high ambient temperature.
MATERIALS AND METHODS
The protocol for the two experiments was approved by the Institutional Animal Care and Use Committee of Chungbuk National University, Cheongju, Korea.
In this experiment, a total of 10 crossbred (Duroc × Landrace × Yorkshire) barrows were assigned to five treatments over five periods in a 5 × 5 Latin square design with two replicates per period. The pigs (average of initial body weight was 16.2 ± 1.2 kg) with T-type cannulas transplanted at approximately 15 cm before the distal ileum were individually confined to metabolic cages (0.8 m × 0.5 m × 0.5 m) in an environment-controlled room. The experiment was carried out in summer climate (August) in South Korea and the average relative humidity was 85.4%. Surgery and recovery were conducted according to Sauer et al. [15]. The temperature and relative humidity were based on experimental piggeries in the summer seasons in Korea (Fig. 1).
Table 1 represents the nutrient contents and the feed formulation used in this experiment. During the five weeks of the experiment, pigs were assigned to one of five dietary treatments, representing supplementation with two types of amino acids (Arg and Met) and two different amounts of amino acids (0.2% and 0.4%). The daily feed ration was regulated to 2.7 times the maintenance requirement for digestible energy (2.7 × 110 kcal of DE/kg BW0.75; [16]). The daily feed ration was divided their allowance in half and fed at 08:00 and 20:00 hours. The diets were blended with water in a 1:1 ratio (Wt/Wt) before feeding and pigs had free approach to water during the experiment.
The pigs were weighed at the start of each period and the amount of feed consumed during each period was recorded. Each experiment period is 7 days and it consists of a 4 days adaptation and a 3 days sampling for collecting feces and urine. The ileal digesta was continuously collected from all pigs for 12 hours starting at 08:00 hours. Plastic bags were equipped to the T-cannula until filled with ileal digesta but no longer than 15 minutes. Ileal digesta was collected for each pig and frozen at −20°C immediately after sampling. Before analyses, the digesta samples were thawed, homogenized, subsampled and freeze-dried. Diets and ileal digesta were analyzed for dry matter (AOAC method 930.15) and crude protein (AOAC method 990.03). The gross energy of diets and ileal digesta were analyzed using an oxygen bomb calorimeter (Parr Instruments, Moline, IL, USA). The urinary nitrogen was analyzed (AOAC method 990.03).
The data for effects of Arg and Met supplementation on growth performance, nutrient digestibility and plasma profile were subjected to an analysis of variance using PROC GLM of SAS (Statistical Analysis System 9.1, SAS Institute, Cary, NC, USA). Duncan’s multiple range test was used to compare differences among the treatment groups including the control group. Significant difference among feeding treatments was shown as p < 0.05, while no significant differences were shown as p > 0.05. Polynomial orthogonal contrasts were performed to compare differences in type (Arg vs. Met) and level (0.2 vs. 0.4).
