INTRODUCTION
During pig breed domestication, breeding has focused on lean tissue deposition, feed conversion efficiency, and above all, on prolificacy (reviewed by [1] and [2]). The larger the litter, the better the profitability for the farmer. Average litter sizes may have increased by 0.2–0.3 piglets/year [3]. However, increased litter size is associated with negative aspects such as high energy demand for milk production [4], prolonged farrowing duration [3], and pre-weaning mortality [5].
Based on 20 different studies carried out between 1990 and 2019, litter size has increased from ca. 10 to 20 piglets and farrowing duration has increased from 1.5–2 to 7–8 h (Fig. 1) [3,6]. While the described tendency is subject to differences in breeds and farrowing housing environments, the overall tendency is rather convincing. The extended duration of farrowing appears as intensive breeding for prolificacy in the pig [3].
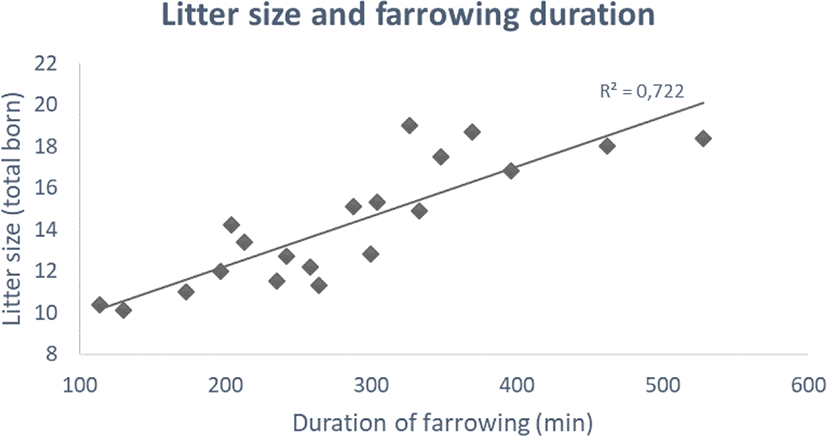
The increasing litter size and prolonged farrowing present as an immunological challenge for the sow and especially the newborn piglets [3,5,7]. With prolonged farrowing , the last 20%–30% of the foetuses to be born seems not to have access to high-quality colostrum, as its quality (i.e., immunoglobulin G [IgG]) rapidly declines after the onset of parturition [8]. They also have less time to suckle on colostrum due to a decreased opportunity for colostrum intake, increased competition for teats, and reduced birth weight [3]. These factors may result in reduced immunity and the emergence of diseases during the growing phase of piglets/fattening pigs.
The metabolic challenge related to the hyper-prolific sow production model begins during gestation and proceeds beyond farrowing and lactation. The sow is supposed to eat enough to meet the nutrient requirements of growing litters prior to farrowing, which may cause some of the problems seen around the time of farrowing [9,10]. During the early stage of lactation, sows with large litters loose more energy while producing more milk that cannot match up with the energy from their feed, ending up in a negative energy balance (NEB) [11,12]. Negative energy balance impacts follicle development after weaning [13–15], oocyte quality [13,14], embryo development [11,16], and piglet birth weight [17]. Thus, pre-mating diets or optimizing the sow metabolic state during lactation may be options for improving subsequent sow fertility.
Growing litter size and production intensity as such appear as items for management issues. This review will explore sow reproduction and piglet survival focusing on large litters and suggest possible management strategies.
PIGLET COLOSTRUM INTAKE AND MORTALITY
Piglets’ first suckling behaviour is the most important factor for colostrum intake, which is crucial for their survival and growth. Studies have shown that the average time of first suckling ranged from 27 to 62 min [18–23] and the interval from udder touch to first suckling averaged 9 min [22]. Yun et al. [21] and Balzani et al. [22], showed that the times of the first udder contact (range from 4 to 215 min) and colostrum intake (range from 0 to 116 min) also varied among individual piglets. The piglets’ first suckling behaviour depends on piglet characteristics such as body weight, size and vitality [19,24]. If piglets take a longer time until first suckling, they experience more heat and energy loss, lower colostrum intake and a higher mortality rate during lactation [19,21,25]. Thus, the physical characteristics and vitality of piglets can play a crucial role in their survival and growth.
The energy requirement of newborn piglets is very high because of high physical activity and thermoregulation directly after birth [26,27]. Piglets acquire energy mainly from colostrum [8,28], which is mainly composed of moisture, protein, fat and lactose [8,29]. The energy content (e.g., fat and lactose) of colostrum has a major impact on short-term piglet survival during lactation (reviewed by [8]). Colostrum also contains a high concentration of IgG [29,30], which is essential for piglet immune systems and thereby for their long-term survival during lactation [31]. The composition of colostrum changes nearly hourly. Theil et al. [8] showed that during the first 24 h after birth, lactose content increased from 3.5% to 4.4%, fat content increased from 5.1 to 6.9%, and energy content increased from 260 to 346 kJ/100 g. The concentration of IgG, on the other hand, decreased rapidly by 50% during the first 6 h after birth of the first piglet [32] and continued to decrease further during farrowing and until 24 h after farrowing (e.g., 62.3 vs. 16.8 mg/mL, respectively for at birth and 24 h after birth [33]). In modern sows with large litters, changes in energy and IgG content in colostrum are also similar to those of sows with relatively small litter size despite the increases in litter size and duration of parturition [3,8,34]. In terms of optimizing energy intake, late colostrum (around 12 h after farrowing) therefore seems more advantageous compared to early colostrum [8]. On the other hand, early colostrum may play a more crucial role in the passive immunity of piglets than late colostrum [32]. Piglet colostrum intake has been shown to positively relate with weaning and inversely related with pre- and post-weaning mortality of the piglets [35]. Declerck et al. [36] and Hasan et al. [37] reported that the colostrum intake of each additional piglet in a large litter decreased by approximately 9 g. This could be due to a limited colostrum yield from the sows [37] and increased competition within litters [38]. Colostrum also contain bioactive factors such as insulin, epidermal growth factor and insulin-like growth factor-1 (IGF-1) [39], which are beneficial for piglet growth and survival. Considering that the energy mobilisations during late gestation are prioritised for mammary growth and colostrum production [8], feeding strategies focusing on late gestation can be one option for improving sow colostrum yield. Also, providing energy source to piglets right after birth has been recommended from many studies (will be discussed below). Therefore, to optimize sow colostrum yield and piglet colostrum intake, nutritional management during late gestation and lactation should be considered more carefully in large litters.
Increased mortality in large litters is of considerable economic and welfare concern in modern pig farming. High pre-weaning mortality in large litters may result from decreased piglet birth weight and increased within-litter birth weight variation (i.e., litter uniformity; Table 1) [5,40,41]. Correspondingly, the number of piglets weaned has not perfectly matched with increased litter size. Recent studies showed that total pre-weaning mortality, including stillbirths, ranged from 13% to 15% in large litters [42–44]. In severe case, sows kept under risky conditions with a large litter of an average 19 piglets have 17.9% of piglet mortality during the first day of lactation in open farrowing crate [21]. Among pre-weaning mortality, 72 h of postnatal life is the most critical period (for review, see [45]). The great majority of piglet mortality is caused by crushing, starvation and hypothermia [46]. In particular, starvation and hypothermia, which can be derived mainly from piglet characteristics, may cause piglet crushing and death during lactation [47]. Low birth weight in piglets may be linked to lower vitality/viability [48], a longer time to the first suckle [25], and less ability to compete for colostrum intake with littermates (for a review, see [35]). Moreover, limited capacity to ingest colostrum of low-birth-weight piglets [49] could be one of the reasons for impaired colostrum intake [50]. Furthermore, Baxter et al. [19] have demonstrated that piglets that die before weaning had lower birth weights and lower rectal temperatures at birth and 1 h after birth compared to piglets that survived. This may imply that hypothermia can also be an important mortality factor in low-birth-weight piglets. Indeed, Herpin et al. [27] showed that smaller piglets may experience greater heat loss and thus a decreased ability to thermoregulate when compared to larger piglets. Considering that low-birth-weight piglets showed higher mortality, especially during the first 24 h after birth [51,52], certain supportive management routines around parturition will be needed in the management of large litters will be discussed.