RESULTS
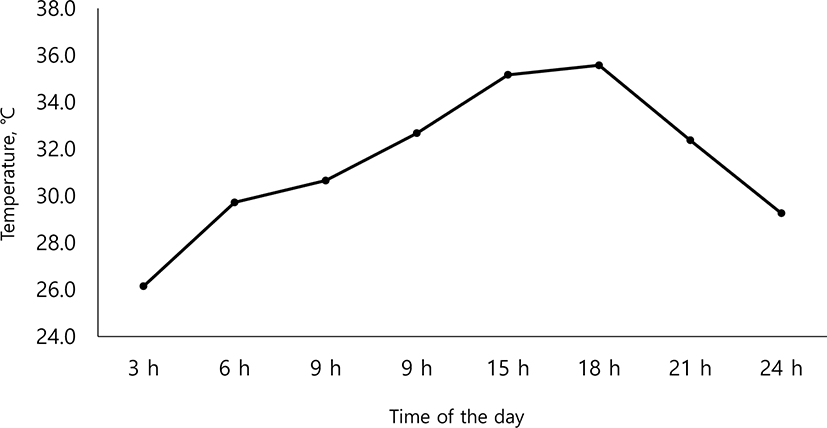
Fig. 2 represents the mean values of variations in temperature, at intervals of 3 hours during the experimental period (35 d). At 18:00 hours to a maximum of 35.6°C and the lowest temperature was 26.1°C at 03:00 hours. Growth performance data are summarized in Table 2. Pigs fed with additional Arg were significantly higher in average daily gain (ADG) than the CON group (p < 0.05), and ADG increased linearly with increasing Arg supplementation (p < 0.05). But there was no significant difference with Met supplementation (p > 0.05). Average daily feed intake (ADFI) has no significant difference according to additional supplementation with Arg and Met (p > 0.05). Feed efficiency increased linearly, with increasing (p < 0.05) amounts of additional Arg and Met. Table 3 presents the effects of Arg and Met supplementation level on the apparent total tract digestibility under the HS condition. The apparent total tract digestibility (ATTD) of dry matter (DM) and crude protein (CP) were not significantly different among treatments (p > 0.05). Table 4 presents the effects of Arg and Met supplementation on plasma profile in weaning pigs under HS condition. Protein, blood urea nitrogen (BUN) and cortisol concentration in plasma, did not significantly differ (p > 0.05), while d-ROMs concentration in treatments with Arg supplementation were significantly higher (p < 0.05) than other treatments. Table 5 presents the effects of arginine and methionine supplementation on apparent ileal digestibility (AID) of amino acids in weaning pigs under high ambient temperature. The AID of amino acid had no significant differences among treatments (p > 0.05).
DISCUSSION
The experimental environment was based on the temperature rising due to the summer climate and the temperature of the experimental piggery recorded the highest temperature at 18 hours. Pigs fed with additional Arg were significantly higher in ADG than the CON group. Also, ADG and feed efficiency increased linearly with increasing Arg supplementation. The HS reduced an animal’s intestinal efficiency for nutrient absorption as proved by decreased height of intestinal villi in pigs [17] and decreased feed intake [1]. These results cause degradation of nutrient use because of the high ambient temperature. When pigs are under HS condition, blood is moved to the peripheral tissue to increase thermo diffusional in the body [4]. Accordingly, HS may cause hypoxia, hyperthermia and inflammation in gastrointestinal tract [18], all of which can trigger oxidative stress. Although depends on the intensity and the duration of HS, it has been reported that markers of intestinal oxidative stress increase in the intestine of HS pigs [2]. The Arg is a nutritionally important amino acid and has various physiological functions in the animal body [19]. One of these functions is to reduce superoxide release and increase antioxidant ability [20]. The Arg produces proline, polyamines, nitric oxide and glutamine during metabolic process [21]. For example, these metabolites can alleviate the oxidative stress damage [22], enhance the immune function [23] and regulate protein synthesis [24]. The ROS is sustainedly produced as a byproduct of cell respiration in the mitochondria [25]. The HS causes overproduction of ROS, which increases oxidative damage to muscle cells [26]. Also, Liu et al. [27] reported significant mitochondrial damage in the jejunal epithelium of heat-stressed pigs. The Arg seems to facilitate restoration of intestinal epithelia [13] and destroy ROS [14]. Additionally, Arg is a synthetic precursor of nitric oxide [13], a potent vasodilator that increases blood flow for body heat dissipation [28]. Large amounts of Arg are used for production of creatine whose anti-oxidative function [14] may facilitate in ameliorating the impact of HS-related overproduction of mitochondrial ROS [12]. At thermoneutral temperature, Arg content in the feed of pigs will be sufficient. Because Arg is synthesized in several organs, especially in kidneys using citrulline as a precursor [13]. Conversely, under high ambient temperature, requirement of Arg may increase to remove ROS caused by HS. Hence, additional supplementation of Arg is used to cope with HS that affects reduction of damage caused by ROS. In conclusion, exposure of pigs to HS does not affect AID of amino acid. However, results of ADG and feed efficiency in treatments fed with additional Arg were greater than other treatments. Thus, pigs fed with supplementation of Arg may facilitate reduction of negative effects such as increasing oxidative stress according to HS.