| Total number of piglets born (n) | ||||
|---|---|---|---|---|
| Milligan et al. [40]1) | Wienjtes et al. [5]2) | Wientjes et al. [17]3) | Han et al. [41]4) | |
| Litter characteristics | ||||
| Mean birth weight (g) | −46*** | −40*** | −41*** | −37*** |
| CV of birth weight (%) | 0.39*** | 0.76*** | 0.83*** | 0.60** |
| Piglets < 1,000 g (%) | - | 2.4*** | 1.9*** | 2.0*** |
Litter uniformity, in addition to individual birth weight, can be a major factor affecting piglet mortality. Increased litter size resulted in poor litter uniformity, which elicited a higher proportion of small piglets (< 1,000 g; Table 1) [5,17,41]. Results by Wientjes et al. [5] support this finding, as they showed the coefficient of variation (CV) of birth weight to positively relate to mortality during the first three days after birth in large litters. Furthermore, poor litter uniformity (i.e., large variation of within-litter birth weights) resulted in less colostrum yield by sows [50] and unevenly distributed colostrum intake by piglets (reviewed by [36]). Poor uniformity at birth causes not only high mortality but also poor uniformity at weaning [40,52]. Thus, improving litter uniformity, either by pre-mating nutritional strategies or breeding, is of great interest with regard to large litters.
Stillborn piglets are also of great concern in large litters. Generally, stillborn rates in piglets have been in the range of 5%–10% in recent studies (reviewed by [53]). Stillborns can be classified into two types, depending on their time of death [54]. Piglets in one group die before parturition (antepartum or prepartum death; type 1), while piglets in the second group die during parturition, which represents a great majority of all cases (intra-partum death; type 2; [55]). Increased farrowing duration with higher litter size (Fig. 1) may increase type 2 stillborn rates. Canario et al. [56] reported a potentially higher risk of stillborn piglets with a litter size of more than 14 piglets. A recent study also found that a higher stillborn rate was related to larger litter size [57], which is in accordance with earlier studies [58,59]. This may be explained by the greater risk of asphyxiation after detachment of the placenta [60], possibly due to increased farrowing duration.
Based on the findings of high mortality in large litters, management strategies for increasing piglet survival rate should focus on strategies applicable during late gestation and before parturition and strategies applicable after birth. In the review of Theil et al. [8], they addressed the importance of sow nutrition in late gestation on colostrum yield and composition. Briefly, different dietary composition during late lactation may alter both colostrum yield and quality. Before parturition, high-fibre diets seems to result in an improved farrowing process [10,61] and colostrum production [8], and thereafter in reduced pre-weaning mortality [61]. Frequent daily meals (more than thrice daily) before farrowing are recommended for improving both the energy status and farrowing process of sows with large litters [62]. For example, Feyera et al. [62] observed that sows with a shorter time from the last meal until the onset of farrowing had a shorter farrowing duration, less probability of requiring farrowing assistance, and a low number of stillbirths. This finding may suggest that decreasing serum glucose levels may be one of the mechanisms through which farrowing duration is prolonged.
Dewey et al. [63] found that farms that provided oral administration of colostrum or glucose to piglets and performed split-nursing showed higher survival rates compared to farms with less intensive management. Especially for weak piglets, helping to establish breathing, assisting them in reaching the udder, and keeping them warm may also be recommended, as suggested by Herpin et al. [60]. These management routines can reduce the time of first suckling [20,60,64], thereby leading to an increase in colostrum intake and survival rate. Vasdal et al. [20] stressed that drying piglets and placing them onto the udder of the sow directly after birth is a key point for optimizing neonatal survival in large litters. They found less than 10% mortality (of total born) in a litter with over 15 total piglets in the open-farrowing system with intensive piglet management routines. This mortality rate is indeed low when compared with a mortality rate of 17.9% observed during the first 24 h after birth in litters of hyper-prolific sows that had not been given management routines at birth [21].
Providing energy supplementation to small piglets by hand has also been recently studied as a means to cope with the insufficient energy intake of piglets in large litters [42,65–68]. Declerck et al. [65] showed that pre-weaning mortality was reduced when small piglets were fed with energy supplementation (e.g., soy oil and coconut oil) directly after birth. Glycerol-rich supplementation and colostrum replacers also seemed to be beneficial for small piglet survival [68]. On the other hand, some studies did not find an increased survival rate with energy supplementation (sow colostrum and coconut oil) [42,67]. Thus, both drying piglets and providing them with energy supplementation, and thereafter moving them to the sow’s udder may be the most effective management routines for optimizing piglet survival in large litters.
SOW LACTATIONAL BODY CONDITION LOSS AND SUBSEQUENT FERTILITY
Sows lose their body condition mostly during lactation. The losses consist of both protein and lipid. In practical situations, backfat thickness is widely measured to predict sow lipid status. Loin muscle depth , which represents protein status, contains relevant information on sow metabolic state and reproductive performance, especially if lean sow lines are used for breeding [11,14,15]. The increased number of suckling piglets in large litters resulted in sows being in severe NEB (attributed to the loss of proteins, lipids, or both) during lactation [4]. This is caused by the high metabolic demands for milk production [69]. Severe NEB (e.g., approximately 10%–12% body weight loss) may compromise subsequent fertility, causing e.g., extended weaning-to-oestrus intervals (WEI), lower pregnancy rates, and lower subsequent litter size [70]. In modern hyper-prolific sows, however, severe NEB appears to associate with a lower ovulation rate (OR) or embryo survival rather than extended WEI (reviewed by [71]).
Impaired OR or embryo survival can be explained by compromised follicle development at weaning. Severe NEB resulted in smaller follicle diameter at weaning [13–15,72]. This may originate from the detrimental effect of NEB on luteinizing hormone (LH) and follicle development. In early lactation, LH is suppressed by sucking-induced inhibition of the GnRH (reviewed by [73]). As lactation progressed, LH pulsatility is normally restored [74], which stimulates follicle development. However, sows with low feed intake had lower LH pulsatility and smaller follicles at weaning compared to sows with high feed intake during lactation.
In large litters, follicle diameter at weaning is approximately 4–5 mm [14,15]. After weaning, pulsatile GnRH release may induce the release of both LH and follicle-stimulating hormone (FSH), which are important for follicle growth and ovulation [75]. As a result, follicles grow to reach the pre-ovulatory size (7–8 mm) [15,76,77] usually within seven days after weaning (reviewed by [71]). Smaller follicle diameter at weaning is related to longer WEI and weaning-to-ovulation interval (WOI) [15,78–80]. This is because smaller follicles take more time to reach the pre-ovulatory phase [79], after which oestrogens produced by pre-ovulatory follicles result in oestrus and ovulation (reviewed by [78]).
Further, sow metabolic state may represent the follicular fluid metabolic state, as follicular fluid can be considered an exudate of sow blood. In the study by Costermans et al. [14], plasma IGF-1 level, which is negatively related to sow body condition loss during lactation [14,15], was strongly related to follicular fluid IGF-1 level after weaning. As follicular IGF-1 is important for follicle and oocyte development [14,15], the importance of sow metabolic state on follicle and oocyte development seems to be clear.
A schematic drawing of the relationship between sow NEB during lactation and litter uniformity at subsequent parturition is described in Fig. 2. [5]. This may be explained by the detrimental effect of sow body condition loss on follicle development and subsequent fertility. Follicle development before ovulation plays a major role in oocyte quality, embryo development and, eventually, piglet characteristics at birth in sows (reviewed by [81]).
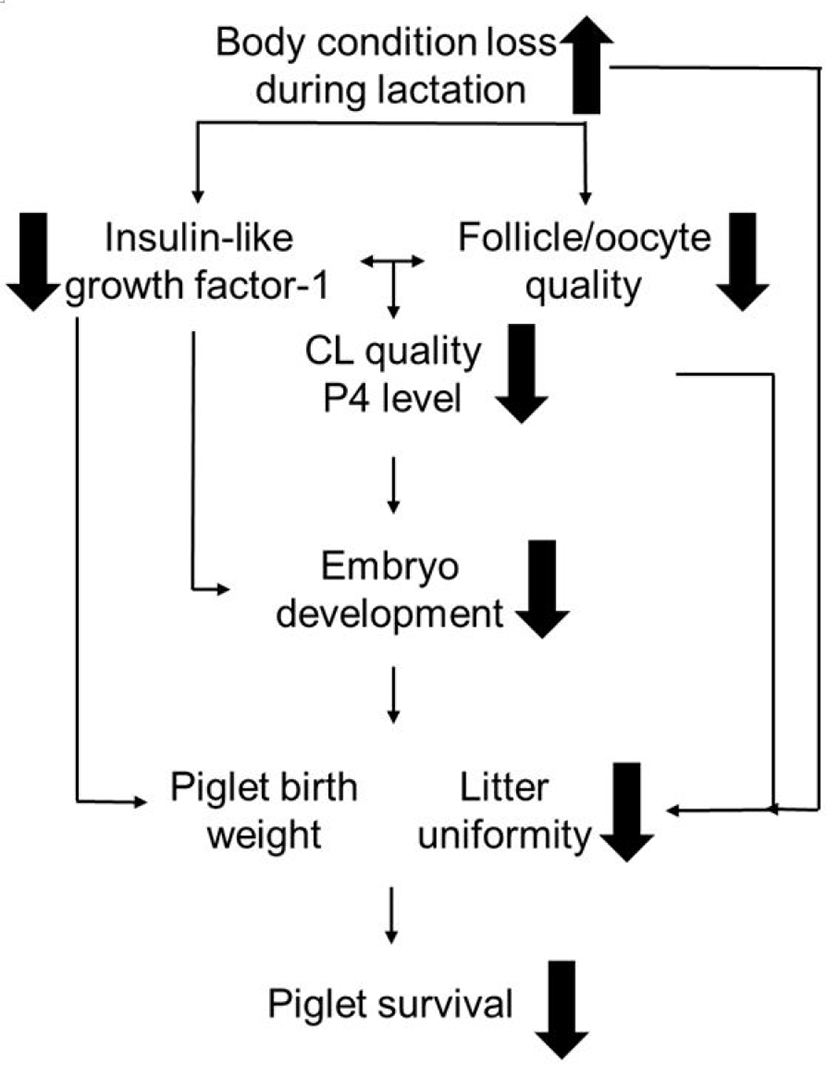
Studies have shown that impaired follicle development at weaning can result in a compromised follicle pool before ovulation [72] and a lower oocyte maturation rate [13,14]. Further, there is a positive relationship between follicle diameter at ovulation and corpus luteum (CL) diameter after ovulation [76,82]. Good CL development is necessary for embryo development during early pregnancy [2,83,84], as CL has been shown to positively relate with progesterone level and pulse [85–88]. Smaller follicles at ovulation may therefore be detrimental for early embryo development. Considering that piglet characteristics are largely determined at the early embryo developmental stage [89], we suggest that follicle diameters at weaning may also be related to piglet characteristics. Likewise, the heterogeneity of the follicle pool before ovulation may have an impact on litter uniformity at birth with a similar mechanism (reviewed by [71]).
IGF-1 is a possible mediator affecting follicle and oocyte development. It is very important in follicular fluid, as it can bind to IGF-1 receptors on the oocytes and granulosa cells. Once bound, it may synergize with FSH so as to activate follicular growth, steroidogenesis, and the oocyte cleavage rate [90–93]. A recent study also found that IGF-1 in the follicular fluid is positively related to follicle diameter before ovulation [14]. During WEI, sow plasma IGF-1 level is strongly related to the follicular IGF-1 level [14] and its levels at weaning are positively related to those during WEI [15,94]. Thus, higher IGF-1 and larger follicles at weaning appear to favour higher oocyte quality. The IGF-1 level around ovulation is also positively related to CL diameter and the increment of progesterone level after ovulation [95], and to embryo survival during early pregnancy [11]. Our recent study observed that higher plasma IGF-1 before ovulation (at oestrus) was positively related to piglet mean birth weight [41]. Thus, IGF-1 -mediating follicle development, which was affected by NEB [14,15], has a major impact on subsequent sow fertility. In addition, extracellular vesicles may be among further mechanisms through which NEB-driven reduction in follicle development can affect the developing ova within the follicle, as shown for canines in vitro [96].
As a consequence of breeding for a large litter, the ovulation rate (OR) has increased and is currently approximate to 25–30 (reviewed by [97]). Embryonic and piglet mortality have increased with increased OR [97,98]. However, the number of piglets could only increase to a certain limit because of the higher embryonic mortality associated with increased OR (reviewed by [97]). Early embryonic mortality occurs before implantation (around 12 or 13 days of gestation), while late embryonic mortality occurs after implantation between 13 and 35 days of gestation. In sows, early embryonic mortality increased with increasing OR and was approximately 59% of the total embryonic mortality [97]. Embryonic heterogeneity within litters may be a major reason for early embryonic mortality. Less-developed embryos cannot develop further in a uterine environment, which is advanced by the more-developed embryos (reviewed by [99]). In detail, oestradiol produced from more-developed embryos stimulates uterine secretions for their own implantation but this results in an unfavourable environment for less-developed embryos [100,101]. Synchrony between developing embryos and the uterine environment is important for successful implantation. Embryos lagging behind in development may experience a uterine environment that is asynchronous with their own development and implantation may therefore fail [102]. Considering that embryonic heterogeneity is largely affected by follicle heterogeneity [99,103], the importance of follicle development before ovulation is once again highlighted. However, increased OR also seems to associate with compromised follicle development. Sows with increased OR showed decreased CL diameters, which were derived from a decreased follicle diameter [76,82]. This implies that breeding for a large litter likely contributed to compromised follicle development. Although less-developed embryos may survive through the implantation process, they may be more vulnerable to dying later during gestation. Late embryonic mortality was ca. 42% of the total mortality and it also increased as OR increased [97]. Limited uterine capacity and competition for space and/or nutrients are major reasons for late embryonic mortality (reviewed by [101]). Da Silva et al. [97] showed that embryos with small size and small implantation sites had higher mortality at a late stage of pregnancy. The small size of the implantation site can be linked to a small placental site [104], which may be harmful to foetal development.
Only five or six days of WEI appears too short to recover from severe NEB in hyper-prolific sows and to support their follicles in reaching the pre-ovulatory size and high-quality oocytes. Thus, skipping the first heat and inseminating at the second oestrus may be recommended for sows with severe body condition loss during lactation. This recommendation stems from the study showing that a longer weaning-to-pregnancy interval (WPI; > 21 day) resulted in better litter uniformity (i.e., lower SD and CV at birth weight; [17]. Wientjes et al. [17] explained that this may be due to the longer recovery of metabolic states and the restoration of follicle development, which is beneficial for subsequent fertility.
Pre-mating diets are one option for stimulating follicle and oocyte development. A fibre-rich pre-mating diet (e.g., sugar beet pulp) before ovulation can have a positive impact on oocyte quality and maturation in the gilts [105]. Furthermore, supplementing insulin- or IGF-1-stimulating diets (dextrose and lactose) during lactation and WEI can improve litter uniformity [106,107]. Nevertheless, only a few nutritional factors have been evaluated as components of pre-mating diets. Considering that sow IGF-1 levels after weaning are positively related to pre-weaning levels [15,94], pre-mating diets during the late or whole lactation period may prove effective. Optimizing sow metabolic state during lactation is also recommended. This may be done by identifying the ideal feed composition of lactation diets, such as protein and amino acids levels, especially in a hyper-prolific situation.
CONCLUSION
Large litters do not come without a catch. Increased litter size creates problems with piglet survival during lactation and sow reproduction that need addressing. Large litters only occur through increased ORs. These rates are associated with compromised follicles that appear to negatively affect early embryonic development and pregnancy-supporting mechanisms such as CL development. These impaired developments result in increased embryonic and foetal mortality. At the end of pregnancy, the process of parturition also seems tightly linked with litter size. Increased litter size prolongs the process of parturition, leaving a proportion of the litter with reduced chances for suckling high-quality colostrum for a reduced period of time under increased competition Therefore, farrowing, early lactation management procedures and late lactation nutritional management are keys to tackling the increasing problems associated with large litters. In particular, feeding strategies before farrowing can be recommended for reducing farrowing duration. For neonatal piglets, additional management routines during parturition may increase piglet colostrum intake and their survival. Nutritional management of the sow around the end of lactation, involving IGF-1-driven follicle development, seems to be important for piglet birth weight and survival at subsequent farrowing.
















